Published online Dec 18, 2017. doi: 10.4254/wjh.v9.i35.1278
Peer-review started: July 17, 2017
First decision: August 7, 2017
Revised: August 25, 2017
Accepted: November 3, 2017
Article in press: November 3, 2017
Published online: December 18, 2017
Processing time: 146 Days and 18 Hours
To investigate whether the use of proton pump inhibitors (PPIs) increases the incidence of spontaneous bacterial peritonitis (SBP) in patients with cirrhosis and ascites.
An historical cohort study was carried out in cirrhotic outpatients with ascites followed in a specialized clinic at a tertiary hospital in Southern Brazil. Patient charts were reviewed to collect information on the variables of interest as the use of PPIs. Primary outcome was defined as development of SBP during the study period. SBP was diagnosed based on ascitic fluid polymorphonuclear cell count ≥ 250 cells/mm³ without evidence of an intra-abdominal, surgically treatable source of infection.
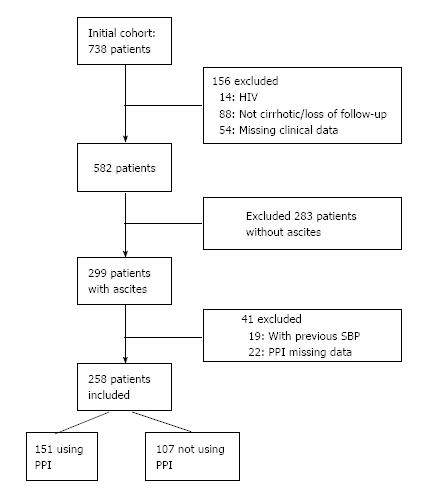
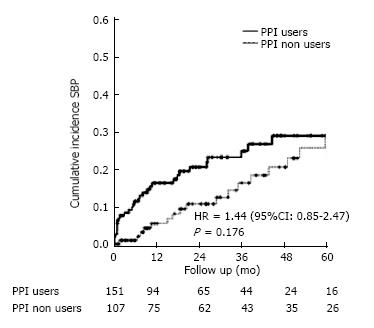
Of 738 cirrhotic patients, 582 (58.2% male) were enrolled, with mean age of 53.6 ± 12 years. Hepatitis C virus infection (36.2%) and alcohol abuse (25.6%) were the main etiologies of cirrhosis. The presence of ascites was detected in 299 (51.4%) patients during the development of the study. Nineteen patients with previous diagnosis of SBP undergoing secondary prophylaxis and 22 patients with insufficient PPI data were further excluded. Of 258 patients with ascites, 151 used PPIs, and 34 developed SBP (22.5%). Among 107 non-users of PPIs, 23 developed SBP (21.5%) (HR = 1.44, 95%CI: 0.85-2.47, P = 0.176). The median follow-up time of patients using PPI was 27 mo vs 32 mo for non-users. Univariate analysis of the risk factors associated with the development of SBP revealed a significant association of SPB with the severity of liver disease according to the Child-Turcotte-Pugh (CTP) score. Multivariate analysis confirmed that CTP score was the only independent variable influencing the occurrence of SBP. Survival at 60 mo (Kaplan-Meier analysis) was similar in users and non-users of PPI, independently of the presence of SBP (58.4% vs 62.7% respectively, P = 0.66). For patients with SBP, survival at 60 mo was 55.1%, vs 61.7% in patients without SBP (P = 0.34).
In conclusion, the rate of SBP was not significantly different in users or non-users of PPIs in this cohort of cirrhotic with ascites.
Core tip: The aim of the present study was to investigate whether the use of proton pump inhibitors (PPIs) increases the incidence of spontaneous bacterial peritonitis (SBP) in patients with cirrhosis and ascites. An historical cohort study was carried out with cirrhotic patients. The primary outcome was development of SBP. Of 258 patients with ascites, 151 used PPIs, and 34 developed SBP (22.5%). Among 107 non-users of PPIs, 23 developed SBP (21.5%) (HR = 1.44, 95%CI: 0.85-2.47, P = 0.176). In conclusion, the use of PPIs does not increase the incidence of SBP in patients with cirrhosis and ascites.
- Citation: Miozzo SAS, John JA, Appel-da-Silva MC, Dossin IA, Tovo CV, Mattos AA. Influence of proton pump inhibitors in the development of spontaneous bacterial peritonitis. World J Hepatol 2017; 9(35): 1278-1285
- URL: https://www.wjgnet.com/1948-5182/full/v9/i35/1278.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i35.1278
The incidence and severity of bacterial infections have been reported to be greater in cirrhotic patients as compared to the general population[1]. In fact, there is evidence that bacterial infections are the cause of death in up to 25% of patients with cirrhosis[2], leading to a four-fold increase in mortality in this population[3]. Supporting this information, a study conducted in our center analyzed 541 consecutively hospitalized cirrhotic patients, revealing the presence of infection in 25% of the cases. In that study, the mortality of infected patients was also four-fold higher as compared to non-infected patients[4]. In addition, infection may trigger other typical complications associated with increased morbidity and mortality in cirrhosis[5,6].
Spontaneous bacterial peritonitis (SBP) is the most characteristic infection in cirrhosis, and prompt recognition and treatment are required to reduce the associated morbidity and mortality.
Bacterial translocation has been described as a key mechanism in SBP development. Small intestinal bacterial overgrowth potentially promotes bacterial translocation[7,8]. Thus, it has been speculated that chronic acid suppression by proton pump inhibitors (PPIs) - which favors gastric and duodenal bacterial colonization - may contribute to small intestinal bacterial overgrowth and consequently increase the incidence of SBP[9].
Nevertheless, there is some controversy regarding the role of PPIs in SBP. The findings of observational studies suggesting PPIs as a risk factor for SBP[10-12] have been supported by retrospective studies[13-19] and meta-analyses[20,21] providing evidence of increased SBP incidence associated with PPI use; however, recent studies by Mandorfer et al[22] and Terg et al[23] have not observed this relationship. The present study aimed to investigate the association of PPI treatment with the incidence of SBP in a cohort of outpatients with cirrhosis and ascites.
This historical cohort study included outpatients with a diagnosis of cirrhosis treated in the Portal Hypertension Clinic at Hospital Santa Casa de Misericórdia de Porto Alegre, a tertiary hospital in the Southern Brazil, between March 2005 and March 2014.
The diagnosis of cirrhosis was confirmed by clinical, laboratory, and imaging data, endoscopy or histologic examination. Outpatient follow-up of at least 1 year was required for inclusion in the study. Primary outcome was defined as development of SBP during the study period.
Patient charts were reviewed to collect information on the variables of interest: Age, sex, etiology of liver disease, Child-Turcotte-Pugh (CTP) score[24] and Model for End-Stage Liver Disease (MELD) score[25], comorbidities, continuous medications (including but not restrict to PPIs), lifetime, hospital admissions, and complications including ascites, SBP, upper gastrointestinal bleeding. At each outpatient visit, serum levels of albumin, creatinine, bilirubin, platelets, and prothrombin time were recorded.
Exclusion criteria were lack of diagnostic confirmation of cirrhosis, co-infection with human immunodeficiency virus (HIV), diagnosis of advanced hepatocellular carcinoma (beyond the Milan criteria)[26] at the first outpatient consultation, and missing clinical data. In addition, in patients with ascites at the moment of enrolment and those undergoing secondary prophylaxis due to prior diagnosis of SBP were excluded. PPI treatment was defined as continuous when in use for at least 3 mo. Indications for PPI treatment were determined based on chart review.
The primary outcome, SBP, was diagnosed based on ascitic fluid polymorphonuclear cell count ≥ 250 cells/mm³ without evidence of an intra-abdominal, surgically treatable source of infection[7,27,28]. The study was approved by the Research Ethics Committee at Hospital ISCMPA (protocol 3675/11).
Continuous data were expressed as means and SD or medians and interquartile range in case of non-Gaussian distribution. Categorical variables were expressed as numbers and percentage. Student’s t test was used for comparison of means, and Mann-Whitney’s U test for comparison of medians. Categorical data were compared using the χ2 test or Fisher’s exact test. The incidence of SBP during the follow-up period was estimated using the Kaplan-Meier (KM) method. The comparison of KM curves of users vs non-users of PPI was performed using the log-rank test. The magnitude of the association between PPI use and presence of SBP was expressed as hazard ratio (HR) with 95%CI, and calculated using a Cox proportional hazards model adjusted for CTP and MELD scores and the presence of upper gastrointestinal bleeding. Data were processed and analyzed using SPSS v. 22.0 at a significance level of P = 0.05.
Of 738 eligible patients, 156 were excluded: 14 patients with HIV, 88 without diagnostic confirmation of cirrhosis or loss of follow-up, and 54 with missing clinical data. The mean age of the 582 patients included in the initial sample was 53.6 ± 12 years, and 58.2% were male. Hepatitis C infection (36.2%) and alcohol abuse (25.6%) were the main etiologies of cirrhosis. Median outpatient follow-up was 5 years.
The presence of ascites was detected in 299 (51.4%) patients during the development of the study. A further 19 patients with a previous diagnosis of SBP undergoing secondary prophylaxis and 22 patients with insufficient PPI data were excluded. Thus, 258 patients with ascites were selected for follow-up (Figure 1). The median follow-up time of patients using PPI was 27.1 (3-60) mo vs 32.2 (7-60) mo for non-users of PPI. The patients were using a standard dose of 20 mg qd of omeprazole, the medication available free of charge in the public health system.
Demographic, clinical, and laboratory data of users and non-users of PPI are shown in Table 1. No significant differences were detected between the groups. Of 151 users of PPI, 34 (22.5%) developed SBP vs 23 (21.5%) of 107 non-users of PPI. This comparison was not statistically significant (HR = 1.44, 95%CI: 0.85-2.47, P = 0.176) (Figure 2).
| Characteristic | Use of PPI | P | |
| Yes (n = 151) | No (n = 107) | ||
| Age (yr) | 54.7 ± 11.2 | 53.1 ± 11.3 | 0.261 |
| Male sex (%) | 63.30% | 62.60% | > 0.992 |
| Etiology of liver disease (%) | 0.532 | ||
| Hepatitis C virus | 34.50% | 34.00% | |
| Alcohol | 27.00% | 34.90% | |
| Alcohol + hepatitis C virus | 24.30% | 19.80% | |
| Other | 14.20% | 11.30% | |
| Platelet count, × 103/mm3 | 126 ± 81 | 112 ± 56 | 0.131 |
| Creatinine, mg/dL | 1.07 ± 0.69 | 0.97 ± 0.27 | 0.151 |
| Albumin, g/dL | 3.4 ± 0.6 | 3.3 ± 0.6 | 0.701 |
| Total bilirubin, mg/dL | 1.30 (0.80-2.60) | 1.40 (0.90-2.60) | 0.593 |
| Prothrombin time, INR | 1.34 ± 0.29 | 1.41 ± 0.26 | 0.241 |
| Child-Turcotte-Pugh score (%) | 0.372 | ||
| A | 42.40% | 36.40% | |
| B | 42.40% | 51.40% | |
| C | 15.20% | 12.10% | |
| MELD score | 12.5 ± 3.9 | 12.7 ± 3.8 | 0.711 |
| Upper gastrointestinal bleeding (%) | 21.90% | 18.70% | 0.642 |
Univariate analysis of the risk factors associated with the development of SBP revealed a significant association with the severity of liver disease according to the CTP score. Multivariate analysis confirmed that CTP score was the only independent variable influencing the occurrence of SBP. Patients with CTP-B and C had a two-fold and three-fold increase, respectively, in the risk of SBP as compared to patients with CTP-A (HR = 2.16, 95%CI: 1.14-4.09, P = 0.018 in CTB B patients and HR 3.77, 95%CI: 1.66-8.59, P = 0.002 in CTP C patients) (Table 2). Using the COX model, the events occurred in Child A 18.2%; Child B 35.6%; and Child C 52.7%; P < 0.001. Throughout the follow-up period, the Child C patients presented a higher mortality.
| Variable | n | Events n (%) | Bivariate analysis | Multivariate analysis | ||
| HR (95%CI) | P | HR (95%CI) | P | |||
| PPI use | ||||||
| Yes | 151 | 34 (22.5) | 1.44 (0.85-2.47) | 0.176 | 1.50 (0.87-2.58) | 0.142 |
| No | 107 | 23 (21.5) | 1 | 1 | ||
| CTP | ||||||
| A | 103 | 15 (26.3) | 1 | 1 | ||
| B | 119 | 30 (52.6) | 2.10 (1.12-3.92) | 0.020 | 2.16 (1.14-4.09) | 0.018 |
| C | 36 | 12 (21.1) | 3.62 (1.69-7.78) | 0.001 | 3.77 (1.66-8.59) | 0.002 |
| MELD | ||||||
| ≥ 15 | 78 | 19 (33.3) | 1.41 (0.81-2.45) | 0.226 | 0.95 (0.52-1.72) | 0.854 |
| < 15 | 180 | 38 (66.7) | 1 | 1 | ||
| UGB | ||||||
| Yes | 53 | 11 (19.3) | 0.92 (0.48-1.79) | 0.808 | 0.99 (0.51-1.92) | 0.967 |
| No | 205 | 46 (83.7) | 1 | |||
Survival at 60 mo (Kaplan-Meier analysis) was similar in users and non-users of PPI, independently of the presence of SPB (58.4% vs 62.7% respectively, P = 0.66). For patients with SBP, survival at 60 mo was 55.1%, vs 61.7% in patients without SBP (P = 0.34).
In the group of 151 patients using PPI, 19 patients had a diagnosis of peptic ulcer (12.6%), 20 presented gastric esophageal reflux (13.1%) and 17 used PPI to treat dyspepsia (11.3%). Evidence of formal indication for PPI treatment was not found in the chart of 95 patients (63%).
Given the importance of SBP in the context of liver disease, the identification of possible risk factors is crucial to prevent this infection. Among possible risk factors, the role of PPIs has been recently discussed. To our knowledge, this is the first study conducted in Brazil in a cohort representing a population with typical environment and demographic characteristics as racial heterogeneity, probably traducing a differentiation in the gut microbiota.
The gastric acidity exerts a defense of the host against enteric pathogens, supporting the hypothesis of an influence of acid suppression on the development of secondary infections resulting from increased bacterial populations in the gastrointestinal tract. As in the pathogenesis of other bacterial infections in patients with cirrhosis, bacterial translocation plays a key role in the genesis of SBP, and has been described as the main trigger of SBP development[29-31]. The increased prevalence of bacterial overgrowth and intestinal dysmotility in cirrhotic patients with SBP when compared to cirrhotic patients without SBP underscores the role of intestinal microbiota in the pathogenesis of this infection[32]. A prospective study with 70 patients with cirrhosis analyzed jejunal secretion cultures and observed an association of bacterial overgrowth with acid-suppressive therapy (P = 0.01) and hypochlorhydria (P < 0.001); nevertheless, no statistical association was detected between the presence of SBP and bacterial overgrowth or acid-suppressive therapy[8]. With regard to the microbiota, few studies[33-35] were carried out in Brazil, making interesting the pioneer knowledge of the impact of the PPIs in cirrhosis.
In the present study, a cohort of patients with cirrhosis was followed-up, allowing the estimation of the incidence of SBP in users or non-users of PPI. We did not observe an association between the use of PPI and the incidence of SBP. However, the degree of liver dysfunction expressed as CTP score was strongly related to incidence of SBP, with a three-fold increase in risk of SBP in patients with more severe disease (CTP C), as reported in other studies[22,36]. This association is also emphasized by previous observations showing that liver dysfunction is related to increased bacterial translocation[7,37].
It should be noted that some studies suggesting an association between PPIs and SBP did not achieve statistically significant results[8,13], or were unable to confirm this association in multivariate analyses[17]. It is important to emphasize that the studies linking the use of anti-secretory therapy to increased frequency of SBP are mostly retrospective or case-control in design[13-19,38].
Bajaj et al[38] have not observed significant associations between the use of PPI and the rate of severe infections (HR = 1.08, 95%CI: 0.90-1.30) or infections related to acid-suppressive therapies (HR = 1.22, 95%CI: 0.97-1.52), except when the duration of PPI treatment was taken into account. In this study the authors do not describe the severity of liver disease of patients.
Min et al[39] reported an association between PPIs and SBP based on results from 1554 patients with cirrhosis and ascites. There were 90 cases of SBP among 512 users of PPI (10.6%) and 146 cases of SBP among 1042 non-users (5.8%). The annual incidence rate of SBP was higher in those using PPIs (HR 1.396, 95%CI: 1.057-1.843, P = 0.019).
Regarding the influence of acid-suppressive therapies on the development of SBP, some works have described different results for PPI and histamine-2 receptor antagonists (H2RA)[15,21,38], with no reported influence of H2RA. This has prompted a discussion regarding whether the difference between these acid-suppressive therapies results from a stronger acid-suppressive effect and greater delay in gastric emptying with PPIs[40,41] or from weaknesses in the hypothesis of acid-suppressive therapy as an independent risk factor for SBP. In the present study, all patients received omeprazole 20 mg qd, since this is the medication available free of charge in the Public Health System.
Meta-analyses[20,21,42] carried out to evaluate the association between acid-suppressive therapies and SBP have confirmed a relationship. The first of these[20] meta-analyzed case-control and retrospective studies with hospitalized patients. The meta-analyzed studies involved 772 individuals with cirrhosis using PPIs, for and odds ratio (OR) of 2.77 (95%CI: 1.82-4.23). A second meta-analysis[21] involved 3815 patients with cirrhosis, and showed significantly higher risk of SBP in users of PPIs vs non-users (OR = 3.15, 95%CI: 2.09-4.74, P < 0.00001); however, once again that study included mostly retrospective, case-control studies of hospitalized patients. Other limitations included the lack of information regarding dose and duration of PPI and H2RA treatment. The more recent meta-analysis[42] evaluated 7822 patients from 14 studies (6 case-control studies with 817 patients and 8 cohort studies with 7005 patients). The authors found statistically significant but quantitatively small associations between SBP and the use of PPIs. After adjustment for publication bias, there was very low-quality evidence per the GRADE approach in favor of this association. Therefore, they suggest that patients with cirrhosis who have indications for the use of PPI should not be denied because of concern for precipitating SBP.
In the same way, van Vlerken et al[36] did not observe an influence of PPIs on bacterial infection in a prospective analysis of cirrhotic patients receiving outpatient follow-up (HR = 1.2, 95%CI: 0.5-3.0, P = 0.72). It should be noted, however, that those authors had only a small number of cases of SBP. More recently, Mandorfer et al[22] carried out a retrospective cohort analysis of 607 patients submitted to paracentesis and did not identify PPIs as a risk factor for SBP. Similarly, in a multicenter study with 521 cirrhotic patients, Terg et al[23] reported similar SBP rates in patients at increased risk of SBP infection - 79.5% in users and 78.7% in non-users of PPIs.
The low mortality observed in patients with SBP in relation to the group without this infection is probably related to the fact that these infections are community-acquired, which results in a lower severity. We recently published a study showing the relevance of multiresistant bacteria in patients with nosocomial SBP, which certainly worsens the prognosis of these patients[43]. However, when patients with a greater impairment of hepatocellular function were evaluated (Child C), mortality was higher.
One aspect that deserves attention is the high prevalence of PPI use (58%) in our patients, and the fact that 63% of those using PPI did not have evidence of formal indication for PPI therapy. Similar data have been previously described, with PPI used by as many as 86% of patients[23] and used by as many as 63% patients without documented indications[16,19,23,36,44-46]. PPIs have been used to prevent gastroesophageal reflux and worsening of inflammation and esophageal ulceration following band ligation and sclerotherapy in cirrhotic patients; however, this practice is questionable[45-47]. As possible limitations of the present study we should note that most of the data were obtained from reviewing the charts, which is important to remark thus we are aware of the potential biases.
In conclusion, considering the current uncertainty regarding PPIs as a risk factor for SBP in patients with cirrhosis, the present study evaluated an historical cohort of cirrhotic outpatients with ascites and did not find evidence of increased incidence of SBP with the use of PPIs. In addition, the CTP score was strongly related to incidence of SBP.
Spontaneous bacterial peritonitis (SBP) is the most characteristic infection in cirrhosis, and has been associated to morbidity and mortality. Small intestinal bacterial overgrowth potentially promotes bacterial translocation. Thus, it has been speculated that chronic acid suppression by proton pump inhibitors (PPIs) - which favors gastric and duodenal bacterial colonization - may contribute to small intestinal bacterial overgrowth and consequently increase the incidence of SBP. Nevertheless, there is some controversy regarding the role of PPIs in SBP.
The increased prevalence of bacterial overgrowth and intestinal dysmotility in cirrhotic patients with SBP when compared to cirrhotic patients without SBP underscores the role of intestinal microbiota in the pathogenesis of this infection. However, few studies evaluating the gut microbiota were carried out in cirrhotic patients, mainly in Brazil, making interesting the pioneer knowledge of the impact of the PPIs in cirrhosis.
To the knowledge, this is the first study conducted in Brazil in a cohort representing a population with typical environment and demographic characteristics as racial heterogeneity, probably traducing a differentiation in the gut microbiota. Considering the current uncertainty regarding PPIs as a risk factor for SBP in patients with cirrhosis, the present study evaluated an historical cohort of cirrhotic outpatients with ascites and did not find evidence of increased incidence of SBP with the use of PPIs.
One aspect that deserves attention is the high prevalence of PPI use (58%) in the patients, and the fact that 63% of those using PPI did not have evidence of formal indication for PPI therapy. Similar data have been previously described in the literature, with PPI used by as many as 86% of patients and used by as many as 63% patients without documented indications. So, it is possible that these results may alert and promote the correct use of PPI in cirrhotics.
The study is well conducted and statistical methods are sound.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Acevedo JG, Bock CT, Khan MA, John S, Schwabl P, Singh S, Trifan A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 2. | Deschênes M, Villeneuve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol. 1999;94:2193-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1-1256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 4. | de Mattos AA, Coral GP, Menti E, Valiatti F, Kramer C. [Bacterial infection in cirrhotic patient]. Arq Gastroenterol. 2003;40:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 5. | Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2173] [Article Influence: 181.1] [Reference Citation Analysis (5)] |
| 7. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 8. | Bauer TM, Steinbrückner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, Kist M, Blum HE. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 10. | Compare D, Pica L, Rocco A, De Giorgi F, Cuomo R, Sarnelli G, Romano M, Nardone G. Effects of long-term PPI treatment on producing bowel symptoms and SIBO. Eur J Clin Invest. 2011;41:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Fried M, Siegrist H, Frei R, Froehlich F, Duroux P, Thorens J, Blum A, Bille J, Gonvers JJ, Gyr K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci. 2008;53:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Bajaj JS, Zadvornova Y, Heuman DM, Hafeezullah M, Hoffmann RG, Sanyal AJ, Saeian K. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Choi EJ, Lee HJ, Kim KO, Lee SH, Eun JR, Jang BI, Kim TN. Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol. 2011;46:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Goel GA, Deshpande A, Lopez R, Hall GS, van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol. 2012;10:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | de Vos M, De Vroey B, Garcia BG, Roy C, Kidd F, Henrion J, Deltenre P. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int. 2013;33:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Ratelle M, Perreault S, Villeneuve JP, Tremblay L. Association between proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Can J Gastroenterol Hepatol. 2014;28:330-334. [PubMed] [DOI] [Full Text] |
| 19. | Kwon JH, Koh SJ, Kim W, Jung YJ, Kim JW, Kim BG, Lee KL, Im JP, Kim YJ, Kim JS. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2014;29:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Trikudanathan G, Israel J, Cappa J, O’Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract. 2011;65:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Deshpande A, Pasupuleti V, Thota P, Pant C, Mapara S, Hassan S, Rolston DD, Sferra TJ, Hernandez AV. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol. 2013;28:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Summereder C, Hagmann M, Blacky A, Ferlitsch A, Sieghart W. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS One. 2014;9:e110503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, Niveyro C, Benavides J, Marino M, Colombato L. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 25. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1865] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 26. | Sociedad Española de Trasplante Hepático. [Consensus document of the Spanish Society of Liver Transplantation]. Gastroenterol Hepatol. 2008;31:82-91. [PubMed] |
| 27. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1132] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 28. | Runyon BA. AASLD Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. Available from: https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. |
| 29. | Chesta J, Silva M, Thompson L, del Canto E, Defilippi C. [Bacterial overgrowth in small intestine in patients with liver cirrhosis]. Rev Med Chil. 1991;119:626-632. [PubMed] |
| 30. | Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, Riba J, Cabré E, Santos J, Luque T, Ausina V. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Passos MDCF, Moraes-Filho JP. Intestinal microbiota in digestive diseases. Arq Gastroenterol. 2017;54: 255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Martins CP, Chaves CHA, Castro MGB, Gomes IC, Passos MDCF. Prevalence of small intestine bacterial overgrowth in patients with gastrointestinal symptoms. Arq Gastroenterol. 2017;54:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Mello CS, Carmo-Rodrigues MS, Filho HB, Melli LC, Tahan S, Pignatari AC, de Morais MB. Gut Microbiota Differences in Children From Distinct Socioeconomic Levels Living in the Same Urban Area in Brazil. J Pediatr Gastroenterol Nutr. 2016;63:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | van Vlerken LG, Huisman EJ, van Hoek B, Renooij W, de Rooij FW, Siersema PD, van Erpecum KJ. Bacterial infections in cirrhosis: role of proton pump inhibitors and intestinal permeability. Eur J Clin Invest. 2012;42:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 38. | Bajaj JS, Ratliff SM, Heuman DM, Lapane KL. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther. 2012;36:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Min YW, Lim KS, Min BH, Gwak GY, Paik YH, Choi MS, Lee JH, Kim JJ, Koh KC, Paik SW. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther. 2014;40:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 251] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 41. | Takahashi Y, Amano Y, Yuki T, Ose T, Miyake T, Kushiyama Y, Sato S, Ishihara S, Kinoshita Y. Influence of acid suppressants on gastric emptying: cross-over analysis in healthy volunteers. J Gastroenterol Hepatol. 2006;21:1664-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Khan MA, Kamal S, Khan S, Lee WM, Howden CW. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol. 2015;27:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Costabeber AM, Mattos AA, Sukiennik TC. Prevalence of bacterial resistance in hospitalized cirrhotic patients in southern brazil: a new challenge. Rev Inst Med Trop Sao Paulo. 2016;58:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Gawron AJ, Rothe J, Fought AJ, Fareeduddin A, Toto E, Boris L, Kahrilas PJ, Pandolfino JE. Many patients continue using proton pump inhibitors after negative results from tests for reflux disease. Clin Gastroenterol Hepatol. 2012;10:620-625; quiz e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Chavez-Tapia NC, Tellez-Avila FI, Garcia-Leiva J, Valdovinos MA. Use and overuse of proton pump inhibitors in cirrhotic patients. Med Sci Monit. 2008;14:CR468-CR472. [PubMed] |
| 46. | Kalaitzakis E, Björnsson E. Inadequate use of proton-pump inhibitors in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Bittencourt PL, Farias AQ, Strauss E, Mattos AA; Pannel of the 1st Brazilian Consensus of Variceal Bleeding, Brazilian Society of Hepatology. Variceal bleeding: consensus meeting report from the Brazilian Society of Hepatology. Arq Gastroenterol. 2010;47:202-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |