Published online Jan 28, 2017. doi: 10.4254/wjh.v9.i3.131
Peer-review started: August 23, 2016
First decision: October 28, 2016
Revised: November 11, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: January 28, 2017
Processing time: 154 Days and 2 Hours
The Cockcroft-Gault (CG) equation has become perhaps the most popular practical approach for estimating renal function among health care professionals. Despite its widespread use, clinicians often overlook not only the limitations of the original serum creatinine (SCr) based equation, but also may not appreciate the validity of the many variations used to compensate for these limitations. For cirrhotic patients in particular, the underlying pathophysiology of the disease contributes to a falsely low SCr, thereby overestimating renal function with use of the CG equation in this population. We reviewed the original CG trial from 1976 along with data surrounding clinician specific alterations to the CG equation that followed through time. These alterations included different formulas for body weight in obese patients and the “rounding up” approach in patients with low SCr. Additionally, we described the pathophysiology and hemodynamic changes that occur in cirrhosis; and reviewed several studies that attempted to estimate renal function in this population. The evidence we reviewed regarding the most accurate manipulation of the original CG equation to estimate creatinine clearance (CrCl) was inconclusive. Unfortunately, the homogeneity of the patient population in the original CG trial limited its external validity. Elimination of body weight in the CG equation actually produced the estimate closest to the measure CrCl. Furthermore, “rounding up” of SCr values often underestimated CrCl. This approach could lead to suboptimal dosing of drug therapies in patients with low SCr. In cirrhotic patients, utilization of SCr based methods overestimated true renal function by about 50% in the literature we reviewed.
Core tip: For many health care professionals in the United States, the Cockcroft-Gault (CG) equation has become perhaps the most popular practical approach for estimating renal function. Despite its widespread use, clinicians often overlook not only the limitations of the original serum creatinine (SCr) based equation, but also may not appreciate the validity of variations used to compensate for these limitations. For cirrhotic patients in particular, the underlying disease pathophysiology contributes to a falsely low SCr, thereby overestimating renal function with use of the CG equation in this population.
- Citation: Scappaticci GB, Regal RE. Cockcroft-Gault revisited: New de-liver-ance on recommendations for use in cirrhosis. World J Hepatol 2017; 9(3): 131-138
- URL: https://www.wjgnet.com/1948-5182/full/v9/i3/131.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i3.131
In order to optimize efficacy and minimize potential toxicity of pharmacologic agents, appropriate patient-specific dosing of medications remains an inherent responsibility of all healthcare providers. Proper assessment of a patient’s renal function is essential when managing medications that are primarily renally excreted[1]. With an estimated 14% of adults in the United States experiencing varying degrees of chronic kidney disease (CKD), optimization of drug therapies poses a frequent challenge to clinicians[2]. Renal impairment may significantly alters the pharmacokinetic (PK) properties of many medications[3]. Therefore, reasonably accurate yet convenient quantification of the degree of renal impairment is an essential tool for clinicians implementing renal dose adjustments[3].
In the majority of clinical settings, calculating the creatinine clearance (CrCl) using the Cockcroft-Gault (CG) equation has become the most popular and practical approach for estimating renal function[4,5]. Many institutions provide dosing recommendations based on calculated CrCl, and even often utilize electronic health record (EHR) software that automatically calculates CrCl based on the CG equation. Unfortunately, some clinicians fail to realize the inherent limitations of a serum creatinine (SCr) based equation and the subsequent variations that stem from many of these limitations[1,4,6].
SCr concentrations can be altered by patient specific factors including age, sex, weight, muscle mass, disease state, diet, and certain drug therapies, thus limiting the generalizability of the CG equation[1,4-6]. For example, patients with hepatic impairment not only experience altered drug metabolism, but also have secondarily reduced creatinine production. If not taken into consideration, these SCr-based formulas can lead to an overestimation of GFR in cirrhotic patients[7].
To help clarify the true applicability of the CG equation, we will discuss the origins of the CG equation and the evidence and reasoning behind specific alterations to the equation used in current practice.
Data included in this review were identified from a PubMed search of publications starting in 1970 through June of 2016. Searches included the keywords “Cockcroft-Gault”, “serum creatinine”, “creatinine clearance”, “renal function”, “cirrhosis” and related search terms. Publications were considered for review if they were designed as meta-analyses, retrospective, or prospective studies that compared different methods of estimating CrCl using the CG equation.
The CG equation (Table 1A; equation I) was derived from 236 patients (96% male), aged 18-92 years old in 1976 at the Queen Mary Veterans’ Hospital in Canada. SCr values used in the equation were the mean values calculated from two 24-h SCr levels obtained from blood for each patient at steady state. The CrCl was calculated using 4 different formulas (Table 1A; equations I-IV) that were compared against each other and with each patient’s 24-h urine creatinine excretion. The CG equation was found to provide an estimated CrCl that was 80% ± 30% of the actual creatinine clearance calculated from the 24-h urine creatinine excretion test[5].
| A: Formula = CrCl (mL/min) | B: Formula = weight (kg) | ||
| I1 | [(140 - age)(weight in kg)] (72 × SCr) | IBWmale | 50 + (2.3 kg × inches > 60) |
| II | (100/SCr) - 12 | IBWfemale | 45.5 + (2.3 kg × inches > 60) |
| III | 98 - 16 × [(age - 20)/20] SCr | AdjBW | IBW + [(TBW - IBW) × C2] |
| IV | (94.3/SCr) - 1.8 | LBWmale | 9270 × TBW 6680 + (216 × BMI) |
| V | (140 - age) SCr | LBWfemale | 9270 × TBW |
| VI | 100 SCr | FFW | Calculated using BIA[15] |
Limitations acknowledged at the end of this trial included requirements for SCr to be at steady state, the need for normal relationship between muscle mass and total body weight, and factors related to age, sex, and height. In addition to this, the formula was tested in a patient population that was 96% male, which obviously limits the external validity of the results in female cohorts. To compensate for females having different relative amounts of fat and muscle compared to males, a somewhat arbitrary 15% reduction of predicted CrCl was considered appropriate based on previous study estimations[8-10]. Furthermore, it was noted that certain patients had predictably low creatinine excretion for age and body weight. Examples included paraplegics and patients with marked obesity or ascites. To correct for these patients, although no data were presented to support this decision, the authors suggested using ideal body weight (IBW).
Finally, due to the delay in SCr fluctuations and the time needed to establish a new steady state, the authors acknowledged that CrCl can be significantly overestimated in early phases of acute renal failure[5]. This is an extremely important concept for clinicians to grasp. In patients with excellent renal function, the t ½ is on the order of 4 h, and a new steady state could be reached in about 1 d. However, a 75% reduction in GFR would increase the half-life to about 15 h, and the time to steady state would increase to about 2 ½ d[11]. In the case of oliguric or anuric renal failure, it may take several days to reach a new steady state for the SCr[11,12].
Despite the above acknowledged limitations of the CG equation, it has become the most popular renal function prediction method used for renal dosing by clinicians[1,13,14]. Attempts to validate CrCl calculated using the CG equation have produced mixed results[6,13]. In their 2010 Guidance for Industry, the Food and Drug Administration (FDA) advocated for the use of the CG equation in drug development because it has been widely used in PK studies[14]. For instance, where CrCl may be inaccurate (muscle wasting, malnutrition, amputation, etc.), alternative methods of calculating CrCl are suggested but not required[14].
As clinicians, it is important to understand that attempts to modify the equation to compensate for some of these patient-specific factors often lead to variable results. In the sections that follow, we discuss the rationale and results that these various adjustments yield in predicting actual CrCl.
Once again, recall that the CG equation was derived based on the assumption that SCr represents muscle mass as a definite percentage of the patient’s body weight, and that both of these values decline in a linear manner as patients age[13]. In obese patients, these assumptions may not be true, as body fat becomes the major contributor to body mass[13]. Given that over 50% of the United States population > 20 years old are overweight or obese, reviewing available literature comparing accuracy of different weights used in the CG equation may help clinicians optimize dose selection[1,15].
The CG equation was derived from a population of normal weight individuals (mean = 72 kg) using actual body weight; and therefore, its use in obese patients may lead to significant estimation errors[5,16]. Despite 40 years of clinical experience and numerous studies evaluating different weight calculations in obese patients (Table 1B), no uniform consensus appears to exist for estimating CrCl using the CG equation in this patient population[1,15-17].
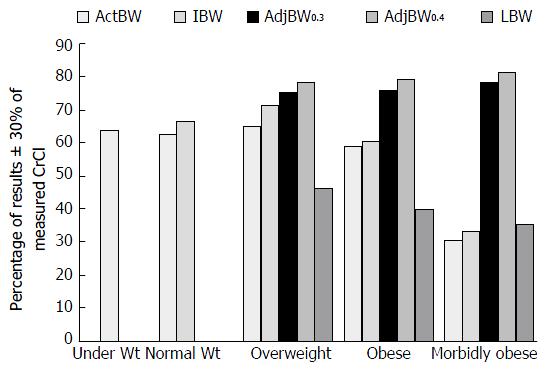
Winter et al[1] studied the impact of various body weights used when calculating CrCl in obese and non-obese patients. They estimated CrCl using the CG equation with actual body weight (actBW) for body mass index (BMI) < 18.5 kg/m2; IBW and actBW for BMI 18.5-24.9 kg/m2; and actBW, IBW, adjusted body weight (adjBW0.3), adjBW0.4, and lean body weight (LBW) for all patients with BMI > 25 kg/m2. The calculated CrCl was compared to a CrCl derived from a measured 24-h urine collection for all 952 patients in the study. ActBW was shown to underestimate CrCl by 0.221 mL/min in underweight patients (BMI < 18.5 kg/m2); in normal weight patients (BMI: 18.5-24.9 kg/m2), IBW was shown to be more accurate than actBW (IBW underestimated CrCl by 1.3 mL/min vs actBW overestimated by 4.7 mL/min); and in patients with a BMI > 25 kg/m2, adjBW0.4 was shown to be the most accurate method of predicting CrCl (BMI 25-29.9 kg/m2 -2.4 mL/min; BMI 30-39.9 kg/m2 -6.2 mL/min; BMI > 40 kg/m2 -5.9 mL/min) (Figure 1).
In a similar study, Demirovic et al[15] prospectively evaluated the impact different body-size descriptors would have on the accuracy of the CG equation when compared to a timed 24-h urine collection. They estimated the CrCl in only obese patients with a BMI ≥ 40 kg/m2 and used ActBW, IBW, AdjBW0.3, AdjBW0.4, fat free weight (FFW), and LBW in the CG equation. Bioelectric impedance analysis (BIA) was used to estimate the FFW in patients. The calculated CrCl was compared to a CrCl derived from a measured 24-h urine collection for all 54 patients in the study (Table 2). On average, the CG equation using a patient’s actBW overestimated the CrCl by 107.4 mL/min; using IBW underestimated CrCl by 24.3 mL/min; using AdjBW0.3 and AdjBW0.4 both overestimated the CrCl by 19.8 and 32.3 mL/min, respectively; FFW and LBW were found to be the most accurate estimate of the measured CrCl, as the FFW underestimated CrCl by 6.8 mL/min and LBW underestimated by 8.1 mL/min (Table 2)[15,18,19].
| Method1 | Mean estimated CrCl ± SD | Mean bias (mL/min) | ± 30% of measured CrCl | ± 50% of measured CrCl |
| Measured CrCl | 109.5 ± 44.4 | |||
| ActBW | 217 ± 113 | -107 | 13% | 30% |
| IBW | 85 ± 29 | +24 | 48% | 89% |
| AdjBW0.3 | 129 ± 55 | -20 | 54% | 76% |
| AdjBW0.4 | 142 ± 63 | -33 | 52% | 67% |
| FFW | 103 ± 48 | +7 | 61% | 83% |
| LBW | 102 ± 43 | +8 | 56% | 87% |
| MDRD4 | 96.3 ± 29.4 | +13.3 | 51.90% | 87% |
| Salazar-Corcoran | 155.2 ± 65.1 | -45.7 | 46.20% | 55.60% |
A meta-analysis by Wilhelm et al[17] analyzed a total of 1197 patients from 13 different trials and compared CrCl calculated with CG using ActBW, IBW, AdjBW0.3, AdjBW0.4, and no body weight (NBW) with a measured 24-h urine collection. For NBW, the authors assumed the patient weight to be 72 kg, as this was the average weight from the original CG trial[5]. NBW slightly modified the CG equation as it only incorporates age and SCr (Table 1A; Equation V). When using actBW, the mean difference in the CG estimated CrCl was an overestimation of 15.91 mL/min; using IBW underestimated CrCl by 5.15 mL/min; using adjBW0.3 slightly underestimated the CrCl by 4.55 mL/min whereas the adjBW0.4 considerably underestimated the CrCl by 19.94 mL/min. The most accurate method of estimating CrCl was using the modified CG equation without a variable for body weight (NBW), which underestimated CrCl by 0.43 mL/min.
The studies presented above reiterate the challenges faced by many clinicians when estimating CrCl using the CG equation. The CG equation was not originally studied in obese patients, and therefore, has limited applicability in this population. The study by Winter et al[1] showed that in patients with a BMI > 25 kg/m2, use of adjBW0.4 was the most accurate method of estimating CrCl when using the CG methods. Unfortunately, Demirovic et al[15] did not come to the same conclusion with the results found by Winter et al[1] and Demirovic et al[15]. They found that FFW and LBW provided the most accurate estimate of CrCl when compared to a measured 24-h urine collection. These findings support what was originally assumed by CG. That is, that SCr can best be used as a surrogate marker for renal function when an accurate assessment of a patient’s muscle mass is used to calculate CrCl[5]. Unfortunately, calculating FFW and LBW on a daily basis is not practical in most clinical settings. Finally, Wilhelm’s study illustrated that in a large, heterogeneous sample, removing the weight variable from the CG equation actually produced the estimate closest to the measured CrCl[17]. Although body weight remains controversial, utilizing the NBW equation assumes SCr predictable declines with age. Like the original CG equation, the NBW equation may be of limited use in patients with low SCr or falsely low SCr due to muscle mass or underlying disease. Certainly, the evidence presented by the authors of this review reiterate the potential limitations of the CG equation, and why this equation cannot be used as the sole means of estimating renal function in all patients.
It is well known that, due to a decrease in muscle mass beyond about age 40, SCr and CrCl decline as a patient ages. The CG equation assumes this decline is linear[5,20]. In patients with a SCr ≤ 0.6 mg/dL, CrCl estimation using the CG equation often overestimate CrCl, and may consequently lead to supratherapeutic dosing of renally excreted drugs[21]. To compensate for this, clinicians often arbitrarily round a SCr ≤ 0.6 mg/dL to a closer-to-normal value (0.8-1 mg/dL)[21]. Although “rounding up” of SCr is a widely used technique by many clinicians, it has not been robustly validated[21].
Dooley et al[21] performed a study comparing measured GFR using diethyl triamine penta-acetic acid (DTPA) to an estimated CrCl calculated using the CG equation in patients with low SCr levels (< 0.6 mg/dL), and determined the impact of rounding SCr to 0.6 mg/dL. This retrospective study analyzed 26 patients with an average age of 57 years old. When compared to the measured GFR, the CG equation, using actual SCr overestimated CrCl by 12.9%, whereas the rounded SCr of 0.6 mg/dL underestimated CrCl by 7% (Table 3). Although rounding of SCr to 0.6 mg/dL was more accurate when calculating CrCl in this study, it was noted by the authors that clinicians typically round to either 0.8 or 1 mg/dL which would increase the underestimation when calculating CrCl. Furthermore, in patients with a measured CrCl that was > 100 mL/min, the rounding of SCr to 0.6 mg/dL underestimated CrCl by 18.9% vs 0.1% using the actual SCr.
| Mean ± SD (mL/min) | Range (mL/min) | Mean % error | P value | ||
| DTPA | All | 111 ± 46 | 45-256 | ||
| ≤ 100 mL/min | 77 ± 14 | 45-96 | |||
| > 100 mL/min | 140 ± 45 | 103-256 | |||
| CG (no rounding) | All | 117 ± 38 | 55-207 | 12.9 | 0.352 |
| ≤ 100 mL/min | 98 ± 28 | 55-152 | 29.2 | 0.024 | |
| > 100 mL/min | 135 ± 38 | 86-207 | -0.1 | 0.631 | |
| CG (rounding SCr to 0.6 mg/dL) | All | 97 ± 30 | 46-172 | -7.0 | 0.029 |
| ≤ 100 mL/min | 82 ± 23 | 46-127 | 7.9 | 0.543 | |
| > 100 mL/min | 110 ± 29 | 72-172 | -18.9 | 0.003 |
Smythe et al[22] performed a prospective study in elderly patients, but chose to round SCr to 1.0 mg/dL when calculating CrCl using the CG. This study included 23 patients (age 69.2 ± 8.1 years old) and compared the calculated CrCl using various body weights with or without rounding of SCr to 1 mg/dL with a 24-h measured CrCl. The results of this study showed that of all three examples of calculating CrCl, using the actual SCr values produced the most accurate estimate of CrCl (Table 4).
| Method | Bias = CrClmeas - CrClcalc (CI) | Precision |
| CG using IBW without gender adjustment | ||
| Actual SCr | 2.3 (-10.3-14.8) | 22.5 |
| Rounded SCr | 28.8 (19.1-38.4) | 17.4 |
| CG using ActBW without gender adjustment | ||
| Actual SCr | -13.6 [-26.8-(-0.43)] | 23.6 |
| Rounded SCr | 16.3 (4.5-28.1) | 21.2 |
| CG using ActBW with gender adjustment | ||
| Actual SCr | -5.2 (-17.2-7.1) | 22.1 |
| Rounded SCr | 22.6 (11.5-33.7) | 19.9 |
The inverse relationship between SCr and CrCl has lead clinicians to further deviate from the studied CG equation in order to broaden the applicability of the equation[22]. Rounding of low SCr values (≤ 0.6 mg/dL) when calculating CrCl is often used by clinicians to prevent overestimation of renal function and over-dosing of renally excreted drugs[21]. The fact that “rounding up” of SCr has not been validated by strong evidence, clinicians who routinely round low SCr in patients may underestimate CrCl and consequently overcompensate for a perceived problem[21,22]. The studies presented in this section confirm the limitations of the CG equation in elderly patients and the use of SCr as a surrogate marker of GFR. Unfortunately, the limitations of SCr based equations extend to additional populations where SCr is falsely low due to underlying disease.
Among the many complications that arise in patients with liver cirrhosis, renal dysfunction has become a well-established predictor associated with poor prognosis and increased mortality[7,23]. The overall survival of patients with cirrhosis who develop hepatorenal syndrome (HRS) is approximately 50% at 1 mo and 20% at 6 mo[24]. Given the frequency at which cirrhotics demonstrate “cryptic” renal impairment and so often go on to develop HRS, it is critical that clinicians appropriately dose all drugs in cirrhotic patients, particularly those that are nephrotoxic[25]. Unfortunately, due to the underlying disease pathophysiology producing a falsely low SCr, SCr based calculations of CrCl are of limited use in cirrhotics[7].
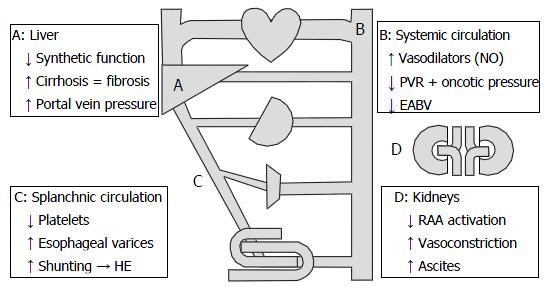
Underlying CKD in cirrhotics results from alterations in hemodynamics, renal autoregulatory mechanisms, and cardiac function (Figure 2)[26,27]. Hemodynamically, because of increased portal vein pressure, compensatory vasodilators such as nitrous oxide (NO) decrease peripheral vascular resistance and dilate the splanchnic circulation[26,27]. Progressive vasodilation in the presence of portal hypertension results in a decrease in effective arterial blood volume and activation of sodium retention mechanisms such as the renin-angiotensin-aldosterone system (RAAS)[26,27]. Unfortunately, these compensatory mechanisms lead to renal vasoconstriction and reduced GFR[26,27].
In addition to the above hemodynamic changes, cirrhotics have falsely low-to-normal levels of SCr, thus further complicating a clinician’s assessment of renal function. Creatine is originally produced in the liver before it is transferred to the skeletal muscles to be stored for energy. In the muscles, it is then phosphorylated, converted to creatinine, and then transferred back into the bloodstream[28]. As cirrhosis progresses, creatine production declines and becomes inconsistent[28]. Furthermore, due to malnutrition and low androgen levels, muscle wasting in cirrhotics limits the storage capacity and phosphorylation of creatine, thereby further decreasing the serum concentration of creatinine[28-30]. Finally, detecting early acute kidney injury (AKI) in cirrhotics using SCr is already tenuous, and may require far greater than 24 h given the pharmacokinetic properties of creatinine in patients with reduced GFRs[11].
MacAulay et al[31] compared estimates of GFR using three SCr based formulas (CG, MDRD and SCrrec) with standard radionuclide measurements (DTPA) of GFR in patients with advanced liver disease. Of the 57 patients in their trial, the mean GFR via DTPA was 83 mL/min per 1.73 m2 (range 28-173 mL/min per 1.73 m2). On average, estimation using the MDRD was most accurate (mean difference % = +4.0; CI = -5.73-20.39), followed by the CG equation (mean difference % = +18.56; CI = 8.48-22.83), and the SCrrec (mean difference % = +28.68; CI = 14.3-30.28). The authors concluded that using the CG and SCrrec (Table 1A equation VI) equations to estimate GFR in this population can lead to a significant overestimation of GFR.
A similar study by Rognant et al[32] compared GFR estimates using the CG and MDRD equations to a measured GFR using inulin. Estimating CrCl using the CG was normalized to 1.73 m2 body surface area (Table 1A equation I). The 143 patients in this study all had decompensated alcoholic cirrhosis. The mean measured GFR using inulin was 76.9 ± 28 mL/min per 1.73 m2, and 30.4% of patients had a GFR ≥ 90 mL/min per 1.73 m2 (group 1), 39.2% had a GFR between 60-89.9 mL/min per 1.73 m2 (group 2), 26.3% had a GFR < 60 mL/min per 1.73 m2 (group 3) with 4.1% of these patients having a GFR ≤ 30 mL/min per 1.73 m2. Mean GFR estimates using the CG and MDRD equations were 98.7 ± 32 mL/min and 99.4 ± 34 mL/min per 1.73 m2. The mean estimates using the CG and MDRD equations both overestimated the GFR mean by 21.8 mL/min (28.3%) and 22.5 mL/min (29.3%), respectively. For patients in group 1, the mean absolute bias for the CG was 20 ± 25 mL/min and 18 ± 23 mL/min per 1.73 m2. In group 2, the mean absolute bias for the CG was 25 ± 18 mL/min and 27 ± 19 mL/min per 1.73 m2 using the MDRD. For those in group 3, the mean absolute bias using the CG was 21 ± 19 mL/min and 19 ± 25 mL/min per 1.73 m2. The authors of the study concluded that although the differences between the CG and MDRD estimations were not statistically significant, their findings suggest both equations significantly overestimated renal function in cirrhotics, particularly in those with lower GFRs.
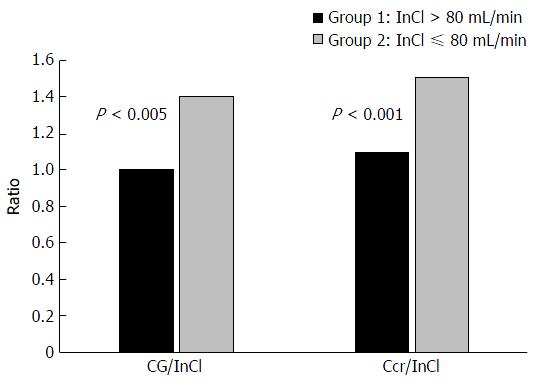
A third study assessing renal function in cirrhotics by Caregaro et al[33] was designed to evaluate the sensitivity of SCr and CrCl in detecting renal insufficiency and the magnitude of overestimation of GFR by CrCl. Estimation of CrCl was made using the CG equation and a 24-h urine collection. Estimates of CrCl were compared to measured GFR using inulin (Inulin Clearance = InCl). Patients in this study were divided into 2 groups based on measured GFR; group 1 (n = 29) had a GFR > 80 mL/min per 1.73 m2 and group 2 (n = 27) had a GFR ≤ 80 mL/min per 1.73 m2. For the patients in groups 1 and 2, the mean measured GFR (InCl) was 113.5 ± 27.9 mL/min per 1.73 m2 and 56.8 ± 19.8 mL/min per 1.73 m2, respectively. Estimating CrCl using the CG and 24-h urine collection provided an adequate assessment of measured GFR (InCl) in group 1 (CG = 106.3 ± 34.0 mL/min; 24-h = 121.5 ± 28.8 mL/min) but significantly overestimated measured GFR in group 2 (CG = 75.9 ± 40.1 mL/min; 24-h = 78.7 ± 39.2 mL/min) (Figure 3). Only 18.5% of patients in group 2 had a SCr level above normal limits and 81.5% of patients with a GFR ≤ 60 mL/min per 1.73 m2 had normal SCr levels. Overall, the sensitivity of SCr, CrCl estimated using the CG equation and 24-h urine collection in detecting renal insufficiency was 18.5%, 51% and 74%, respectively.
The authors concluded renal failure in cirrhotic patients is greatly underestimated because of the low sensitivity and accuracy of SCr levels in this population (Figure 3). Based on the data presented in this study, utilization of SCr based methods overestimated true renal function by about 50% in cirrhotic patients with a GFR ≤ 80 mL/min per 1.73 m2.
The importance of accurate and appropriate dosing of all medications remains a critical component of healthcare to maximize efficacy while limiting toxicity. For renally excreted medications, assessment and interpretation of renal function often dictates dosage selection. Due to the impractical nature of a 24-h urine collection, SCr has become a widely accepted surrogate marker of renal function used in several equations including the CG equation. With now over 40 years since its development, the CG equation remains one of the most widely used methods of assessing renal function. Unfortunately, because of its seemingly ubiquitous use and acceptance, many have forgotten its limitations and provider-specific variations used to compensate for these limitations.
Many of the limitations of the CG equation largely stem from the original study supporting its accuracy. The homogenous sample population limits the external validity and creates opportunity for the implementation of empiric correction factors that may or may not be supported by data[5]. As outlined in this review, selection of appropriate weight and rounding of low SCr levels are two examples of techniques used to broaden the applicability of the CG equation. These techniques vary among clinicians, largely because of a lack of evidence unanimously supporting one method over another. Unfortunately, additional limitations in the CG equation extend beyond body composition and habitus to include the subtle manifestations of the underlying disease process in a patient.
In cirrhotic patients, in addition to declining liver function, secondary physiological hemodynamic changes lead to a resultant reduction in GFR. Meanwhile, reductions in creatinine production and reduced muscle mass result in low SCr levels. Because of this, a sort of “cryptic renal failure” picture ensues, whereby SCr-based formulae will overestimate actual GFR by an average of about 50%[28-30]. Based on the evidence presented in this review and in the authors experience, multiplying the SCr by 1.5 in patients with decompensated cirrhosis provides a better CrCl estimate using the CG equation.
Utilization of the CG equation plays a significant role in the dosing decisions of many clinicians. In order to appropriately utilize this equation, clinicians have an inherent responsibility to understand its origins and limitations. True clinicians comprehensively assess each patient and consider SCr and CrCl as two variables that carry equal weight with several other parameters. Regardless of one’s approach to dosing medications, sole reliance on CrCl will undoubtedly lead to the ultimate realization that there is indeed fault in the CG.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jin B S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Winter MA, Guhr KN, Berg GM. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy. 2012;32:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3736] [Cited by in RCA: 3545] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 3. | Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008;83:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Dowling TC. Controversies in assessing kidney function. Pharmacotherapy Self-Assessment Program. 6th ed. Nephrology I. Kansas: American College of Clinical Pharmacy 2007; 1-10. |
| 5. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [PubMed] |
| 6. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-S266. [PubMed] |
| 7. | Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Jelliffe RW. Estimation of creatinine clearance when urine cannot be collected. Lancet. 1971;1:975-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Jelliffe RW. Letter: Creatinine clearance: bedside estimate. Ann Intern Med. 1973;79:604-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 336] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Edwards KD, Whyte HM. Plasma creatinine level and creatinine clearance as tests of renal function. Australas Ann Med. 1959;8:218-224. [PubMed] |
| 11. | Chiou WL, Hsu FH. Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol. 1975;15:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Ronco C, Grammaticopoulos S, Rosner M, De Cal M, Soni S, Lentini P, Piccinni P. Oliguria, creatinine and other biomarkers of acute kidney injury. Contrib Nephrol. 2010;164:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Jones GR. Estimating renal function for drug dosing decisions. Clin Biochem Rev. 2011;32:81-88. [PubMed] |
| 14. | US Food and Drug Administration, Center for Drug Evaluation and Research. March 2010. Guidance for Industry. Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis and Impact on Dosing and Labeling. Draft guidance, revision I. Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, MD. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformatio/Guidances/UCM204959.pdf. |
| 15. | Demirovic JA, Pai AB, Pai MP. Estimation of creatinine clearance in morbidly obese patients. Am J Health Syst Pharm. 2009;66:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Brown DL, Masselink AJ, Lalla CD. Functional range of creatinine clearance for renal drug dosing: a practical solution to the controversy of which weight to use in the Cockcroft-Gault equation. Ann Pharmacother. 2013;47:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11817] [Article Influence: 454.5] [Reference Citation Analysis (0)] |
| 19. | Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988;84:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | O’Connell MB, Dwinell AM, Bannick-Mohrland SD. Predictive performance of equations to estimate creatinine clearance in hospitalized elderly patients. Ann Pharmacother. 1992;26:627-635. [PubMed] |
| 21. | Dooley MJ, Singh S, Rischin D. Rounding of low serum creatinine levels and consequent impact on accuracy of bedside estimates of renal function in cancer patients. Br J Cancer. 2004;90:991-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Smythe M, Hoffman J, Kizy K, Dmuchowski C. Estimating creatinine clearance in elderly patients with low serum creatinine concentrations. Am J Hosp Pharm. 1994;51:198-204. [PubMed] |
| 23. | Cholongitas E, Shusang V, Marelli L, Nair D, Thomas M, Patch D, Burns A, Sweny P, Burroughs AK. Review article: renal function assessment in cirrhosis - difficulties and alternative measurements. Aliment Pharmacol Ther. 2007;26:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 25. | Solà E, Ginès P. Challenges and Management of Liver Cirrhosis: Pathophysiology of Renal Dysfunction in Cirrhosis. Dig Dis. 2015;33:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Teneva BH. Pathogenesis and assessment of renal function in patients with liver cirrhosis. Folia Med (Plovdiv). 2012;54:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Ho HL, Huang HC. Molecular mechanisms of circulatory dysfunction in cirrhotic portal hypertension. J Chin Med Assoc. 2015;78:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Martini GA. Some Endocrine Changes in Liver Disease. Postgrad Med J. 1963;39:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | O’Brien A, Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology. 2008;134:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | MacAulay J, Thompson K, Kiberd BA, Barnes DC, Peltekian KM. Serum creatinine in patients with advanced liver disease is of limited value for identification of moderate renal dysfunction: are the equations for estimating renal function better? Can J Gastroenterol. 2006;20:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Rognant N, Bacchetta J, Dubourg L, Ahmed SN, Radenne S, Dumortier J, Hadj-Aïssa A. What is the best alternative to inulin clearance to estimate GFR in patients with decompensated alcoholic cirrhosis? Nephrol Dial Transplant. 2010;25:3569-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 173] [Article Influence: 5.6] [Reference Citation Analysis (0)] |