Published online Oct 8, 2017. doi: 10.4254/wjh.v9.i28.1133
Peer-review started: January 7, 2017
First decision: March 13, 2017
Revised: July 23, 2017
Accepted: September 14, 2017
Article in press: September 15, 2017
Published online: October 8, 2017
Processing time: 203 Days and 9.3 Hours
To explore the applicability of the Asia-Pacific Association for the Study of the Liver (APASL) and European Association for the Study of the Liver (EASL) guidelines for acute-on-chronic liver failure (ACLF) in profiling patients and determining the outcome.
Patients admitted to a tertiary hospital in Singapore with acute decompensation of liver disease from January 2004 to July 2014 are screened for ACLF according to the APASL and EASL criteria. The patients’ data (including basic demographics, information about existing chronic liver disease, information about the acute decompensation, relevant laboratory values during admission, treatment, and outcome) are retrospectively analyzed to determine the background, precipitating factors and outcome.
A total of 458 liver patients is analyzed, and 78 patients with ACLF are identified. Sixty-three patients (80.8%) meet the APASL criteria, 64 patients (82.1%) meet the EASL criteria, and 49 patients (62.8%) fulfilled both criteria. The most common causes of acute liver injury are bacterial infections (59.0%), hepatitis B flare (29.5%), and variceal bleeding (24.4%). The common aetiologies of the underlying chronic disease included hepatitis B (43.6%), alcoholic (20.5%) and cryptogenic (11.5%) liver disease. The overall mortality rate is 61.5%. Increased age, the number of organ failures (as per CLIF-SOFA score), peak creatinine, INR, and amylase levels are associated with increased mortality or the need for liver transplantation. 14.3% of patients undergo liver transplantation with a 100% 1-year survival rate.
Both APASL and EASL criteria have identified ACLF patients with high three-month mortality, but those who fulfill APASL criteria alone have a better survival.
Core tip: Acute-on-chronic liver failure (ACLF) is a distinct disease entity with a high short-term mortality. Utilizing both the Asia-Pacific Association for the Study of the Liver (APASL) and European Association for the Study of the Liver criteria, our study shows that the clinical profile of ACLF patients in Singapore appears to have mixed features compared with similar studies reported in the rest of Asia and the West. Patients with ACLF fulfilling only the APASL criteria in our study had significantly better survival rates. We also analyzed the prognostic factors of ACLF in our study.
- Citation: Selva Rajoo A, Lim SG, Phyo WW, Tun T, Dan YY, Lee YM, Low HC, Lim K, Tan PS, Lee GH. Acute-on-chronic liver failure in a multi-ethnic Asian city: A comparison of patients identified by Asia-Pacific Association for the Study of the Liver and European Association for the Study of the Liver definitions. World J Hepatol 2017; 9(28): 1133-1140
- URL: https://www.wjgnet.com/1948-5182/full/v9/i28/1133.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i28.1133
Acute-on-chronic liver failure (ACLF) is a distinct disease entity characterized by the acute deterioration of liver function in patients with chronic liver disease[1]. It describes a condition in which two hepatic insults liver operate simultaneously, one of them being ongoing and persistent (e.g., chronic hepatitis C) while the other being an acute precipitating event (e.g., hepatotoxic drug, variceal bleed)[2]. Patients with ACLF have a statistically higher mortality rate (30%-40%) compared with patients without ACLF, at the same baseline Model for End-Stage-Liver Disease (MELD) score[3].
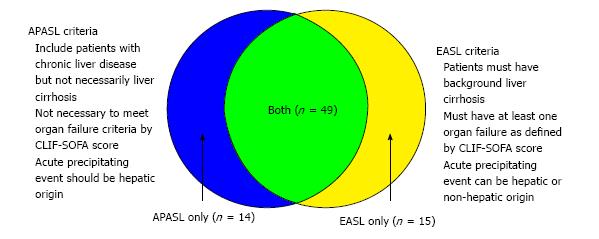
There are currently two widely accepted diagnostic criteria for ACLF: The Asia-Pacific Association for the Study of the Liver (APASL) in 2014[2], and the European Association for the Study of the Liver (EASL) consensus definitions in 2011[4]. Although these definitions describe the same disease entity, there are some crucial differences between them (summarised in Figure 1). APASL focuses more on signs of ascites and encephalopathy within a time frame of 4 wk with chronic liver disease. EASL underlines the occurrence of organ failure in patients with cirrhosis resulting in 3-mo mortality. Furthermore, these two definitions are based on populations with different disease patterns[5].
The objectives of this study are first, to understand the clinical profile of the patients with ACLF in Singapore. From this, the precipitating risk factors for ACLF could be treated or prevented. Secondly, this study aims to analyze the prognostic indicators of ACLF thereby discussing ways to improve the outcome.
There is an increasing concern about ACLF, due to its high short-term mortality and lack of clear understanding of the natural history and clinical profile of the patients, which vary across different countries and regions in the world. This study provides preliminary data on the local ACLF patient profile and outcome of this condition. We also examined the relevance and applicability of the current two guidelines for ACLF diagnosis and prognosis in the local context.
This retrospective cohort study was performed with existing data of patients admitted to the National University Hospital (NUH) in Singapore from January 2004 to July 2014. The data is part of an approved database of all patients admitted to the hepatology service or referred to liver transplant service. All patients were followed up for at least three months. All relevant data had been recorded in the hospital electronic medical records, Intensive Care Unit (ICU) monitoring system, and the patients’ case files. In this study, the diagnosis of ACLF was made by utilizing either the APASL or EASL definitions.
Data were retrospectively analyzed, but the clinicians prospectively collected the data through their inpatient lists and anonymously transferred to the study administrator. Confidentiality of the patients was preserved by anonymising the data collected. The subject data was assigned code numbers which do not reflect personal identifiers and were entered into a computerized database. Data collection included basic demographic information (age, gender, body mass index), information about existing chronic liver disease, information about acute decompensation, other relevant laboratory values of the patient during admission (white cell count, creatinine, bilirubin, international normalized ratio, C-reactive protein, etc.), treatment and outcome. This study protocol had been reviewed and approved by the National Healthcare Group Domain Specific Research Board (Domain E) (DSRB reference: 2014/01194).
Data entry and analysis were carried out using SPSS 20. Inter-group comparisons for categorical variables were made using the χ2 test or Fisher’s exact test, and those for quantitative variables were compared using the Student’s t-test, one-way ANOVA. A P-value less than 0.05 was considered statistically significant.
A total of 458 liver patients were screened. One hundred and forty-seven were found to have an acute decompensation of chronic liver disease, and 78 of these patients were found to have ACLF that fulfilled either the APASL or EASL criteria. Sixty-three patients (80.8%) met the APASL criteria, and 64 patients (82.1%) met the EASL criteria. Forty-nine patients (62.8%) fulfilled both criteria (summarized in Figure 1 and Table 1).
| APASL only (n = 14) | EASL only (n = 15) | Both (n = 49) | P value3 | Total (n = 78) | |
| AGE (mean) | 53 (48-57) | 66 (61-71) | 57 (54-60) | 0.003 | 58 (55-61) |
| Race | |||||
| Chinese | 9 (64.3) | 9 (60) | 36 (73.5) | 0.171 | 54 (69.2) |
| Malay | 1 (7.1) | 2 (13.3) | 5 (10.2) | 8 (10.3) | |
| Indian | 0 | 3 (20) | 5 (10.2) | 8 (10.3) | |
| Others | 4 (28.6) | 1 (6.7) | 3 (6.1) | 8 (10.3) | |
| Male gender | 12 (85.7) | 11 (73.3) | 36 (73.5) | 0.625 | 59 (75.6) |
| Diabetes mellitus | 7 (50) | 5 (33.3) | 16 (32.7) | 0.478 | 28 (35.9) |
| Cause of acute liver injury4 | |||||
| Infection | 4 (28.6) | 14 (93.3) | 28 (57.1) | 0.002 | 46 (59) |
| Hepatitis B flare | 9 (64.3) | 0 | 14 (28.6) | 0.001 | 23 (29.5) |
| Variceal bleeding | 4 (28.6) | 2 (13.3) | 13 (26.5) | 0.535 | 19 (24.4) |
| Unknown cause | 2 (14.3) | 1 (6.7) | 8 (16.3) | 0.642 | 11 (14.1) |
| Alcohol | 1 (7.1) | 0 | 5 (10.2) | 0.429 | 6 (7.7) |
| TCM | 1 (7.1) | 0 | 5 (10.2) | 0.429 | 6 (7.7) |
| TIPSS | 0 | 0 | 1 (2) | 0.741 | 1 (1.3) |
| Underlying chronic liver disease | |||||
| Hepatitis B | 11 (78.6) | 1 (6.7) | 22 (44.9) | < 0.0001 | 34 (43.6) |
| Alcohol | 2 (14.3) | 3 (20) | 11 (22.4) | 0.799 | 16 (20.5) |
| Cryptogenic | 0 | 3 (20) | 6 (12.2) | 0.234 | 9 (11.5) |
| Hepatitis C | 0 | 2 (13.3) | 3 (6.1) | 0.339 | 5 (6.4) |
| NASH | 0 | 2 (13.3) | 2 (4.1) | 0.23 | 4 (5.1) |
| Hepatitis B + alcohol | 1 (7.1) | 0 | 1 (2) | 0.444 | 2 (2.6) |
| Others1 | 0 | 4 (26.7) | 5 (10.2) | 0.072 | 9 (11.5) |
| Liver cirrhosis | 6 (42.9) | 15 (100) | 49 (100) | < 0.0001 | 70 (89.7) |
| HCC (Milan’s criteria) | 0 | 1 (6.7) | 4 (8.2) | 0.627 | 5 (6.4) |
| Other cancers2 | 0 | 1 (6.7) | 4 (8.2) | 5 (6.4) | |
| No malignancy | 14 (100) | 13 (86.7) | 41 (83.7) | 68 (87.2) | |
| Previous decompensation | 4 (28.6) | 10 (66.7) | 20 (43.5) | 0.11 | 34 (45.3) |
Table 1 shows the profile of patients with ACLF. The age range of the 78 patients included in the study was 55 to 61 years. Sixty-nine point two percent of these patients were Chinese, which is consistent with the local racial demographics of the population (74.3% Chinese, 13.4% Malays, and 9.1% Indians[6]). Seventy-five point five percent of the patients were male. Patients with ACLF meeting the EASL criteria were more likely to be older than those in the other two groups (P = 0.003).
Bacterial infection (59.0%), hepatitis B flare (29.5%) and variceal bleeding (24.4%) were the most common causes leading to the acute insult. Patients fulfilling ACLF-EASL criteria were more likely to have had a bacterial infection triggering ACLF compared to those in the other two groups (P = 0.002). On the other hand, patients fulfilling ACLF-APASL criteria were more likely to have had hepatitis B flare triggering ACLF compared to those in the other two groups (P = 0.001). Patients admitted with ACLF most frequently have hepatitis B (43.6%), alcoholic liver disease (20.5%) and cryptogenic liver disease (11.5%) as their underlying chronic liver diseases. Patients fulfilling the APASL criteria were more likely to have Hepatitis B compared to patients in the EASL group.
Table 2 shows the outcome of patients with Acute-on-Chronic Liver Failure. The overall mortality rate at the point of admission and three-month mortality rate were expectedly high at 57.7% and 61.5% respectively. Patients with ACLF fulfilling both criteria were more likely to have a fatal outcome at the point of admission (67.3% mortality) as well as in 3 mo [71.4% mortality (P = 0.033 and P = 0.041 respectively)]. Transplant rate was 14.3%, and all the transplant patients survived and lived for more than one year (P ≤ 0.0001).
| APASL only (n = 14) | EASL only (n = 15) | Both (n = 49) | P value | Total (n = 78) | |
| MELD score (mean-range) | 27 (23-31) | 18 (13-23) | 25 (22-28) | 0.020 | 24 (33-26) |
| Transplant | 4 (28.6) | 0 | 7 (14.6) | 0.089 | 11 (14.3) |
| Mortality (during admission) | 4 (28.6) | 8 (53.3) | 33 (67.3) | 0.033 | 45 (57.7) |
| Three month mortality | 5 (35.7) | 8 (53.3) | 35 (71.4) | 0.041 | 48 (61.5) |
Patients were further classified by ACLF grade (ACLF 0-3) according to the EASL-CLIF Consortium definitions[5,7], which classifies the severity of ACLF by the number of organ failures[8]. Table 3 shows information regarding organ failures, laboratory parameters and outcome of ACLF patients to the ACLF grade. Higher mortality rates were associated with an increased ACLF grade. Three-month mortality for ACLF 0 to 3 was 0%, 42.9%, 41.7% and 84.8% respectively. Patients with 3 or more organ failures (i.e., ACLF 3) had a significantly higher mortality rate than all other patients at the point of admission and at three months (P ≤ 0.0001, P < 0.0001 respectively). Patients who fulfill the APASL criteria for ACLF exclusively (i.e., no organ failures or ALCF 0) had a 0% mortality rate.
| ACLF0 (n = 6) | ACLF1 (n = 7) | ACLF2 (n = 24) | ACLF3 (n = 33)1 | P value | |
| Organ failures-clif-sofa score | |||||
| Liver | 1 (16.7) | 1 (14.3) | 12 (50) | 27 (81.8) | < 0.0001 |
| Kidney | 0 | 4 (57.1) | 10 (41.7) | 25 (75.8) | 0.002 |
| Cerebral | 0 | 1 (14.3) | 2 (8.3) | 13 (39.4) | 0.018 |
| Coagulation | 1 (16.7) | 1 (14.3) | 16 (66.7) | 25 (75.8) | 0.002 |
| Circulation | 0 | 0 | 7 (30.4) | 26 (78.8) | < 0.0001 |
| Respiration | 0 | 0 | 2 (8.3) | 7 (21.2) | 0.22 |
| Chronic renal disease | 0 | 2 (28.6) | 5 (20.8) | 3 (9.1) | 0.245 |
| Laboratory data (mean) | |||||
| Leucocyte count at baseline, × 109/L | 8 (6-11) | 11 (6-16) | 10 (7-12) | 12 (10-13) | 0.256 |
| Platelet count at baseline | 127 (42-212) | 61 (40-82) | 104 (78-129) | 149 (105-193) | 0.105 |
| Bilirubin at baseline, mg/dL | 7.0 (4.2-9.8) | 7.7 (-3.1-18.5) | 10 (4.9-15.1) | 13.9 (9.9-17.8) | 0.29 |
| Peak bilirubin, mg/dL | 11.3 (4.1-18.5) | 10.7 (-4.2-25.5) | 14.1 (9.1-19) | 23 (18.7-27.3) | 0.009 |
| Creatinine at baseline, mg/dL | 0.9 (0.4-1.3) | 2.1 (1.3-2.9) | 1.4 (1.1-1.8) | 1.8 (1.1-2.4) | 0.232 |
| Peak creatinine, mg/dL | 0.9 (0.5-1.2) | 2.2 (1.4-3.1) | 1.8 (1.4-2.1) | 3.1 (2.6-3.7) | < 0.0001 |
| Lactate at baseline, mmol/L | 2.1 (1.4-2.7) | 2.3 (2.1-2.6) | 2.4 (1.7-3.2) | 4.1 (2.4-5.7) | 0.225 |
| MELD score at baseline (mean) | 23 (20-26) | 21 (11-31) | 20 (17-24) | 26 (23-30) | 0.113 |
| Liver transplantation | 1 (16.7) | 0 | 6 (26.1) | 1 (3) | 0.043 |
| Mortality during admission | 0 | 3 (42.9) | 10 (41.7) | 28 (84.8) | < 0.0001 |
| 90-d mortality | 0 | 3 (42.9) | 11 (45.8) | 29 (87.9) | < 0.0001 |
The demographics, type of organ failure and laboratory parameters of ACLF patients who survived vs those who met a fatal outcome have been analyzed and summarised in Table 4. Patients with fatal outcomes were more likely to be older (mean age 60 vs 55, P = 0.044). Patients with renal (68.9%, P = 0.001), cerebral (37.8%, P = 0.012), circulatory (63.6%, P ≤ 0.0001) and respiratory (17.8%, P = 0.044) failure were more likely to have a fatal outcome compared to those without these organ failures. Also, higher serum creatinine and INR and baseline amylase were strongly associated with the poor prognosis compared to other laboratory tests (P ≤ 0.0001, 0.018 and 0.026 respectively).
| Deaths (n = 45) | Survivors (n = 33) | P value | |
| Age (mean-range) | 60 (56-64) | 55 (52-58) | 0.0441 |
| Liver transplantation | 0 | 11 (34.4) | < 0.00012 |
| Race | |||
| Chinese | 33 (73.3) | 21 (63.6) | 0.0041 |
| Malay | 6 (13.3) | 2 (6.1) | |
| Indian | 6 (13.3) | 2 (6.1) | |
| Others | 0 | 8 (24.2) | |
| Male gender | 33 (73.3) | 26 (78.8) | 0.5791 |
| Diabetes mellitus | 13 (28.9) | 15 (45.5) | 0.1321 |
| Previous hepatic decompensation | 16 (37.2) | 18 (56.2) | 0.1011 |
| Potential events leading to acute insult | |||
| Infection | 28 (62.2) | 18 (54.5) | 0.4961 |
| Hepatitis B flare | 15 (33.3) | 8 (24.2) | 0.3841 |
| Variceal bleeding | 10 (22.2) | 9 (27.3) | 0.6081 |
| Unknown cause | 5 (11.1) | 6 (18.2) | 0.2872 |
| Alcohol | 2 (4.4) | 4 (12.1) | 0.2042 |
| TCM | 5 (11.1) | 1 (3) | 0.1892 |
| TIPSS | 1 (2.2) | 0 | 0.5772 |
| Underlying chronic liver disease | |||
| Hepatitis B | 20 (44.4) | 14 (42.4) | 0.8591 |
| Alcohol | 10 (22.2) | 6 (18.2) | 0.6621 |
| Cryptogenic | 4 (8.9) | 5 (15.2) | 0.3072 |
| Hepatitis C | 3 (6.7) | 2 (6.1) | 0.6462 |
| NASH | 2 (4.4) | 2 (6.1) | 0.5672 |
| Hepatitis B + alcohol | 1 (2.2) | 1 (3) | 0.6702 |
| Others1 | 6 (13.3) | 3 (9.1) | 0.4192 |
| Liver cirrhosis | 41 (91) | 29 (87.9) | 0.4592 |
| HCC+ (Milan criteria) | 3 (6.7) | 2 (6.1) | 0.7092 |
| Other cancers2 | 2 (4.4) | 3 (9.1) | |
| None | 40 (88.9) | 28 (84.8) | |
| Organ failures-clif-sofa score | |||
| Liver | 31 (68.9) | 17 (51.5) | 0.1191 |
| Kidney | 31 (68.9) | 10 (30.3) | 0.0011 |
| Cerebral | 17 (37.8) | 4 (12.1) | 0.0121 |
| Coagulation | 31 (68.9) | 19 (57.6) | 0.3031 |
| Circulation | 28 (63.6) | 7 (21.2) | < 0.00011 |
| Respiration | 8 (17.8) | 1 (3) | 0.0442 |
| Chronic renal disease | 9 (20) | 2 (6.1) | 0.0752 |
| Leucocyte count at baseline (mean-range) | 11 (10-12) | 10 (8-12) | 0.2733 |
| Platelet count at baseline (mean-range) | 135 (109-160) | 120 (79-160) | 0.5283 |
| Amylase (mean-range) | 117 (90-144) | 72 (38-105) | 0.0263 |
| Maximal total bilirubin (mean-range) | 20.2 (16.3-24) | 15.9 (11.5-20.2) | 0.1373 |
| Maximal creatinine (mean-range) | 2.9 (2.4-3.3) | 1.6 (1.3-1.9) | < 0.00013 |
| Maximal INR (mean-range) | 4.3 (3.5-5.1) | 3 (2.5-3.6) | 0.0183 |
| MELD at baseline (mean-range) | 26 (23-29) | 22 (19-25) | 0.1193 |
| Lactate at baseline (mean-range) | 3.5 (2.4-4.6) | 2.8 (2-3.7) | 0.3113 |
One of the compelling reasons for the lack of a unifying definition for ACLF is the difference in etiologies for both the acute insults and underlying chronic liver diseases in the East and West[9], and much of this can be attributed to the socioeconomic status of the countries in Asia. In Singapore, with endemic chronic hepatitis B as the dominant chronic liver disease, coupled with a Westernised lifestyle and standard of living, understanding the mixed profiling of local ACLF patients and the prognostic factors will be important in better prevention and management of such high-risk patients.
Bacterial infection, hepatitis B flare, and variceal bleeding are the most common causes for the acute component of ACLF. Patients fulfilling the EASL criteria are more likely to have bacterial infections that triggered ACLF while patients meeting the APASL criteria are more likely to have a hepatitis B flare as the trigger. This difference is in keeping with the differences in underlying etiologies of acute deterioration of liver disease between the East and the West published in the literature. In the Asia-Pacific region, which is the demographic that the APASL guidelines are based upon, the majority of ACLF is precipitated by viral hepatitis. In developed European countries, these viral etiologies are mostly supplanted by non-viral insults such as bacterial infections[10]. We note that more than half of the study population had a bacterial infection as the precipitating factor of ACLF, reflecting the developed health care standards enjoyed by the Singaporean population. There is no consensus as to whether variceal bleeding qualifies as a precipitant of ALCF under the APASL guidelines[10]. However, this study shows that it is a prominent cause of ACLF (24.4%) should it be included.
Patients with ACLF in this study most frequently have chronic hepatitis B infection, alcoholic liver disease, and cryptogenic liver cirrhosis as their underlying chronic liver disease. The prevalence of HBV is expected given that Singapore lies within the Asia-Pacific region and in most Asian countries, hepatitis B constitutes about 70% of all chronic etiologies of ACLF. Alcoholic liver cirrhosis represents 50%-70% of all underlying etiologies of ACLF in Western countries[5,11,12]. The fact that alcoholic liver cirrhosis constitutes such a high proportion of the study population suggests a Western influence on the local community as well. However, some studies do indicate that alcohol-related ACLF is equally represented worldwide[10,13].
One of the unexpected findings of this study is the narrow age range (52-64 years) of all 78 consecutive ACLF subjects. All 45 deaths were older (mean 60 years, range 56-64 years), with little overlap with the survivors (mean age 55 years, range 52-58 years) who were younger. Thus, a higher index of suspicion for progression to ACLF should be applied when patients above 50 years of age present with acute liver decompensation. Deterioration of the patient should be expected and pre-empted, especially for those between 55 to 65 years of age who are still eligible for liver transplantation. This age-related incidence and prognosis still await future validation studies for confirmation.
Patients with ACLF have a high mortality rate at 57.7% and 61.5% (at 0 and 3 mo respectively), which is comparable to the documented mortality rate of 50%-90%[14]. Higher mortality rates have been associated with an increase in ACLF grade based on the CLIF-SOFA score (i.e., more organ failures) in line with existing literature[15]. Furthermore, patients with ACLF and no organ failure had a 0% mortality rate. These results suggest that the CLIF-SOFA organ failure score may be a useful predictor of death in our local ACLF population, in keeping with publications which identify the correlations between the number of organ failure(s) in patients with cirrhosis with mortality[5]. In this study, peak creatinine, INR, and amylase levels are independently associated with increased mortality or the need for liver transplantation. Peak creatinine level, in particular, is most strongly associated with increased mortality, which is expected given the association between renal failure and death in ACLF[16].
Liver transplantation is an important definitive treatment for patients with severe ACLF, who usually have underlying liver cirrhosis[17-20]. This study has shown that patients with ACLF who subsequently underwent liver transplantation had a 100% 1-year survival rate. This promising result suggests that high-urgency allocation of liver transplantation should be considered for ACLF patients[19,21]. Nonetheless, we note that not all patients with ACLF are transplant candidates for numerous reasons, which may include advanced age, active alcoholism, or concomitant diseases. The presence of non-liver organ failures may sometimes be a contraindication to liver transplantation[3].
In conclusion, ACLF is a life-threatening syndrome and both the APASL and EASL criteria have identified ACLF patients with high short-term mortality. The clinical profile of ACLF patients in Singapore appears to have mixed features compared with similar studies reported in the rest of Asia and the West. This would not be unique to Singapore, but applicable to many growing cities in Asia undergoing a rapid transformation from traditional disease epidemiology and lifestyle to improved living standards and widespread modern healthcare standards. Each region will have to re-evaluate their changing patterns of ACLF and address the new needs accordingly. The multi-ethnic composition of the Singapore population also has implications for understanding the variations in the Asian-Pacific region.
Patients with ACLF fulfilling only the APASL criteria in our study had significantly better survival rates compared with patients meeting the EASL criteria only, largely due to the APASL criteria accepting subjects who had chronic hepatitis but not liver cirrhosis (42.9%), while EASL-defined ACLF subjects must be cirrhotic. It is interesting to note that patients meeting the only APASL criteria had a higher MELD score than patients fulfilling the EASL criteria. These patients may have had a more severe acute insult leading to acute decompensation, but they still had higher survival rate due to better baseline liver function. Patients with ACLF fulfilling both criteria were more likely to have a fatal outcome (71.4% 3-mo mortality (P = 0.041). CLIF-SOFA organ failure score, complemented by laboratory parameters such as creatinine, amylase, and INR appear to be promising tools in determining the prognosis of patients with ACLF. Early diagnosis of ACLF and identification of indicators predictive of poor outcome (as discussed above) will help to distinguish between patients with ACLF that would require transplantation from those that will survive with only organ support and intensive medical care[14] and thus optimise treatment and survival.
Acute-on-chronic liver failure (ACLF) is a distinct disease entity with a high short-term mortality. There are two widely accepted diagnostic criteria for ACLF. However, there are crucial differences between them. There is also currently a lack of clear understanding of the natural history and clinical profile of the patients, which vary across different regions in the world.
This is the first study to explore the applicability of the Asia-Pacific Association for the Study of the Liver (APASL) and European Association for the Study of the Liver (EASL) guidelines for ACLF in profiling patients and determining the outcome in Singapore.
EASL criteria may identify patients with a higher mortality. CLIF-SOFA organ failure score, complemented by laboratory parameters such as creatinine, amylase, and INR appear to be promising tools in determining the prognosis of patients with ACLF.
Early diagnosis of ACLF and identification of indicators predictive of poor outcome will help to distinguish between patients with ACLF that would require transplantation from those that will survive with only organ support and intensive medical care and thus optimise treatment and survival.
ACLF: A distinct disease entity characterized by the acute deterioration of liver function in patients with the chronic liver disease.
The manuscript describes a retrospective study investigating ACLF in patients from Singapore. The study compared the EASL and APASL ACLF guidelines in patients with an acute decompensation of liver disease. The manuscript overall is of interest, and the results are enlightening.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bianchini M, Donnelly MC, Marchan-Lopez A, McMillin MA S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 2. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 3. | Arroyo V, Moreau R, Jalan R, Ginès P; EASL-CLIF Consortium CANONIC Study. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62:S131-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 306] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 4. | Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol. 2013;19:349-359. [PubMed] [DOI] [Full Text] |
| 6. | Department of Statistics, Ministry of Trade Industry, Republic of Singapore. Population Trends, 2016. ISSN 1793-2424. |
| 7. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [PubMed] [DOI] [Full Text] |
| 8. | Vincent JL, Sakr Y. SOFA so good for predicting long-term outcomes. Resuscitation. 2012;83:537-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Jindal A, Rastogi A, Sarin SK. Reviewing the diagnostic criteria for acute-on-chronic liver failure. Expert Rev Gastroenterol Hepatol. 2016;10:1385-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Bajaj JS. Defining acute-on-chronic liver failure: will East and West ever meet? Gastroenterology. 2013;144:1337-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2011;17:3448-3452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Pati GK, Singh A, Misra B, Misra D, Das HS, Panda C, Singh SP. Acute-on-Chronic Liver Failure (ACLF) in Coastal Eastern India: “A Single-Center Experience”. J Clin Exp Hepatol. 2016;6:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Amarapurkar D, Dharod MV, Chandnani M, Baijal R, Kumar P, Jain M, Patel N, Kamani P, Issar S, Shah N. Acute-on-chronic liver failure: a prospective study to determine the clinical profile, outcome, and factors predicting mortality. Indian J Gastroenterol. 2015;34:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 2013;33:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care. 2012;16:R227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Bahirwani R, Shaked O, Bewtra M, Forde K, Reddy KR. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation. 2011;92:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Duan BW, Lu SC, Wang ML, Liu JN, Chi P, Lai W, Wu JS, Guo QL, Lin DD, Liu Y. Liver transplantation in acute-on-chronic liver failure patients with high model for end-stage liver disease (MELD) scores: a single center experience of 100 consecutive cases. J Surg Res. 2013;183:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW, Vogel W. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen QQ, Chen YP. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol. 2011;9:351-356.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Putignano A, Gustot T. New concepts in acute-on-chronic liver failure: Implications for liver transplantation. Liver Transpl. 2017;23:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |