Published online Feb 8, 2017. doi: 10.4254/wjh.v9.i4.209
Peer-review started: July 22, 2016
First decision: September 5, 2016
Revised: November 6, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: February 8, 2017
Processing time: 200 Days and 6.7 Hours
To evaluate the effects of aqueous extract of Salep on Paraquat-mediated liver injury.
In this experimental study, 56 adult male Wistar rats were divided randomly to 7 groups as control, sham, and 5 experimental groups. In control group, rats did not receive any substance during experiment. In Sham group, rats were given distilled water according to their body weight and in experimental groups, Paraquat alone and with different doses of Salep aqueous extract (40, 80, 160 and 320 mg/kg) was given intraperitoneal daily for 14 d. After that, liver biochemical parameter and histologic changes were analyzed and compared in different groups.
Paraquat compared to control and sham groups, significantly (P < 0.05) increased serum level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, malondialdehyde (MDA) and total oxidant capacity (TOC); while level of total protein, albumin and total antioxidant capacity (TAC) were remarkably decreased by Paraquat. Salep at doses of 80, 160 and 320 mg/kg significantly decreased serum level of ALT, AST, ALP, bilirubin, MDA and TOC and significantly increased total protein, albumin and TAC level as compared to Paraquat exposed group in dose dependent manner. Aqueous extract of Salep at doses of 40 mg/kg made no significant changes in serum level of mentioned biochemical parameters. Liver microscopic observation revealed that Paraquat could cause hepatocyte necrosis, degenerative changes, proliferation and activation of Kupffer cells (sporadically) which were reduced by Salep treatment.
Salep possesses remarkable hepatoprotection activity against Paraquat-induced hepatic injury by having antioxidant activity and reducing lipid peroxidation and oxidative stress.
Core tip: Oxidative stress has a key role in triggering Paraquat-mediated liver injury. Paraquat causes oxidative stress via modulation of redox cycling, generation of free radicals and reduction of endogenous antioxidant levels. Salep from orchid family (Orchidaceae) used in traditional medicine as a healing agent in the treatment of breast disorders, gastrointestinal disorders, tuberculosis, diarrhea, Parkinson, cancer, fever, and impotency. Salep is used in food engineering for preparation of ice cream and drinks. This study showed that Salep could have a protective effect against Paraquat-induced hepatic injury via reinforcing endogenous antioxidant systems, reduction of lipid peroxidation and free radical scavenging. The antioxidant and protective effect of Salep could be due to presence of flavonoids and polyphenols such as Quercetin, Ferulic Acid and Glucomannan.
- Citation: Atashpour S, Kargar Jahromi H, Kargar Jahromi Z, Zarei S. Antioxidant effects of aqueous extract of Salep on Paraquat-induced rat liver injury. World J Hepatol 2017; 9(4): 209-216
- URL: https://www.wjgnet.com/1948-5182/full/v9/i4/209.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i4.209
With the increasing population of human societies, providing nourishment without the use of advanced scientific farming is impossible. In this modern agriculture, using pesticides, herbicides and chemical fertilizers for more and higher quality crop is inevitable and may be toxic to man and animals. Paraquat (1, 1’dimethyl-4, 4’-bipyridylium-dichloride) is a widely used herbicide for broadleaf weed control[1] and is extremely poisonous for humans and animals and many cases of acute poisoning and death have been reported over the past few decades[2].
Paraquat is a bipyridyl compound with high toxicity for lungs, kidney, brain and liver[3]. When it is given in acute dose (50 mg/kg) in mice, liver necrosis and inflammation will develop[4]. Paraquat toxicity is due to oxidative damage to cells and generation of free radicals[5]. Herbicidal activity of paraquat can be explained by its interfering with photosynthesis and intracellular electron transfer system in plants and prevention of NADP reduction to NADPH. This could disrupt important NADPH-dependent biochemical processes[6,7]. In addition, Paraquat radical forms superoxide anion in presence of oxygen which leads to production of more toxic reactive oxygen species like hydrogen peroxides and hydroxyl radical and would cause oxidative stress[1,8]. Superoxide anion may also attack unsaturated lipids of membrane to form fatty acid hydroperoxide, resulting in lipid peroxidation, membrane injury, cell death and multi-system toxicity[9].
Due to the role of oxidative stress mechanisms in Paraquat toxicity and the lack of an effective antidote, researchers are currently focused on the importance of antioxidant in Paraquat poisoning management[10]. Many herbal compounds have antioxidant properties and can protect the liver from damaging agents like Paraquat. One of these plants is Salep from orchid family (Orchidaceae) which has different species worldwide[11]. Salep contains Quercetin, Nitrogenic materials, Ferulic acid, starch, protein, Glucomannan, Glucose, Daucosterol, Cirsilineol and steroids[11-13]. This plant is used in traditional medicine as a healing agent in the treatment of breast disorders, gastrointestinal disorders, tuberculosis, diarrhea, Parkinson, cancer, fever, and impotency. Salep is used in food engineering for preparation of ice cream and drinks[13-15].
Polyphenols, especially flavonoids such as quercetin, are important antioxidants found in Salep[12]. These compounds have hepatoprotective effects against liver damage caused by toxins and free radicals[16] and can also protect cells against depletion of glutathione by increasing the capacity of antioxidant enzymes such as glutathione reductase, glutathione peroxide and catalase[17]. Furthermore, glucomannan can inhibit oxidative stress and effectively reduce alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels[18]. Therefore, the aim of this survey was to evaluate the effects of aqueous extract of Salep on Paraquat - induced hepatotoxicity.
Paraquate was purchased from Ara Shimi-Iran Company. Paraquat was dissolved in distilled water and animals were given intraperitoneal injection in each case. Malondialdehyde (MDA), total antioxidant capacity (TAC) and total oxidant capacity (TOC) measurement kits were purchased from Diametra Company (Italy), ALT, AST, alkaline phosphatase (ALP), albumin and bilirubin and total protein (TP) measurement kits were purchased from Pars Azmoon Company (Iran).
Salep plants were obtained from farmlands around Yasouj (a city in the southwest of Iran). Salep roots were washed and dried in laboratory and mixed with Ethanol 96° in 1 to 5 proportions, mixed for 24 h at room temperature and a homogeneous mixture was obtained. Then, the uniform solution was filtered and dried for 48 h to obtain solid extract without ethanol. The final dried extract was dissolved with distilled water[12].
Fifty-six adult male Wistar rats (180-200 g) were obtained from the Animal House of Jahrom University of Medical Sciences. The animal house temperature was maintained at 22 °C ± 2 °C with a 12 h light/dark cycle. All animals were kept for two weeks prior to experiment and had free access to food and water. All ethical points regarding working with laboratory animals were considered in this research (Ethical Code: IR.JUMS.1394.722).
The rats were divided randomly to 7 groups, 8 rats each, as followed: Control group: Rats did not receive any substance during experiment; Sham group: Rats were given distilled water according to their body weight during the experiment; Experimental group 1: Rats were given Paraquat 2 mg/kg per BW; Experimental groups 2, 3, 4 and 5: Rats were given Paraquat at a dose of 2 mg/kg per BW daily and Salep at doses of 40, 80, 160 and 320 mg/kg per BW, respectively. Salep doses were selected based on previous studies done on this herbal treatment[12]; Paraquat and Salep aqueous extract were administered intraperitoneally daily for 14 d in all 5 groups.
At the end of the study (day 15) after weighing the animals, blood sample were taken directly from their hearts using 5 cc syringes (rats were anesthetized by barbiturate) and blood serum was collected after centrifugation (15 min, 3000 rpm) and stored at -20 °C until they were tested. Biochemical measurement kits (made in Iran and Italy) using the colorimetric method and an autoanalyzer machine (Selectera XL model made in Holland) were used for assessment of biochemical factors including ALT, AST, ALP, TP, albumin, bilirubin, MDA, TOC and TAC.
After drawing the blood, for histological examination a small part of liver was separated, fixed by 10% formalin and embedded in paraffin wax. Paraffin sections with thickness of 5 μm were prepared, stained employing the haematoxylin and eosin and Masson Trichrome stain methods and histological and pathological changes were studied using a light microscope. Furthermore, The Degree of inflammation in the portal zone, liver necrosis and inflammatory cell infiltration were evaluated in the form of semiquantitative scale, double-blind, according to the method described by Frei et al[19] in 1984. Severity of damage were ranked from zero to four (zero: No damage, 1: Minimum damage, 2: Mild damage, 3: Average damage, 4: Severe damage). Scoring was performed in five microscopic fields of each cut, randomly, with magnification of × 100.
All values were given as mean ± SEM. Statistical analysis was carried out using SPSS 21, One-way analysis of variance followed by Duncan post hoc test. Statistical P-value less than 0.05 was considered significant.
Paraquat compared to control and sham groups significantly (P < 0.05) increased serum level of liver factors including ALT, AST and ALP, Bilirubin, MDA and TOC; while serum level of Total Protein, Albumin and TAC were considerably lower in group receiving Paraquat (Tables 1 and 2).
| Group/parameter | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Bilirubin (mg/dL) | MDA (nmol/L) | TOC (IU/mL) |
| Control | 183.4 ± 2.19 | 95.4 ± 2.52 | 138.4 ± 4.55 | 0.8 ± 0.02 | 0.13 ± 0.005 | 0.18 ± 0.01 |
| Sham | 185.5 ± 2.19 | 94.7 ± 3.00 | 138.9 ± 5.01 | 0.8 ± 0.02 | 0.13 ± 0.007 | 0.18 ± 0.01 |
| Paraquat at 2 mg/kg | 563.2 ± 11.43a | 265.5 ± 7.48a | 736.1 ± 4.21a | 2.4 ± 0.05a | 3.36 ± 0.06a | 2.02 ± 0.05a |
| Paraquat + Salep at 40 mg/kg | 536.4 ± 14.44b | 252.5 ± 6.25b | 730.0 ± 9.30b | 2.3 ± 0.04b | 3.27 ± 0.03b | 1.98 ± 0.05b |
| Paraquat + Salep at 80 mg/kg | 517.2 ± 7.30bc | 234.7 ± 7.14bc | 709.0 ± 9.14bc | 2.0 ± 0.04bc | 2.92 ± 0.13bc | 1.77 ± 0.05bc |
| Paraquat + Salep at 160 mg/kg | 460.5 ± 12.01bc | 209.4 ± 4.55bc | 626.4 ± 6.74bc | 1.7 ± 0.03bc | 2.48 ± 0.05bc | 1.55 ± 0.04bc |
| Paraquat + Salep at 320 mg/kg | 376.5 ± 12.07bc | 166.0 ± 3.75bc | 428.1 ± 7.25bc | 1.1 ± 0.03bc | 1.22 ± 0.04bc | 1.20 ± 0.03bc |
| Group/parameter | Total protein (g/dL) | Albumin (g/dL) | TAC (IU/mL) |
| Control | 8.0 ± 0.19 | 5.0 ± 0.13 | 1.11 ± 0.02 |
| Sham | 7.9 ± 0.26 | 5.1 ± 0.18 | 1.6 ± 0.04 |
| Paraquat at 2 mg/kg | 4.2 ± 0.09a | 2.5 ± 0.06a | 0.40 ± 0.02a |
| Paraquat + Salep at 40 mg/kg | 4.1 ± 0.05b | 2.6 ± 0.5b | 0.43 ± 0.02b |
| Paraquat + Salep at 80 mg/kg | 5.0 ± 0.10bc | 3.1 ± 0.04bc | 0.66 ± 0.03bc |
| Paraquat + Salep at 160 mg/kg | 5.7 ± 0.09bc | 3.6 ± 0.07bc | 0.82 ± 0.05bc |
| Paraquat + Salep at 320 mg/kg | 6.6 ± 0.11bc | 5.0 ± 0.07bc | 1.10 ± 0.03bc |
Paraquat treatment groups with aqueous extract of Salep at doses of 80, 160 and 320 mg/kg significantly decreased serum level of ALT, AST, ALP, Bilirubin, MDA and TOC and significantly increased elevated Total Protein, Albumin and TAC serum level as compared to Paraquat treatment group alone (Tables 1 and 2). Aqueous extract of Salep at doses of 40 mg/kg made no significant changes in serum level of mentioned biochemical parameters while the greatest effect is related to the dose of 320 mg/kg of Salep.
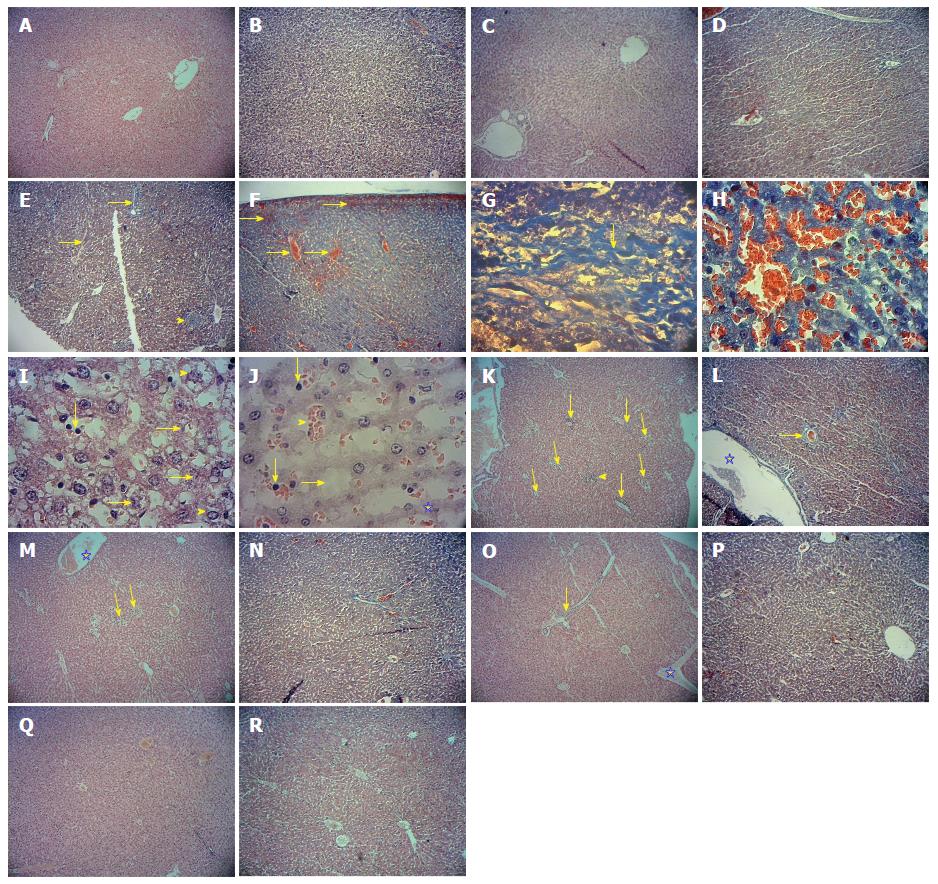
Microscopic examination of liver tissue of control and sham groups showed that liver tissue structure was normal and healthy (normal structure of lobules with normal central venous, sinusoids and Kupffer cells and normal distribution of glycogen and lack of lymphocytic infiltration and congestion in the blood vessels) (Figure 1A-D).
In study group 3 (rats were given Paraquat alone), microscopic observation revealed hepatocyte necrosis, degenerative changes, proliferation and activation of Kupffer cells (sporadically), increased infiltration of inflammatory cells around the portal vein and in sinusoid space, formation of fibrotic inflamed bridges between liver lobules, and sever cellular ballooning and blood congestion in the sinusoids. In this group, progressive liver fibrosis had occurred as evidenced by presence of collagen fibers in the liver parenchyma, the portal space and around the central vein in the centrilobular region (Figure 1E-J).
Treatment with aqueous extract of Salep reduced the damaging effect of Paraquat on liver tissue. This reduction in destructive effect of Paraquat on liver tissue was mild with Salep at doses of 80 mg/kg, moderate at doses of 160 mg/kg and highest at doses of 320 mg/kg as compared to study group that received Paraquat alone (Figure 1K-R). A microscopic observation of liver tissue of rats under study have been brought to a quantitatively in Table 3.
| Group/damage score | Control | Sham | Paraquat 2 mg/kg | Paraquat+ Salep at 40 mg/kg | Paraquat+ Salep at 80 mg/kg | Paraquat+ Salep at 160 mg/kg | Paraquat+ Salep at 320 mg/kg |
| Portal congestion and inflammation | |||||||
| Score 0 | 8 | 8 | 0 | 0 | 0 | 0 | 1 |
| Score 1 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Score 2 | 0 | 0 | 0 | 1 | 2 | 3 | 1 |
| Score 3 | 0 | 0 | 2 | 2 | 2 | 1 | 1 |
| Score 4 | 0 | 0 | 6 | 5 | 4 | 4 | 0 |
| Necrosis | |||||||
| Score 0 | 8 | 8 | 0 | 0 | 0 | 0 | 1 |
| Score 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Score 2 | 0 | 0 | 1 | 2 | 2 | 2 | 3 |
| Score 3 | 0 | 0 | 2 | 1 | 2 | 2 | 0 |
| Score 4 | 0 | 0 | 5 | 5 | 4 | 3 | 0 |
| Interstitial infiltration of inflammatory cells | |||||||
| Score 0 | 8 | 8 | 0 | 0 | 0 | 0 | 1 |
| Score 1 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Score 2 | 0 | 0 | 1 | 1 | 1 | 3 | 2 |
| Score 3 | 0 | 0 | 1 | 2 | 3 | 2 | 1 |
| Score 4 | 0 | 0 | 6 | 5 | 4 | 2 | 0 |
Oxidative stress has a key role in triggering Paraquat- mediated liver injury[20]. Paraquat causes oxidative stress via modulation of redox cycling, generation of free radicals and reduction of endogenous antioxidant levels[21-23]. Furthermore, generation of nitric oxide and reactive oxygen species like superoxide also play a crucial role in Paraquate - induced hepatotoxicity[24]. Salep could have protective effect against chemical induced liver injury via reinforcing endogenous antioxidant systems and free radical scavenging[12,16]. As liver is one of the major sites of Paraquat toxicity, this study was done in order to evaluate protective potential of Salep against Paraquat- induced liver injury.
Remarkable increase of ALT, AST, ALP and bilirubin and significant decrease of total protein and Albumin levels were observed in Paraquat - exposed group in comparison with control group, which confirmed the hepatotoxic potential of Paraquat. These results were in concurrence with previous studies on evaluation of Paraquat induced liver toxicity, which showed increase in serum level of liver enzymes[21,25]. Significant reduction of increased level of ALT, AST, ALP and bilirubin and marked increased in level of Albumin and Total Protein in Paraquat + Salep treated groups showed that Salep could have protective effect against Paraquat-mediated hepatic injury in dose dependent manner. These results are supported by a previous report which also revealed the protective effect of Salep on liver function[12].
Liver pathological examination showed hepatocyte necrosis, proliferation and activation of Kupffer cells, increased infiltration of inflammatory cells around the portal vein and in sinusoid space, formation of fibrotic inflamed bridges between liver lobules, and sever cellular ballooning and blood congestion in the sinusoids in Paraquat - exposed group, which was in accordance with previous reports[3,20]. Remarkable recovery toward normal liver histology in Paraquat + Salep treated groups also favored the protective activity of Salep against Paraquat-induced liver injury.
As oxidative stress has a crucial role in Paraquat- induced liver injury, in this study we evaluated serum level of TOC, which precisely shows the oxidant status of blood, and TAC, as indicator of blood, cells and tissues defense system against free radicals, measures the antioxidant capacity of all antioxidants in a biological sample and not just the antioxidant capacity of a single compound. Measurement of TAC can provide information on overall antioxidant status, which may include those antioxidants not yet recognized or not easily measured[20,26]. Significant augmentation of TOC and reduction of TAC were observed in Paraquat-exposed groups, which confirmed the role of free radical generation and attenuation of antioxidant level in Paraquat-medicated hepatic injury. Significant reduction of TOC and marked increased of TAC in Paraquate + Salep treated groups demonstrated that Salep could have protective effects against Paraquat toxicity by possessing antioxidant activity. These results were supported by a previous study done by Pourahmad et al[12]. The antioxidant effect of Salep could be due to the presence of flavonoids and polyphenols such as Quercetin, Ferulic Acid and Glucomannan[11,16,18]. The two latter components of Salep could also reduce serum level of liver enzymes such as ALT and AST[27,28]. Zhang et al[16] showed that Quercetin could have hepatoprotective and antioxidant activity by decreasing lipoxygenase, free radical scavenging, enhancing the expression of antioxidant transcription factor and antioxidant enzyme such as Thioredoxin and Peroxiredoxin.
Furthermore, part of Paraquat hepatotoxicity is related to lipid peroxidation due to free radical generation including Oxygen Reactive Species[24]. MDA works as an indicator of lipid peroxidation and oxidative stress assessment[29]. MDA level was significantly augmented in Paraquat - exposed group which was in accordance with previous studies[6]. Serum level of MDA was substantially decreased in Paraquat + Salep treated groups as compared with Paraquat - exposed group in dose dependent manner. This result also supported the protective activity of Salep against oxidative stress and lipid peroxidation caused by Paraquat.
In conclusion, based on our results, it could be concluded that Salep possesses remarkable hepatoprotection activity against Paraquat-induced liver injury and could reduce the damaging effect of Paraquat on liver by having antioxidant activity and reducing lipid peroxidation and oxidative stress. Further studies are required to evaluate protective and antioxidant effect of Salep in human.
We would like to thank the Vice Chancellor of Research of Jahrom University of Medical Sciences.
Paraquat is a common herbicide used in agriculture and could cause severe damage to the lungs, liver and other tissues in mammals. Oxidative stress has a key role in triggering Paraquat-mediated hepatotoxicity. Salep could have protective effect against chemical induced hepatotoxicity via reinforcing endogenous antioxidant systems and free radical scavenging.
In the present study, the authors found that Salep possesses remarkable hepatoprotection activity against Paraquat-induced liver injury and could reduce damaging effect of Paraquat on liver by having antioxidant activity and reducing lipid peroxidation and oxidative stress.
This is the first study evaluating the effect of Salep on Paraquat-induced liver injury. This study investigates the protective and antioxidant effect of salep on liver damage caused by Paraquat. The results of current study demonstrated that Salep could ameloriate paraquate-mediated liver injury by having antioxidant activity and reducing lipid peroxidation and oxidative stress.
Salep aqueous extract could reduce damaging effect of Paraquat on liver tissue by having significant antioxidant activity. Therefore, the results of this study showed that Salep can be introduced as an alternative to chemical agents as potential therapeutic strategies for Paraquate-induced liver injury.
Oxidative stress is essentially an disturbance in balance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidant which could cause tissue damange including liver. Lipid peroxidation is a crucial step in the pathogenesis of several disease states in adult and infant patients. Lipid peroxidation is a process mainly caused by the effect of reactive oxygen species including hydroxyl radical and hydrogen peroxide. These reactive oxygen species readily attack the polyunsaturated fatty acids of the cell membrane, initiating a self-propagating chain reaction. The destruction of membrane lipids and the end-products of such lipid peroxidation reactions are dangerous for the viability of cells, even tissues.
The paper by Atashpour et al has an interesting rationale and a good background. The results presented are consistent with the effects of the different components of the Salep.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cardinale V, Grattagliano I S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Burk RF, Lawrence RA, Lane JM. Liver necrosis and lipid peroxidation in the rat as the result of paraquat and diquat administration. Effect of selenium deficiency. J Clin Invest. 1980;65:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 189] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning. An overview of the current status. Drug Saf. 1990;5:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Awadalla EA. Efficacy of vitamin C against liver and kidney damage induced by paraquat toxicity. Exp Toxicol Pathol. 2012;64:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Dragin N, Smani M, Arnaud-Dabernat S, Dubost C, Moranvillier I, Costet P, Daniel JY, Peuchant E. Acute oxidative stress is associated with cell proliferation in the mouse liver. FEBS Lett. 2006;580:3845-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Goldstein BD, Rozen MG, Quintavalla JC, Amoruso MA. Decrease in mouse lung and liver glutathione peroxidase activity and potentiation of the lethal effects of ozone and paraquat by the superoxide dismutase inhibitor diethyldithiocarbamate. Biochem Pharmacol. 1979;28:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Bus JS, Aust SD, Gibson JE. Paraquat toxicity: proposed mechanism of action involving lipid peroxidation. Environ Health Perspect. 1976;16:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 453] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 8. | Cochón AC, Della Penna AB, Kristoff G, Piol MN, San Martín de Viale LC, Verrengia Guerrero NR. Differential effects of paraquat on oxidative stress parameters and polyamine levels in two freshwater invertebrates. Ecotoxicol Environ Saf. 2007;68:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Autor AP. Reduction of paraquat toxicity by superoxide dismutase. Life Sci. 1974;14:1309-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 107] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Vale JA, Meredith TJ, Buckley BM. Paraquat poisoning: clinical features and immediate general management. Hum Toxicol. 1987;6:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 148] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Barone Lumaga MR, Cozzolino S, Kocyan A. Exine micromorphology of Orchidinae (Orchidoideae, Orchidaceae): phylogenetic constraints or ecological influences? Ann Bot. 2006;98:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Pourahmad M, Kargar Jahromi H, Kargar Jahromi Z. Protective effect of salep on liver. Hepat Mon. 2015;15:e28137. [PubMed] |
| 13. | Farhoosh R, Riazi A. A compositional study on two current types of salep in Iran and their rheological properties as a function of concentration and temperature. Food Hydrocolloids. 2007;21:660-666. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Tekinsen KK, Güner A. Chemical composition and physicochemical properties of tubera salep produced from some Orchidaceae species. Food Chem. 2010;121:468-471. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Kayacier A, Dogan M. Rheological properties of some gums-salep mixed solutions. J Food Eng. 2006;72:261-265. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Zhang JQ, Shi L, Xu XN, Huang SC, Lu B, Ji LL, Wang ZT. Therapeutic detoxification of quercetin against carbon tetrachloride-induced acute liver injury in mice and its mechanism. J Zhejiang Univ Sci B. 2014;15:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910-6916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 464] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Donmez N, Keskin E. The Effects of Aflatoxin and Glucomannan on Some Antioxidants and Biochemical Parameters in Rabbits. Acta Veterinaria. 2008;58:307-313. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Frei A, Zimmermann A, Weigand K. The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology. 1984;4:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Ahmad I, Kumar A, Shukla S, Prasad Pandey H, Singh C. The involvement of nitric oxide in maneb- and paraquat-induced oxidative stress in rat polymorphonuclear leukocytes. Free Radic Res. 2008;42:849-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Ahmad I, Shukla S, Kumar A, Singh BK, Kumar V, Chauhan AK, Singh D, Pandey HP, Singh C. Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats. Chem Biol Interact. 2013;201:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Ahmad I, Shukla S, Kumar A, Singh BK, Patel DK, Pandey HP, Singh C. Maneb and paraquat-induced modulation of toxicant responsive genes in the rat liver: comparison with polymorphonuclear leukocytes. Chem Biol Interact. 2010;188:566-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 415] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Hong SY, Yang DH, Hwang KY. Associations between laboratory parameters and outcome of paraquat poisoning. Toxicol Lett. 2000;118:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Ferrari CK. Effects of xenobiotics on total antioxidant capacity. Interdiscip Toxicol. 2012;5:117-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Rukkumani R, Aruna K, Suresh Varma P, Padmanabhan Menon V. Hepatoprotective role of ferulic acid: a dose-dependent study. J Med Food. 2004;7:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Dvorska JE, Pappas AC, Karadas F, Speake BK, Surai PF. Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1862] [Article Influence: 93.1] [Reference Citation Analysis (0)] |