Published online Dec 28, 2017. doi: 10.4254/wjh.v9.i36.1372
Peer-review started: September 25, 2017
First decision: October 9, 2017
Revised: November 20, 2017
Accepted: December 5, 2017
Article in press: December 5, 2017
Published online: December 28, 2017
Processing time: 93 Days and 18.7 Hours
Preoperative radioembolization may improve the resectability of liver tumor by inducing tumor shrinkage, atrophy of the embolized liver and compensatory hypertrophy of non-embolized liver. We describe the case of a cirrhotic Child-Pugh A patient with a segment IV hepatocellular carcinoma requiring a left hepatectomy. Preoperative angiography demonstrated 2 separated left hepatic arteries, for segment IV and segments II-III. This anatomic variant allowed sequential radioembolizations, delivering high-dose 90Yttrium (160 Gy) to the tumor, followed 28 d later by lower dose (120 Gy) to segments II-III. After 3 mo, significant tumor response and atrophy of the future resected liver were obtained, allowing uneventful left hepatectomy. This case illustrates that, when anatomic disposition permits it, sequential radioembolizations, delivering different 90Yttrium doses to the tumor and the future resected liver, could represent a new strategy to prepare major hepatectomy in cirrhotic patients, allowing optimal tumoricidal effect while reducing the toxicity of the global procedure.
Core tip: Preoperative radioembolization may improve resectability of hepatocellular carcinoma in cirrhotic patient, inducing tumor downsizing, atrophy of radio-embolized sector and regeneration of non-embolized liver. We describe a patient with a segment IV hepatocellular carcinoma where the presence of two separated left hepatic arteries permitted to deliver sequentially high-dose 90Yttrium to the tumor and lower dose to future resected liver, allowing uneventful left hepatectomy 3 mo later. This observation suggests that, when different arterial accesses exist to tumor and future resected non-tumor liver, sequential radioembolization with different radiation doses could represent a new preoperative strategy, optimizing the tumoricidal effect while minimizing the risk of radiation-induced liver damage.
- Citation: Vouche M, Degrez T, Bouazza F, Delatte P, Galdon MG, Hendlisz A, Flamen P, Donckier V. Sequential tumor-directed and lobar radioembolization before major hepatectomy for hepatocellular carcinoma. World J Hepatol 2017; 9(36): 1372-1377
- URL: https://www.wjgnet.com/1948-5182/full/v9/i36/1372.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i36.1372
Partial hepatectomy (PH) and tumor destruction with radiofrequency (RF) are the first therapeutic options in patients with hepatocellular carcinoma (HCC) and compensated cirrhosis who are not candidates for liver transplantation (LT)[1,2]. However, the feasibility and efficacy of these treatments are dramatically limited by underlying liver disease and high tumor recurrence rates. At the present time, no neoadjuvant treatment has been validated for improving the safety and efficacy of PH and RF in this setting. In particular, locoregional treatment with transarterial chemoembolization (TACE) has failed to demonstrate significant long-term benefits when used before PH or RF for HCC[3-5]. Furthermore, when a major resection of 3 or more segments is indicated in cirrhotic livers, preoperative homolateral portal vein embolization (PVE) is recommended to induce an atrophy of the future resected liver and a compensatory hypertrophy of the future liver remnant (FLR)[6,7]. This strategy, however, leaves the tumor untreated while waiting for liver regeneration, exposing the patient to the risk of tumor progression before the surgery[8].
Selective internal radiotherapy (SIRT), relying on the transarterial embolization of 90yttrium-loaded microspheres (90Y), has become a new tool for treatment of liver tumors. In HCC, SIRT has been demonstrated to improve survival in patients who are not candidates for curative-intent therapies and to allow tumor control while waiting for LT[9-13]. Furthermore, SIRT can be used preoperatively and the feasibility and safety of post-SIRT surgery has been now assessed[14-17]. The tumoricidal effect of SIRT, leading to tumor downsizing, may significantly modify the extent of surgery or allow the resection of initially unresectable tumors. Moreover, regional intra-arterial hepatic embolization with 90Y could also induce the atrophy of the embolized segments and a compensatory hypertrophy of the non-embolized liver[18,19]. This specificity allows for the design of new therapeutic strategies, integrating neoadjuvant SIRT into current surgical approaches to liver tumors, particularly for HCC in cirrhotic patients.
We describe here the case of a patient with centrally-located HCC, treated with sequential intra-tumor and left lobar 90Y embolization before a left hepatectomy. This case illustrates the new possibilities offered by the use of SIRT as a preoperative therapy before major liver resection for HCC in cirrhotic patients.
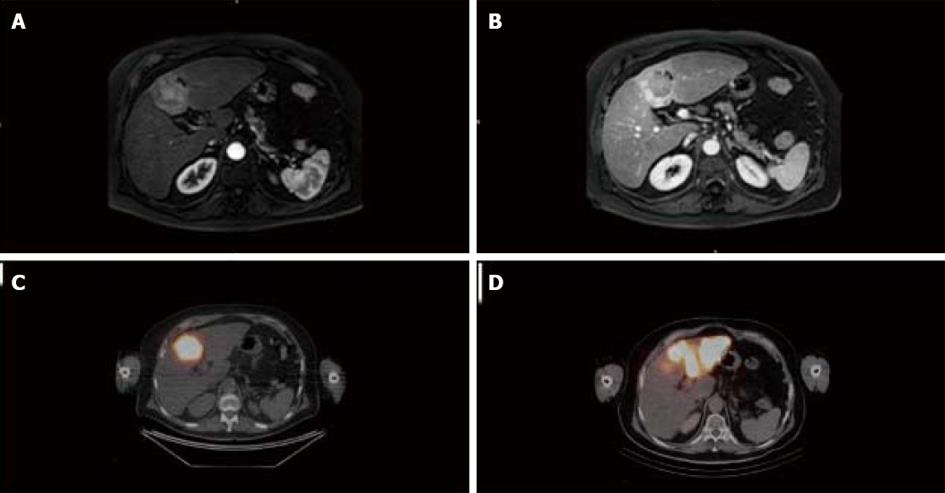
A 70-year-old man with a past history of alcohol consumption presented with a liver tumor. Contrast-enhanced magnetic resonance imaging (MRI) demonstrated a 40 mm mass in segment IV with vascular characteristics of HCC (arterial wash-in and portal wash-out) and features of cirrhosis (Figure 1A and B). Blood tests, including liver function and alpha-fetoprotein, were normal and the patient was classified as Child-Pugh A, with a MELD score of 7. Complete work-up did not demonstrate extra-hepatic metastasis. Accordingly, the tumor corresponded to Okuda stage 1 and BCLC stage A. Due to the patient’s age, the comorbidities, and the patient’s preferences, LT was not recommended during multidisciplinary meeting. Therefore, a left hepatectomy (resection of segments II-III-IV) was proposed and, due to the presence of cirrhosis, preoperative treatment to modulate FLR volume and function was indicated. Analysis of liver volumes on angio-CT scan showed a total liver volume (TLV) of 2339 mL, a tumor volume of 36 mL, a left liver volume (segments II, III, IV) of 812 mL, and an FLR volume (segments I, V, VI, VII, VIII) of 1527 mL, corresponding to a FLR/TLV of 65% and an FLR/body weight ratio of 0.68. On the basis of our previous experience[20] and in relation to the proximity of the tumor to the portal bifurcation that might preclude the chance for resection in case of progression, SIRT was preferred to PVE as preoperative treatment.
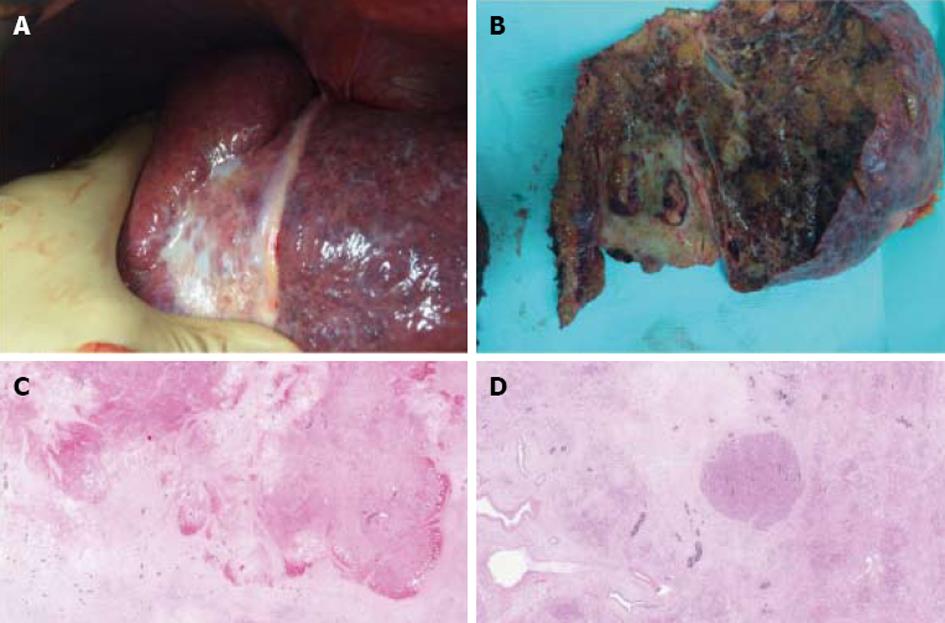
Simulation of SIRT with 99Tc macroaggregated albumin showed no extra-hepatic deposition and excellent tumor targeting. In addition, the angiography demonstrated a variant hepatic arterial anatomy characterized by a left hepatic artery arising from the right gastric artery, a segment IV artery arising from the gastroduodenal artery and a right hepatic artery arising normally from the celiac trunk. Therefore, 2-step SIRT using different 90Y doses was decided upon in order to maximize the dose of 90Y selectively delivered to the tumor and to minimize the potential toxicity related to intense radioembolization of a large liver volume. First, 90Y hyperselective radioembolization of the segment IV artery to the tumor was performed, allowing the delivery 161 Gy to segment IV (Figure 1C). No side effects related to this procedure were observed. Twenty-eight days later, the left hepatic artery was catheterized and 90Y microspheres injected, allowing for the delivery of 120 Gy to segments II and III (Figure 1D). No side effects were observed following this procedure. At day 110 after the second SIRT, contrast-enhanced MRI showed a significant tumor response (size reduction of the tumor diameter from 40 to 34 mm and complete necrosis on arterial phase) (Figure 1D). On the same examination, segments II, III, and IV measured 545 mL, corresponding to a 34% reduction, and FLR measured 1643 mL, corresponding to a minimal increase of 2%. At day 115 after the second SIRT, a left hepatectomy, partially extended to segment V, was performed. Operative exploration confirmed the cirrhosis while the entire left lobe appeared as atrophic and fibrotic (Figure 2A). The surgery proceeded uneventfully. Intraoperative blood losses were 800 mL and no blood transfusions were required. Postoperative course was unremarkable clinically and biologically (minimal values of PT, peak INR, and total bilirubin respectively of 56%, 1.3, and 1.5 mg/dL on day 3 after surgery) and the patient was discharged on day 14. On macroscopic examination of the operative specimen, small foci of cancer cells < 5 mm were observed within a tumor necrotic/fibrotic zone of 55 mm in diameter (Figure 2b). Pathological examination demonstrated a margin-free resection and a major tumor response as indicated by approximately less than 10% of residual cancer cells (Figure 2C and D).
PH remains the treatment of choice in patients with large HCC and compensated cirrhosis without significant portal hypertension and who are not candidates for LT[1]. When a major resection is required, preoperative PVE to adapt the FLR is currently considered as the standard procedure. The present case illustrates that neoadjuvant SIRT before surgery may represent now an alternative to this classical sequence. The rationale for considering the use of SIRT before PH for HCC in cirrhotic patients relies on several factors. The first is that SIRT is an effective local treatment for HCC[13]. Thus, if liver surgery would ultimately be found to be infeasible, the patient would still receive an efficient anti-tumor therapy. Secondly, when 90Y microspheres are administered both selectively in the tumor and regionally in the future resected liver segments (radiation lobectomy), SIRT has the unique capacity to induce an effective tumoricidal effect together with the atrophy of the future resected liver and a compensatory hypertrophy of the FLR. As compared with preoperative PVE, this may reduce the risk of tumor progression while waiting for functional and volumetric adaptation of the FLR. Finally, and as described for TACE[21], response to SIRT may potentially serve as a predictive factor both for the safety and the efficacy of the surgery. The feasibility of major liver resection after 90Y radiation lobectomy has been assessed. However, particularly in cirrhotic livers, such large liver volume irradiation exposes the patient to the risk of radiation-induced liver disease (RILD)[22]. In the present case, the hepatic arterial anatomy allowed to perform a 2-step SIRT, delivering first high 90Y dose to the segment IV tumor, followed by an ablative but safe irradiation dose to left lobe (segments II and III). As a dose-tumor response correlation was demonstrated over 170 Gy[23] and FLR volume modulation was found for doses approximating 120 Gy[18], such sequential procedures may potentially optimize the neoadjuvant effect of the treatment while reducing the toxicity and the risk of RILD. At 3 mo after SIRT, we observed volumetric effects within the embolized regions, as indicated by significant tumor shrinking and left lobe atrophy. In contrast, virtually no increase of the non-embolized FLR was detected, potentially related to the relatively short time period between SIRT and surgery[18]. Despite the absence of significant volumetric regeneration of the right liver, no sign of liver insufficiency has been observed after the left hepatectomy, potentially in relation with favorable initial FLR/TLV ratio. Finally, this case indicates that, despite the so-called ablative 90Y dose given to the tumor, a complete pathological response was not obtained, highlighting the need to still resect these irradiated tumors whenever possible.
In conclusion, when distinct arteries to the tumor and to the future resected liver can be selectively catheterized, sequential 90Y embolization with modulated doses to the tumor and to the future resected liver could represent a new strategy for improving the safety and the efficacy of neoadjuvant radioembolization before major liver resection in cirrhotic patients. The potential oncological benefit of this therapeutic combination remains to be evaluated.
A seventy years old patient presented with a segment IV liver tumor.
Due to the presence of alcohol-related cirrhosis, a diagnosis of hepatocellular carcinoma was suspected.
Differential diagnosis included other solid liver tumors, primary or secondary.
Laboratory data, including alpha-fetoprotein were not contributive.
Contrast-enhanced magnetic resonance imaging demonstrated a 40 mm mass in segment IV of the liver with vascular characteristics of hepatocellular carcinoma, such as arterial phase wash-in and portal phase wash-out and features of cirrhosis. Angiography demonstrated two separated left hepatic arteries, for segment IV and for segments II and III, allowing selective access to the tumor and to the future resected liver.
On operative specimen, pathology confirmed the diagnosis of hepatocellular carcinoma and a major response to preoperative radioembolization as indicated by less than 10% residual cancer cells.
Left hepatectomy was preceded by sequential radioembolizations, delivering high-dose radiation to the tumor and then, lower dose to the future resected liver. This 2-steps approach aimed to maximize tumoricidal effect while limiting the risks for radiation-induced liver disease and liver insufficiency.
In such cases of hepatocellular carcinoma requiring a major hepatectomy in patients with compensated cirrhosis, resectability is dramatically limited by the risk of postoperative liver insufficiency.
This case indicates that, when arterial anatomy allows it, sequential radioembolizations with different radiation doses to the tumor and to the future resected liver could represent a new strategy to maximize the tumoricidal effect while preserving the atrophic effect but reducing the risk of radiation-induced liver injury.
We acknowledge the contribution of a medical writer, Sandy Field, PhD, for editing of this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Bramhall S, Hori T, Kai K, Qin JM S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 2. | Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, Lau WY, Wu MC. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, Hwang S. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Si T, Chen Y, Ma D, Gong X, Yang K, Guan R, Peng C. Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma in Asia area: a meta-analysis of random controlled trials. Scand J Gastroenterol. 2016;51:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Beppu T, Okabe H, Okuda K, Eguchi S, Kitahara K, Taniai N, Ueno S, Shirabe K, Ohta M, Kondo K. Portal Vein Embolization Followed by Right-Side Hemihepatectomy for Hepatocellular Carcinoma Patients: A Japanese Multi-Institutional Study. J Am Coll Surg. 2016;222:1138-1148.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Glantzounis GK, Tokidis E, Basourakos SP, Ntzani EE, Lianos GD, Pentheroudakis G. The role of portal vein embolization in the surgical management of primary hepatobiliary cancers. A systematic review. Eur J Surg Oncol. 2017;43:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812-817; discussion 817-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, Abecassis M, Baker T, Gates V, Nayar R. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Ettorre GM, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Maini CL, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Lastoria S; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 12. | Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (30)] |
| 14. | Cucchetti A, Cappelli A, Ercolani G, Mosconi C, Cescon M, Golfieri R, Pinna AD. Selective Internal Radiation Therapy (SIRT) as Conversion Therapy for Unresectable Primary Liver Malignancies. Liver Cancer. 2016;5:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Justinger C, Kouladouros K, Gärtner D, Tatsch K, Reimer P, Rüdiger T, Binnenhei M, Bentz M, Schön MR. Liver resection after selective internal radiotherapy (SIRT): Proof of concept, initial survival, and safety. J Surg Oncol. 2015;112:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Iñarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D’Avola D, Herrero JI, Rodriguez M, Martí P, Zozaya G. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Pardo F, Sangro B, Lee RC, Manas D, Jeyarajah R, Donckier V, Maleux G, Pinna AD, Bester L, Morris DL. The Post-SIR-Spheres Surgery Study (P4S): Retrospective Analysis of Safety Following Hepatic Resection or Transplantation in Patients Previously Treated with Selective Internal Radiation Therapy with Yttrium-90 Resin Microspheres. Ann Surg Oncol. 2017;24:2465-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, Gaba RC, Mulcahy MF, Baker T, Sato K. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 19. | Garlipp B, de Baere T, Damm R, Irmscher R, van Buskirk M, Stübs P, Deschamps F, Meyer F, Seidensticker R, Mohnike K. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Bouazza F, Poncelet A, Garcia CA, Delatte P, Engelhom JL, Gomez Galdon M, Deleporte A, Hendlisz A, Vanderlinden B, Flamen P. Radioembolisation and portal vein embolization before resection of large hepatocellular carcinoma. World J Gastroenterol. 2015;21:9666-9670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Lei JY, Zhong JJ, Yan LN, Zhu JQ, Wang WT, Zeng Y, Li B, Wen TF, Yang JY; -Liver Surgery Group. Response to transarterial chemoembolization as a selection criterion for resection of hepatocellular carcinomas. Br J Surg. 2016;103:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, Benito A, Dominguez I, D’Avola D, Herrero JI. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57:1078-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, Mulcahy M, Baker T, Abecassis M, Sato KT. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |