Published online Aug 18, 2017. doi: 10.4254/wjh.v9.i23.979
Peer-review started: March 2, 2017
First decision: March 28, 2017
Revised: May 22, 2017
Accepted: June 19, 2017
Article in press: June 20, 2017
Published online: August 18, 2017
Processing time: 169 Days and 19.2 Hours
Innate lymphoid cells (ILCs) are the most recently discovered family of innate immune cells. They are a part of the innate immune system, but develop from the lymphoid lineage. They lack pattern-recognition receptors and rearranged receptors, and therefore cannot directly mediate antigen specific responses. The progenitors specifically associated with the ILCs lineage have been uncovered, enabling the distinction between ILCs and natural killer cells. Based on the requirement of specific transcription factors and their patterns of cytokine production, ILCs are categorized into three subsets (ILC1, ILC2 and ILC3). First observed in mucosal surfaces, these cell populations interact with hematopoietic and non-hematopoietic cells throughout the body during homeostasis and diseases, promoting immunity, commensal microbiota tolerance, tissue repair and inflammation. Over the last 8 years, ILCs came into the spotlight as an essential cell type able to integrate diverse host immune responses. Recently, it became known that ILC subsets play a key role in immune responses at barrier surfaces, interacting with the microbiota, nutrients and metabolites. Since the liver receives the venous blood directly from the intestinal vein, the intestine and liver are essential to maintain tolerance and can rapidly respond to infections or tissue damage. Therefore, in this review, we discuss recent findings regarding ILC functions in homeostasis and disease, with a focus on the intestine and liver.
Core tip: Receiving approximately 70% of blood through the portal vein, the liver represents one of the most important sites of defense against invading pathogens. In addition, the liver and the intestine are important immune organs, as they are often in contact with antigens and endotoxins produced by the gut microbiota. These organs are densely populated by innate immune cells such as natural killer cells, dendritic cells, macrophages, natural killer T cells and innate lymphoid cells (ILCs), which are rapidly activated by commensal and pathogenic antigens, growth factors, cytokines and host metabolites. Recent studies have been focused on discovering the role of ILCs and how these cell populations can regulate the immune response. Our goal is to discuss innovative literature highlighting the importance of ILCs in the context of infectious disease, tissue repair, tolerance of gut microbiota and inflammatory diseases that affect the liver and intestine homeostasis.
- Citation: Ignacio A, Breda CNS, Camara NOS. Innate lymphoid cells in tissue homeostasis and diseases. World J Hepatol 2017; 9(23): 979-989
- URL: https://www.wjgnet.com/1948-5182/full/v9/i23/979.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i23.979
Innate lymphoid cells (ILCs) are the most recent family of innate immune cells discovered among the myriad of factors that make up the immune system. They belong to innate immune system but develop from the lymphoid lineage. However, contrary to T and B lymphocytes, ILCs do not have RAG-mediated recombined antigen receptors[1,2]. Their distribution is ubiquitous, being found throughout the body and enriched in mucosal surfaces[3,4]. These cells are able to communicate with different cell types to orchestrate the immune system during homeostasis and inflammation[5-7].
The non-cytotoxic ILCs consist of three different groups: ILC1, ILC2 and ILC3[5,7-10]. The ILC3s also include the lymphoid tissue inducer (LTi) cells. These cells were uncovered in 1997 and are involved in the formation of secondary lymphoid tissues[4].
Mirroring the Th subsets, the non-cytotoxic ILCs are separated based on cytokine expression, transcription factors during development, surface markers and distinct effector functions[5,6]. Although parallels between ILCs and Th subsets have been observed, ILCs lack pattern-recognition receptors and therefore cannot directly mediate antigen specific responses[3,11]. In fact, given that these cells are not directly activated by pathogen-associated molecular patterns it was unclear how ILCs discern infection, tissue injury or disruption of homeostasis. It is now known that ILCs present within adult tissues constitutively express cytokines, alarmins and growth factor receptors making them more sensitive to these mediators in their environment, enabling immediate ILC activation[3,12]. Despite being present in very low numbers, the wide distribution of ILCs in lymphoid and non-lymphoid tissues across species was seen as an indicator of the fundamental role of these innate cells in regulating multiple physiological processes throughout the body[7].
Several studies have shown that ILCs are easily recovered in areas susceptible to microbial colonization or invasion by pathogens, such as barrier surfaces. Recently, it became known that ILC subsets play a key role in host immune responses to bacteria, fungi, viruses and extracellular parasites at these sites[6,13,14]. In addition, their interaction with the microbiota, nutrients and metabolites[6,13] highlighted important functions for ILCs in triggering tissue repair and inflammation which, if unregulated, can result in exacerbated immune responses.
Based on the emerging roles of ILCs in controlling tissue homeostasis, this review will highlight the advances in understanding how ILCs can participate in host defense in the context of immunity, microbiota, autoimmunity and tissue remodeling, focusing on the intestinal and liver pathophysiology.
Until the discovery of ILCs, conventional natural killer cells (cNKs) were the only innate cells able to respond to cytokines released by antigen presenting cells (APCs). Therefore, NK cells represent the prototypical member of the ILC family[1,15-18]. However, NKs have additional roles that set them apart from other ILCs, such as cytotoxicity and the ability to initiate immune responses against virus and tumor cells[18]. Besides, recent analysis of the progenitor cells and surface markers of the ILC family members indicate that NK cells and non-cytotoxic ILCs group do not come from the same lineage[7-10].
The identification of the ILC precursors and the key factors required for development of the different ILC subsets is quite recent. It was found that the ILCs arise from a common lymphoid progenitor (CLP). Downstream, the precursors can develop into different ILC subsets and NK cells expressing the integrin α4β7 and the transcription factors Nfil3 (nuclear factor- interleukin 3 regulated) and TOX[7,9,19,20]. First, the common helper-like ILC progenitor (CHILP), that expresses the transcriptional regulator inhibitor of DNA binding 2 (Id2), gives rise only to ILC1, ILC2, ILC3 and LTi cells[9,10,19]. Downstream to CHILP, another ILC precursor is able to give rise all ILCs subsets, but not LTi or cNK cells[10]. This precursor can express the transcription factor promyelocitic leukemia zinc finger (PLZF)[21,22].
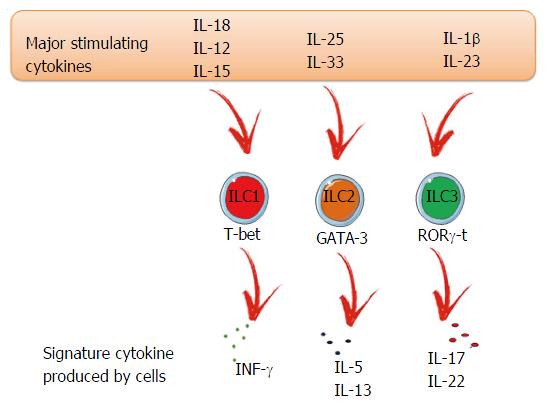
As mentioned before, ILC populations differ based on their transcription factors and production of signature cytokines, similar to Th cells. However, while ILC2s and ILC3s are well characterized, ILC1s are more complex to identify due to many shared characteristics with NK cells[23]. Both are responsive to inflammatory cytokines, such as interleukin (IL)-15 and IL-12, and produce interferon (IFN)-γ and tumor necrosis factor (TNF) after activation[24,25]. ILC1s are enriched in the liver, skin, salivary glands, uterus, thymus and the gut[23,26]. Regarding transcription factors, T-bet is the most important and regulates the ILC phenotype and functions, such as the production of IFN-γ. NK cells can also express T-bet (Figure 1). However, in NK cells, this transcription factor is not expressed in the same level, nor is it important to their development, unlike ILC1s. The T-box transcription factor eomesodermin (Eomes) is the marker of NK cells, but in some organs ILC1s can also express this transcription factor[24,25,27,28]. The general ILC1 surface markers include CD69, NK1.1 and NKp46 but, depending on the environment, we can find distinct ILC1 phenotypes and, consequently, surface markers. ILC1s are important to promote immunity against intracellular bacteria and parasites[9,29-31].
ILC2s are known to produce Th2 signature cytokines, IL-5 and IL-13[32-35] and can also release IL-9, IL-5 and IL-6[36]. They are characterized by their responsiveness to IL-33, IL-25 and thymic stromal lymphopoietin (TSLP)[37-40]. Their main function is to promote type 2 inflammation, which is important during allergies, helminth infection, and tissue repair[32-34,41-43]. ILC2s are found in different tissues including lung and adipose tissue, as well as in the gut, liver and skin[39,44-46]. Their main surface markers are CD127, c-kit, Sca1 and ST2 (the receptor for IL-33)[1]. Regarding transcription factors, experiments with GATA-binding protein 3 (GATA-3) knockout mice have shown that this factor is essential for ILC2 differentiation and maturation (Figure 1)[47,48].
ILC3s are a heterogenous group found mainly in mucosal tissues[49]. However, a small number can be found in the spleen[50], lung[50] and liver[51]. Two different ILC3 subsets can be distinguished based on the expression of T-bet and CCR6. Both express RAR-related orphan receptor gamma (RORγt), but only LTi cells express CCR6+. The LTi population can be further divided based on the expression of CD4 (LTi CD4+ or LTi CD4-). The CCR6- ILC3 population can express T-bet and consists of two subpopulations that are distinguished based on the expression of the natural cytotoxicity receptor (NCR) NKp46[52-54]. The ILC3 subset signature cytokines are IL-22 and IL-17 (Figure 1). The IL-22 acts selectively on stromal and epithelial cells leading to a rapid production of the antimicrobial peptides alpha and beta defensins. ILC3-derived IL-22 is crucial in preventing dissemination of commensal bacteria[52,55,56].
The strategic location of ILCs at the mucosal surfaces ensures the induction of an immune response and shapes the adaptive immune response against invading pathogens. In addition, the presence of these cells in non-lymphoid tissues suggests that, besides regulating the proinflammatory responses, ILCs can also play a role in tissue development and homeostasis (Table 1).
| ILC subtype | Function | Model | Evidence | Ref. |
| ILC1 | T. gondii infection | Oral infection C57BL/6 mice | Immunity to T. gondii infection is IFNγ-dependent; mice lacking T-bet expression had virtually no IFN-g production in response to T. gondii infection and failed to control parasite replication | [9] |
| ILC2 | N. brasiliensis infection | Balb/c subcutaneous infection | Combined absence of IL-25 and IL-33 signaling led to a defect in worm expulsion, that was rescued by ILC2-adoptive transfer | [34] |
| ILC3 | S. Thiphymurium infection | Fut2-deficient C57BL/6 mice | Fucosylation of intestinal epithelial cells is catalyzed by Fut2; IL-22-derived ILC3s induce the expression of Fut2. Disruption of intestinal fucosylation led to increased susceptibility to infection by S. Typhimurium | [57] |
| ILC3 | C. rodentium infection | Oral infection C57BL/6 mice | Mice lacking IL-22-producing ILC3 cells showed heightened susceptibility to the pathogen | [54,62] |
| ILC3 | C. albicans infection | C57BL/6 and BALB/c mice | IL-22 mediates protection in IL-17RA-deficient mice; an early IL-22-dominated response is then followed by Th1/Treg reactivity | [65] |
| ILC2 | Epithelium repair after intestinal inflammation | C57BL/6 DSS-induced colitis | Number of AREG-expressing ILC2s increases following intestinal inflammation. Disruption of the AREG-EGFR pathway exacerbated disease | [74] |
| ILC3 | Repair of lymphoid tissue | C57BL/6 mice | LCMV infection induces the destruction of secondary lymphoid organs RORγ-deficient WT chimeras had impaired rebuilding of stromal cell compartment after LCMV infection | [70] |
| ILC3 | Regeneration of intestinal epithelium | C57BL/6 mice | Intestinal microbiota represses the ILC3-producing IL-22 through the induction of IL-25 by IECs. RAG-2-deficient mice treated with IL-25 showed significant weight loss in response to DSS treatment | [68] |
| ILC3 | Containment of the gut microbiota | C57BL/6 mice | Depletion of IL22-producing ILC3s resulted in peripheral dissemination of commensal bacteria and systemic inflammation, which was prevented by administration of IL-22 | [80] |
| Ablation of LTα in RORgt + cells abrogated IgA production in the gut and altered microbiota composition | [81] | |||
| ILC1 | Crohn’s disease | Human | ILC1 population is increased in the inflamed intestine of people with Crohn’s disease | [29,30] |
| ILC1 | Ulcerative colitis | Anti-CD40 colitis model | IELs from the small intestine of mice treated with anti-CD40 revealed a robust production if IFN-γ by ILC1s. Anti-Nk1.1 treatment reduced inflammatory infiltration and epithelial damage, suggesting that ILC1 can contribute to colitis through IFN-γ secretion | [85] |
| ILC3 | Ulcerative colitis | Anti-CD40 colitis model | ILC3s secrete higher amounts of GM-CSF which in turn recruits pathogenic Ly6C+ inflammatory monocytes, increasing inflammation and tissue damage | [86] |
| ILC3 | Crohn’s disease | Human | Inflamed tissue from patients with CD showed accumulation of IL-23-responive ILCs and increase expression of IL-17 | [91] |
| ILC3 | Colorectal cancer | C57BL/6 mice | Absence of IL-23 promotes tumor development accompanied by increased innate immune cell infiltration; tumorigenesis induced by IL23 could not be initiated in RAG2-/-IL-2R-/- double knockout mice; IL-23R expression was identified in gut associated lymphoid tissue | [49] |
| [93] |
Following pathogen invasion and tissue damage, epithelial cells and innate immune cells produce cytokines and alarmins which cooperatively mobilize and activate ILCs subsets[6]. Studies have shown that ILC-derived cytokines have an important protective function against S. Typhimurium[57], C. rodentium[54,58], N. brasiliensis[32,48], and C. albicans[59] infections.
IFNγ-producing ILC1s contribute to protection against Salmonella enterica subsp. enterica serovar Typhimurium infection in the colon. In addition, ILC3-derived IL-22 is required for the fucosylation of the intestinal epithelium which helps to protect against S. Typhimurium infection. Once bound to the receptor, IL-22 triggers a signaling cascade which induces fucosylation of epithelial cells, activation of the transcription factor STAT3 and consequently secretion of antimicrobial peptides[57,60]. Murine ILC1s are also important to immune function in response to T. gondii[9]. An increase of human ILC1s was shown in patients with chronic hepatitis B infection[61], indicating that this population can contribute to immunity in response to specific pathogens in both mice and humans.
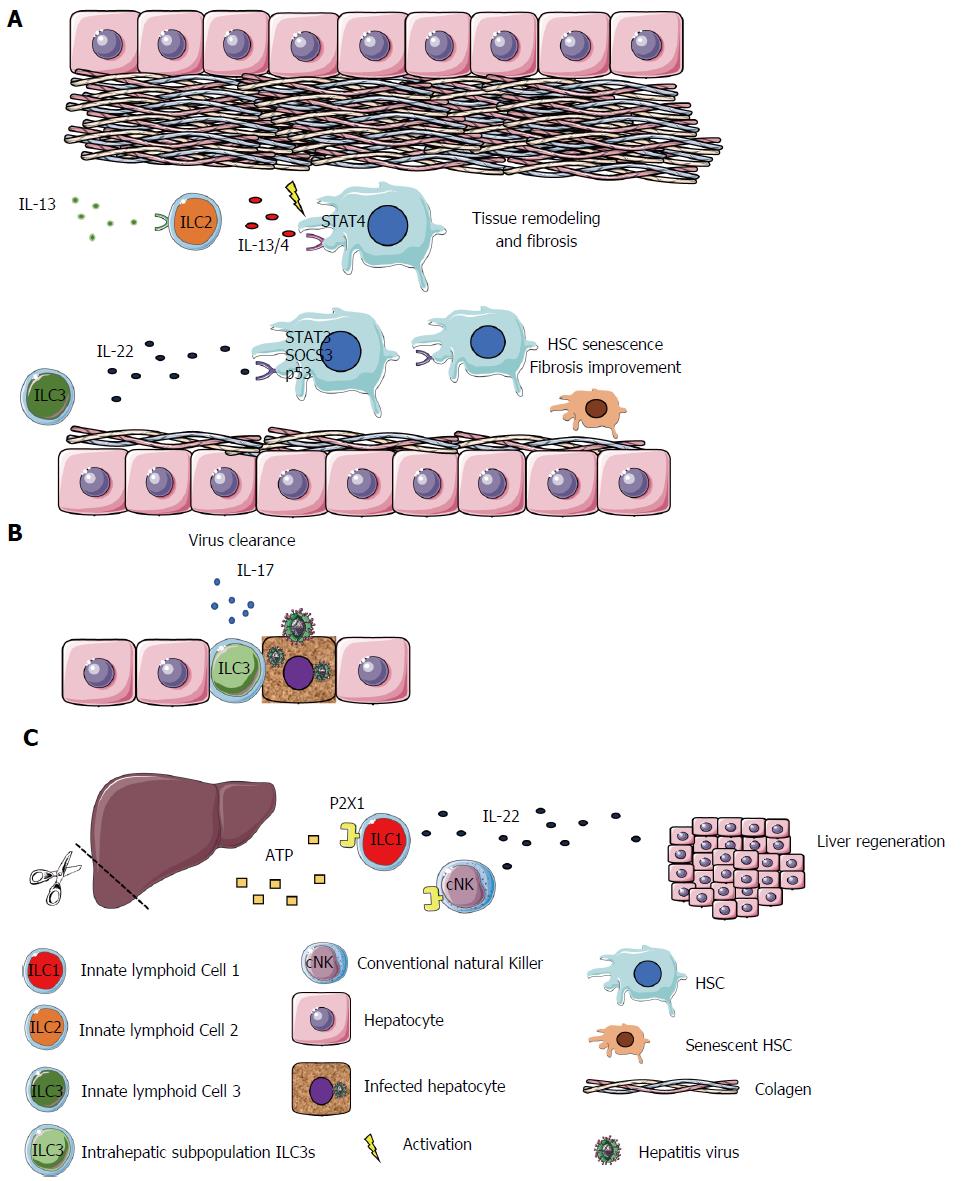
Before the onset of adaptive immune responses, the innate immune response to the enteric pathogen Citrobacter rodentium is critically dependent on ILC3-derived IL-22. C. rodentium is a gram-negative bacterium which causes acute colitis in mice. As mentioned above, the expression of antimicrobial peptides, dependent on the STAT-3 pathway, is induced by IL-22 and contributes to maintenance of the epithelial barrier surface. In addition, mice deficient in IL-22 rapidly succumb to infection due to exacerbated intestinal inflammation, bacterial invasion and proliferation throughout the tissues[60]. IL-23 production by DCs or CX3CR1+ mononuclear phagocytes is necessary for ILC3 activation and it has been shown that ILC3s are the predominant source of IL-22 in the first week of C. rodentium infection[60]. Satpathy et al[62] showed that il23a-/- mice are more susceptible to infection with high concentrations of this bacterium than wild type mice. As IL-23 was found to be crucial for IL-22 production by ILC3s, but not by Th17 cells, this model suggests that ILC3s are essential for resistance to C. rodentium infection. To clarify this phenomenon, experiments with Rag-/-il2rg-/- mice, which lack T cells and ILCs, showed that ILC-deficient mice are more susceptible to infection when compared to Rag-/- mice[54,58]. However, at a later infection stage, it was observed that T cell-derived IL-22 contributes substantially to C. rodentium clearance and tissue repair[63]. Therefore, whether ILC3s and T cells can perform redundant functions cannot be ruled out. In addition to IL-22 and IL-33, IL-17 was also described to play an important role during viral hepatitis. The intrahepatic subpopulation of ILC3s can induce IL-17 signaling to induce T cell responses in viral hepatitis, improving the clearance of the virus[64].
In a similar fashion, in the response against bacterial pathogens, ILC3s seem to be the primary source of IL-22 during Candida albicans infection. C. albicans is a commensal fungus found on mucosal and skin surfaces, but can also cause infection in children younger than 1 mo, elderly and in immuno compromised individuals. A recent study showed that IL-22 acts as the first-line of defense during candidiasis by controlling fungal overgrowth and epithelial integrity. In the second stage, the Th1 response is crucial to prevent fungal dissemination[65].
Interactions between the epithelium and ILC2s mediate immunity to helminth parasites. The type 2 immune response is characterized by the production of IL-4, IL-5, IL-9 and IL-13 cytokines. The immunity to the mouse nematode Nippostrongylus brasiliensis is IL-13-dependent, as this cytokine upregulates macrophage activation, development of goblet cells and smooth muscle contraction that together will induce parasite expulsion[7]. Even though CD4+ T cells are central in a type 2 immune response, it was shown that T cells are not the major producing cells of IL-13 for the expulsion of N. brasiliensis. CD4+ T cells from wild type mice were unable to induce worm expulsion when transferred into IL-4- and IL-13-deficient Rag2-/- mice[66]. The secretion of IL-13 by ILC2s was demonstrated by adoptive transfer of IL-13-/- ILC2s, which were not able to promote worm elimination. Moreover, transferring of wild-type ILC2s into mice deficient in IL-13 supported the data whereby IL-13 secretion from ILC2s is enough for worm expulsion[33].
Although the trigger of the signals is not well understood, these recent studies suggest a crosstalk between epithelial cells and ILCs driving appropriate ILC response. In attempting to explain the mechanisms by which ILCs interact with the non-hematopoietic and other hematopoietic cells, the employment of genetic and imaging tools are necessary to clarify it.
ILCs also promote the maintenance of tissue integrity by contributing to tissue remodeling and healing of tissue injury. During embryonic development, a subset of ILC3s known as LTi, promote the formation of secondary lymphoid organs such as Payer’s Patches in the gut. LTi cells induce the production of chemokines CXCL13, CCL21 and CCL19 by stromal cells and the upregulation of adhesion molecules (VCAM1, MadCam1 and ICAM1) that attract and bind leukocytes to constitute lymphoid structures[67]. ILC3s have also been implicated in repair of lymphoid tissue after damage as a result of graft-vs-host disease, acute viral infection[7], irradiation or treatment with methotrexate. The intestinal epithelial cell regeneration can also be launched through ILC3-derived IL-22 which mediates the regeneration of the cells by acting on intestinal stem cells that express the IL-22 receptor[60]. In addition, IL-22 can be produced in the liver, acting on hepatocytes and hepatic stellate cells (HSCs) exhibiting hepatoprotective properties by diminishing liver fibrosis and improving acute liver injury[68-71]. Kong et al[72] identified high levels of IL-22R1 expression on HSCs. This cell type is the most involved during liver fibrogenesis. They demonstrated that IL-22, via STAT3, SOCS3 and p53 activation, has an antifibrotic effect by inducing the senescence of HSCs, ameliorating liver fibrosis[72]. Matsumoto et al[73] reported that the IL-22-producing ILC3s play a protective role in a murine acute hepatitis model, by potentially blocking the hepatocyte cell death.
Corroborating the IL-22 protective role, Kudira et al[51] recently demonstrated that IL-22 contributes to liver regeneration in a partial hepatectomy (PH) model. However, they demonstrated that the source of IL-22, in this model, is ILC1s and cNK. They showed that IL-22 is essential for liver regeneration, and its production depends on the extracellular adenosine triphosphate (ATP) via P2X1 receptor[51].
The fact that dying and damaged epithelial cells discharge alarmins which can be sensed by ILC2s suggests a close interaction between these two cells types. In fact, ILC2s expressing amphiregulin can regulate cell differentiation and proliferation by binding to the epidermal growth factor receptor (EGFR). IL-33-stimulated ILC2s can induce the repair of intestinal epithelial lesions after DSS-induced colitis by amphiregulin secretion[74]. This cytokine, IL-33, as well as ILC2s, have been in the spotlight due to their contributions to the improvement of obesity-induced insulin resistance. IL-33 can bind the ST2 receptor and induce the production of large amounts of anti-inflammatory cytokines by ILC2s in adipose tissue. These cytokines lead to polarization of the adipose tissue macrophages to an M2 phenotype[75,76]. In the liver, unlike in DSS-induced colitis and adipose tissue, IL-33 was identified as a key mediator of hepatic fibrosis. It is released in response to chronic hepatocellular stress and, after binding to ST2, culminates in ILC2s activation, as mentioned above. These cells produce anti-inflammatory and tissue remolding cytokines, such as IL-13 and IL-4. In turn, IL-13 can activate HSCs in an IL-4Ra- and STAT6 transcription-factor-dependent fashion, a pro-fibrotic cascade. Accordingly, IL-33 plays a role in a profibrotic cascade as the apex of the signaling pathway[77]. Another study showed that HBV infected patients have higher concentrations of IL-33 in serum compared to healthy controls. In addition, that concentration decreases following 12 wk of treatment[78]. These findings indicate that, in certain conditions, ILC2s can be manipulated, avoiding excessive tissue remodeling, when IL-33-stimulated ILC2s secrete IL-13 and IL-4, inducing fibrosis mediated by liver stellate cells (Figure 2)[79].
Besides their tissue repair properties, ILC2s play a role in limiting exacerbation of inflammatory responses. This can occur through the production of type 2 cytokines, that can suppress type 1 and type 17 inflammation[3], showing the diverse roles that ILCs can play.
Complementary to their role in promoting immunity against pathogens, ILCs are also evolved with tolerance mechanisms regarding interactions between the host and the commensal microbiota. Recent studies have begun to disclose how ILC3s interact with gut bacteria, diet-derived factors and various cell types to maintain intestinal homeostasis.
Although the organization of ILC subsets in the gut-associated lymphoid tissues and murine intestinal tissues occur independently of microbiota colonization, the anatomical retention of lymphoid tissue resident bacteria seems to be related with ILC3s function[80]. For example, B cells can be activated by ILC3s through lymphotoxin α1β2 which induces the proliferation and the production of immunoglobulin A (IgA), that subsequently contributes to neutralization of commensal bacteria in the lumen and prevents an inappropriate immune response[81]. Furthermore, it was shown that ILC3s express MHC class II and although they do not express co-stimulatory molecules necessary to activate T-cells, depletion of ILC3s or selective deletion of MHC class II in these cells is associated with exacerbated bacteria-specific Th17 cell response and intestinal inflammation[82]. These data suggest that ILC3s can drive a host immune tolerogenic state in the intestine by controlling the functions of other immune cell types.
Controversial results have been found regarding the influence of microbiota on ILC3 function. The production of protective levels of IL-17A, IL-17F and IL-22 and the responsiveness to IL-23 suggest that both human and mice ILC3s contribute to intestinal homeostasis[14]. Despite some studies that have shown that murine ILC IL-22 production is not affected after alteration of bacterial communities[80], other works show that microbial products influence the level of IL-22 secretion in mice and humans[7,14], and that germ-free mice have decreased IL-22-expressing ILC3s[54]. In addition, epithelial cells stimulated by commensal microbiota release IL-25 which acts on CD11c+ cells to limit IL-22 secretion derived from ILC3s[83]. Future work will be needed to elucidate the mechanisms by which this interaction occurs and how this process is regulated. In attempt to explain how ILC3s communicate with environmental factors in the intestine, recent studies have focused on whether dietary substances can be sensed by ILC3s. Fucose can be used as a carbohydrate source by commensal bacteria. ILC3s facilitate the transfer of fucose to the surface of intestinal epithelial cells which is critical for resistance to infection with Salmonella Thyphimurium and to maintain the appropriate number of bacteria in the lumen[57]. Another example is the relationship between the level of vitamin A and ILC3 functions, whereby vitamin A deficiency was related with impaired ILC3 responses[84], suggesting that these cells sense signals from host-derived nutrients and directly from the microbiota.
Besides their function in promoting tissue homeostasis, the chronic activation of ILCs can also induce inflammation at mucosal surfaces. IL-23 is a powerful activator of ILC3s and this axis is intimately linked to inflammatory bowel disease (IBD). Infection-induced and sterile inflammation models of colitis such as S. typhimurium, Helicobacter hepaticus, Helicobacter typhlonius infectious models or anti-CD40 models have been used to better understand the ILC3 functions, which are thought to be related to stimulation by IL-23 or IL-12 and consequently release of IL-17, GM-CSF and IFN-γ[85-87]. IL-17-producing ILC3s have been shown to play a key role in T-cell independent mouse models and, in this context, CD127 blockade seems to reduce ILC3 numbers and ameliorates disease. It is believed that activation of dendritic cells leads to TNF and IL-23 release which in turn results in an expansion of IL-17 producing ILCs[88]. In contrast, ILC3-derived IL-22 protects mice from intestinal inflammation trigger by C. rodentium infections, DSS-induced colitis and the transfer of T cells[55,86,89]. In some murine colitis models, the blockade of intra-epithelial ILC1s and IFNγ-producing ILC3 ameliorates the inflammation on the mucosal layer[6,90]. Conversely, although a consistent role for ILCs in human IBDs continues tobe discussed, several studies have reported varying numbers of these cells in intestinal samples. Patients with Crohn’s disease presented an increase in ILC1 populations accompanied by decreased levels of IL-22-producing ILC3s in inflamed intestinal tissues[29,30]. In addition, in pediatric patients with Crohn’s disease, a lower expression of MHC class II on ILC3s was observed than in control subjects without IBD; a reduction of MHC class II was correlated with increased numbers of Th17 cells[91]. Together, these data suggest that ILC1s and ILC3s might participate in the establishment and the development of inflammation, and ILC3s might reduce pathogenic T cells trough MHC class II interactions.
Evidence regarding the function of ILCs in tumorigenesis are emerging from studies investigating the pro-carcinogenic role of cytokines and chronic inflammation. Human colorectal cancer (CRC) samples showed increased expression of IL-23 receptor, and the induced expression of IL-23 in mice led to the development of adenomatous tumors originating in the duodenum. Although the contribution of adaptive cells remains unclear, this model would indicate a potential role for ILC3s[92,93]. Moreover, IL-22-producing ILC3s might also be related in human CRC because uncontrolled IL-22 production facilitates tumor-infiltrating lymphocytes and IL-22 levels in the tumor were significantly higher than in non-tumor sections from the same patients[94].
The liver and intestine are complex organs that have multiple interactions with the microbiota, nutrients, metabolites and diverse types of cell to maintain the host homeostasis. The importance of the ILC family in the immunity panel is growing fast. Many studies have been done to elucidate the specific ILCs function in different sites triggering immunity, tissue repair and inflammation. However, the molecular mechanism by which ILC subsets play specific roles and their consequences for the host homeostasis remain unclear. Future studies focusing on how ILC responses are regulated and how they integrate the immune cells in different organs might provide therapeutic potential in the treatment of diverse diseases.
We gratefully acknowledge MSc Christina Adams, from Division of Translational Medicine, “Hospital For Sick Children - Peter Gilgan Centre for Research and Learning”, Toronto, ON, Canada and Tatiana Takiishi, from Immunology Department of Institute of Biomedical Science, University of São Paulo, Brazil for the manuscript editing.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chuang WL, De Ponti F, Shen T S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Cella M, Miller H, Song C. Beyond NK cells: the expanding universe of innate lymphoid cells. Front Immunol. 2014;5:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 541] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 3. | Tait Wojno ED, Artis D. Emerging concepts and future challenges in innate lymphoid cell biology. J Exp Med. 2016;213:2229-2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 5. | Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1678] [Cited by in RCA: 1864] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 6. | Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 661] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 7. | Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1241] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 8. | Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, Hu G, Barron L, Sharma S, Nakayama T. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity. 2014;40:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 310] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 9. | Klose CS, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 877] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 10. | Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 563] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 11. | Ignacio A, Morales CI, Câmara NO, Almeida RR. Innate Sensing of the Gut Microbiota: Modulation of Inflammatory and Autoimmune Diseases. Front Immunol. 2016;7:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Simoni Y, Fehlings M, Kløverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang CL. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2017;46:148-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 13. | Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166:1231-1246.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 448] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 14. | Eberl G. Development and evolution of RORγt+ cells in a microbe’s world. Immunol Rev. 2012;245:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Yokoyama WM. Natural killer cell immune responses. Immunol Res. 2005;32:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1343] [Cited by in RCA: 1269] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 17. | Chijioke O, Münz C. Dendritic cell derived cytokines in human natural killer cell differentiation and activation. Front Immunol. 2013;4:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Vosshenrich CA, Di Santo JP. Developmental programming of natural killer and innate lymphoid cells. Curr Opin Immunol. 2013;25:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med. 2012;209:729-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, Golub R. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat Immunol. 2011;12:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 21. | Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 590] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 23. | Jiao Y, Huntington ND, Belz GT, Seillet C. Type 1 Innate Lymphoid Cell Biology: Lessons Learnt from Natural Killer Cells. Front Immunol. 2016;7:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 454] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 25. | Zook EC, Kee BL. Development of innate lymphoid cells. Nat Immunol. 2016;17:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 26. | Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 27. | Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 543] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 28. | Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 596] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 29. | Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 819] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 30. | Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 762] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 31. | Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity. 2010;33:736-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 581] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 32. | Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1573] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 33. | Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1765] [Cited by in RCA: 1695] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 34. | Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489-11494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 928] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 35. | Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951-2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 329] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 37. | Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 633] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 38. | Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 39. | Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939-2950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 646] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 40. | Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol. 2014;14:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 944] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 42. | Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1102] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 43. | Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 44. | Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 45. | Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, Nakanishi K, Yamanishi K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA. 2013;110:13921-13926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 46. | Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 47. | Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 351] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 48. | Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 697] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 49. | Montaldo E, Juelke K, Romagnani C. Group 3 innate lymphoid cells (ILC3s): Origin, differentiation, and plasticity in humans and mice. Eur J Immunol. 2015;45:2171-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 50. | Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 577] [Cited by in RCA: 603] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 51. | Kudira R, Malinka T, Kohler A, Dosch M, de Agüero MG, Melin N, Haegele S, Starlinger P, Maharjan N, Saxena S. P2X1-regulated IL-22 secretion by innate lymphoid cells is required for efficient liver regeneration. Hepatology. 2016;63:2004-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1031] [Cited by in RCA: 1051] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 53. | Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 549] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 54. | Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 915] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 55. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 687] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 56. | Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 723] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 57. | Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 58. | Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 484] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 59. | Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 60. | Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1119] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 61. | Yang Z, Tang T, Wei X, Yang S, Tian Z. Type 1 innate lymphoid cells contribute to the pathogenesis of chronic hepatitis B. Innate Immun. 2015;21:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Satpathy AT, Briseño CG, Lee JS, Ng D, Manieri NA, Kc W, Wu X, Thomas SR, Lee WL, Turkoz M. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 63. | Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 370] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 64. | Jie Z, Liang Y, Hou L, Dong C, Iwakura Y, Soong L, Cong Y, Sun J. Intrahepatic innate lymphoid cells secrete IL-17A and IL-17F that are crucial for T cell priming in viral infection. J Immunol. 2014;192:3289-3300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 66. | Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 67. | van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Höpken UE, Lipp M, Niederreither K. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 68. | Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 822] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 69. | Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 70. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 503] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 71. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 72. | Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 73. | Matsumoto A, Kanai T, Mikami Y, Chu PS, Nakamoto N, Ebinuma H, Saito H, Sato T, Yagita H, Hibi T. IL-22-producing RORγt-dependent innate lymphoid cells play a novel protective role in murine acute hepatitis. PLoS One. 2013;8:e62853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci USA. 2015;112:10762-10767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 75. | Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, Levings MK. IL-33 Reverses an Obesity-Induced Deficit in Visceral Adipose Tissue ST2+ T Regulatory Cells and Ameliorates Adipose Tissue Inflammation and Insulin Resistance. J Immunol. 2015;194:4777-4783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 76. | Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol. 2015;6:637. [PubMed] |
| 77. | McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 423] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 78. | Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu J, Jiang Y. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res. 2012;32:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 725] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 80. | Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 81. | Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, Eberl G, Littman DR, Heikenwalder M, Tumanov AV. Nonredundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342:1243-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 82. | Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, Mantegazza AR, Ma HL, Crawford A, Angelosanto JM. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 618] [Cited by in RCA: 617] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 83. | Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 499] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 84. | Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Wang J, Ramalingam TR. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 85. | Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 924] [Cited by in RCA: 916] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 86. | Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, Mack M, Cella M, Colonna M. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212:1869-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 87. | Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver Y. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 2013;494:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 589] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 88. | Powell N, Walker AW, Stolarczyk E, Canavan JB, Gökmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Jenner RG. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 89. | Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211:1571-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 90. | Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014;124:700-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 281] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 91. | Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 522] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 92. | Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 773] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 93. | Chan IH, Jain R, Tessmer MS, Gorman D, Mangadu R, Sathe M, Vives F, Moon C, Penaflor E, Turner S. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 2014;7:842-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 94. | Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O, Powrie F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (0)] |