Published online Aug 8, 2017. doi: 10.4254/wjh.v9.i22.973
Peer-review started: February 28, 2017
First decision: April 18, 2017
Revised: April 29, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: August 8, 2017
Processing time: 160 Days and 21 Hours
Identification of extrahepatic metastases (EHM) of hepatocellular carcinoma (HCC) has been paradoxically increasing due to an increase in the survival of HCC patients. However, metastasis of HCC to the skeletal muscle tissue is extremely rare. We describe a unique case of HCC metastasizing to the paravertebral muscle. A 55-year-old man with a history of hepatitis B cirrhosis underwent partial liver resection with complete removal of HCC. Three months later, a computed tomography (CT) scan showed intrahepatic recurrence. The tumors were treated with yttrium-90 microspheres, trans-catheter arterial chemoembolization, and sorafenib. Six months later, a CT scan showed an enhancing lesion of the left paravertebral muscle that on biopsy were consistent with metastatic HCC. The tumor was treated with stereotactic hypo-fractionated image-guided radiation therapy (SHFRT). A follow-up scan 3 mo post-radiotherapy revealed a stable appearance of the paravertebral muscle metastasis. Because of the progression in the intrahepatic tumors, the patient was treated with capecitabine, which was changed to dasatinib 6 mo later. The patient passed away three years after the primary surgical resection. Management of EHM poses an extreme challenge. This is the first case of HCC with EHM to the paravertebral muscle in which stability of disease was achieved using SHFRT. This case highlights the importance of early detection of hepatitis B viral infection and initiation of anti-viral therapy to decrease recurrence of HCC and prevent EHM.
Core tip: Extrahepatic metastases (EHM) of hepatocellular carcinoma (HCC) to skeletal muscle are extremely rare. We describe the first case of HCC with EHM to the paravertebral muscle, in which stability of disease was achieved using stereotactic hypo-fractionated image-guided radiation therapy. A literature review revealed the strong relationship between hepatitis B viral infection and EHM. This case highlights the importance of early detection of viral infection and initiation of anti-viral therapy to decrease recurrence of HCC and prevent EHM.
- Citation: Takahashi K, Putchakayala KG, Safwan M, Kim DY. Extrahepatic metastasis of hepatocellular carcinoma to the paravertebral muscle: A case report. World J Hepatol 2017; 9(22): 973-978
- URL: https://www.wjgnet.com/1948-5182/full/v9/i22/973.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i22.973
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third most common cause of cancer-related death in the world[1-3]. World-wide incidence is between 250000 and 1000000 new cases per year, and it has been rapidly increasing due to the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections[1-3]. In the United States, HCC related to HCV infection has become the fastest rising cause of cancer-related death, and the incidence has tripled during the past two decades. Survival time in patients with HCC has recently increased as a consequence of advanced diagnostic modalities and treatment methods; however, the 5-year survival rate still remains low at approximately 16%[1,3,4]. Current available treatment methods include surgical resection, radio-frequency ablation, trans-catheter arterial chemoembolization (TACE), yttrium-90 microspheres, liver transplantation, chemotherapy, and radiotherapy[5].
Because of the improvement in survival, extra-hepatic metastases (EHM) are becoming more commonly recognized in patients with HCC, with a reported incidence of 15%-17%[6,7]. The most common sites of EHM are lungs, lymph nodes, bones, and adrenal glands; however, HCC can metastasize to the skeletal muscles and subcutaneous tissues, albeit rarely[7]. In this report, we describe a unique case of HCC metastasizing to the paravertebral muscle, which was treated with stereotactic hypo-fractionated image guided radiation therapy (SHFRT) and achieved disease stability. We report this case along with a review of the recent literature.
A 55-year-old male with a history of HBV-associated liver cirrhosis had an incidental right lobe liver mass 6.0 cm in size identified during a routine computed tomography (CT) scan. His serum alpha-fetoprotein (AFP) level was within the normal range. A magnetic resonance imaging (MRI) scan showed a hyper-intense irregular T2 focus, which distorted the contours of the liver. This focus demonstrated moderate enhancement on the initial phase post-Gadolinium images, with a central hypo-intense area. These imaging characteristics were most compatible with focal nodular hyperplasia, and follow-up at the outpatient clinic was advised. However, the patient was non-compliant and did not visit the clinic until three years later. MRI scan at that time showed that the tumor had increased in size to 9.4 cm, and the patient had a mild elevation in AFP level (15.1 ng/mL). His HBV DNA level was 12.7 × 106 copies/mL and he had not received any anti-viral therapies. The patient then underwent partial liver resection with complete removal of the tumor. Histopathological examination revealed the tumor to be a moderate-to-poorly differentiated HCC with vascular invasion. According to the Union for International Cancer Control guidelines, the final stage of the tumor was stage II (pT2N0M0). Due to the elevated viral titer, entecavir 1 mg daily was instituted postoperatively.
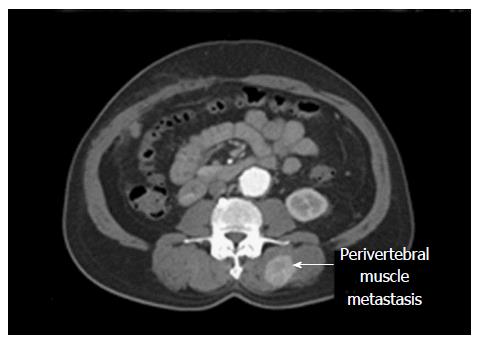
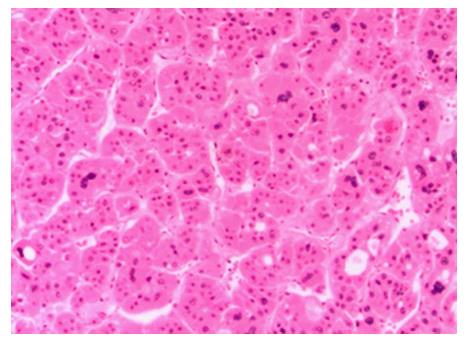
Three months later, CT scan showed recurrence of the tumor as three foci: 4 mm in size along the resected plane, 7 mm at S4, and 6 mm at S7. The patient’s HBV DNA level was less than 300 copies/mL. The tumors were treated with yttrium-90 microspheres (TheraSphere®, BTG IM, London, United Kingdom). A total dose of 90 Gy was delivered. One year later, he developed multiple enhancing lesions in the liver. He received three sets of TACE with adriamycin, and finally sorafenib (Nexavar®, Bayer HealthCare AG, Leverkusen, Germany) 200 mg twice daily. Six months later, he complained of back pain, and CT scan showed an enhancing lesion 3.7 cm in size in the left paravertebral muscle (Figure 1). A biopsy of the mass showed moderate-to-poorly differentiated HCC, consistent with metastatic HCC (Figure 2). The tumor was treated with four rounds of SHFRT at 10 Gy per fraction with a total dose of 40 Gy. A follow-up scan at 3 mo post-radiotherapy revealed a stable appearance of the paravertebral muscle metastasis. Because of progression of the intrahepatic tumors, the patient was switched to capecitabine (Xeloda®, Roche, Basel, Switzerland) 1500 mg twice daily once a week for 2 wk. He was later enrolled in a clinical trial and started on dasatinib. The patient passed away more than three years after the primary liver resection.
Despite significant advances in the treatment of HCC, the prognosis remains poor. Median survival times for patients with HCC who have EHM are 4.9-7.0 mo. One, three, and five year survival rates are 21.7%-31.0%, 7.0%-7.1%, and 4.0%, respectively[8]. Currently, there is no standardized treatment for HCC patients with EHM. Sorafenib is the first systemic agent that has demonstrated a significant improvement in survival time in patients with advanced HCC; however, the modest improvement of 3 mo is far from satisfactory[9]. Systemic cytotoxic chemotherapy agents, such as adriamycin, fluorouracil, cisplatin, etc. are considered palliative treatment options for advanced HCC but have low response rates of less than 10%. Recently, there have been some reports on the efficacy of capecitabine as a second-line treatment following sorafenib[10,11]. However, these studies are retrospective in nature with low levels of evidence. Other target agents such as regorafenib, c-Met inhibitor, and check point inhibitors are promising, but still under investigation. Dasatinib, an Src family kinase inhibitor, is reported to have effects on human HCC cell lines[12,13], however, the results of a recent clinical study showed insufficient response rates[14]. Due to lack of highly effective systemic chemotherapy for HCC, enrolling in a clinical trial with a new chemotherapeutic agent is the only option for patients with advanced HCC[15,16].
Several authors have reported long-term survivors after aggressive surgery for EHM[17,18]. From the viewpoint of reducing tumor burden, loco-regional therapy may be a reasonable strategy when the target lesions account for a major portion of the total tumor volume. These reports suggest a potential benefit to loco-regional treatment for intra and/or extrahepatic tumor in HCC patients with EHM. Patients with T1/2 primary tumor or less than two EHM were described as good candidates for aggressive local therapy[19,20]. A retrospective analysis reported that surgical resection of peritoneal or thoraco-abdominal wall implants from HCC in selected patients (limited number of implanted lesions; intrahepatic lesions absent or predicted locally controllable; and the absence of ascites with sufficient hepatic functional reserve) improved long-term survival, with 1, 3 and 5 year overall survival rate of 71%, 44% and 39%, respectively[21]. On the other hand, the cause of death in HCC patients with extrahepatic metastasis were mostly related to problems as a consequence of intrahepatic tumors, such as liver failure[8,18]. In our case, SHFRT was selected for local treatment of EHM, in addition to sorafenib as a systemic treatment, since the tumor invaded deeply into the paravertebral muscle and multiple intrahepatic recurrent HCC foci were identified, suggesting a poor prognosis even after the resection. Although the primary purpose for this radiation was for pain control, it was also effective in the control of disease progression. Our case is the first report of EHM treated by a non-surgical method which led to extrahepatic disease stability.
Vascular invasion of HCC has proven to be a strong determinant of EHM. Hematogenous spread to the lungs, lymph nodes, bones, and adrenals are reported to be the most common sites for EHM. Metastasis of HCC to muscle tissue is an infrequent phenomenon. Skeletal muscle and cardiac muscle are classified as striated muscles, which contain sarcomeres that are arranged into highly organized bundles. The infrequency of muscle metastasis seen in HCC may be attributed to the contractility of muscle, the local pH environment, and the presence of tumor suppressors in the muscle tissue[22]. Over 40 cases of cardiac muscle metastasis of HCC have been reported, whereas only found 17 cases of skeletal muscle metastasis of HCC have been reported (Table 1)[17,23-37]. All these cases were reported after 2005, two years before sorafenib was approved by the Food and Drug Administration for the treatment of HCC. Skeletal muscle recurrence occurred in various locations throughout the body, the trunk, and the peripheral musculature, with one case of extraocular muscle metastasis[28]. The majority of patients were male (16/18 cases) and had a history of HBV infection (10/13 cases, excluding 5 cases with unknown etiology). HBV viral load and anti-viral treatment were not recorded except in our case. Most cases underwent surgical resection as a local treatment (9/17 cases, excluding one case with unknown treatment), and some received radiation therapy as palliative therapy (three cases). In cases with simultaneous recurrence similar to ours, sorafenib or another chemotherapeutic agent was used as systemic therapy[17,23,25,27,32]. However, even with these treatments, prognosis was extremely poor, ranging from a few weeks to 6 mo.
| Ref. | Year | Age/gender | Background | Treatment (primary lesion) | Muscle recurrence site | Recurrence time (mo)1 | Treatment (metastasis) | Other lesions2 | Simultaneous systemic treatment |
| This case | 2017 | 55/M | HBV | Resection | Paravertebral muscle | 21 | SHFRT | Multiple intrahepatic HCC | Sorafenib |
| [23] | 2014 | 36/M | Unknown | Chemo- radiotherapy | Chest wall | 0 | Chemo- radiotherapy | Liver, peripancreatic region, brain, cervical lymph node | Chemo- radiotherapy |
| [23] | 2014 | 31/M | HBV HIV | Cisplatin/adriamycin | Chest wall, pectoral muscles | 0 | Cisplatin/adriamycin | Intrahepatic HCC | Cisplatin/adriamycin |
| [24] | 2014 | 47/M | Unknown | Resection | Rectus muscle | 13 | Resection | None | None |
| [17] | 2013 | 55/M | HBV HCV | Resection | Pectoralis major Deltoid, left teres minor | 54 | Radiotherapy | Brain metastasis | Sorafenib |
| [25] | 2013 | 61/M | Alcohol | None | Iliac muscle | 0 | Chemotherapy | Diffuse intrahepatic HCC | Chemotherapy |
| [26] | 2012 | 65/M | HBV | RFA TACE | Intercostal muscle | 24 | Resection | None | None |
| [27] | 2012 | 72/M | Alcohol | None | Medial pterygoid muscle | 0 | Radiotherapy | Multiple intrahepatic HCC | Sorafenib |
| [28] | 2012 | 44/M | Unknown | Resection | Extraocular muscle | 17 | Radiotherapy | None | None |
| [29] | 2011 | 70/M | HBV | Resection | Humorous muscle | 108 | Resection | None | Unknown |
| [30] | 2009 | 82/M | Unknown | Resection | Diaphragm | 30 | Resection | None | None |
| [31] | 2009 | 62/unknown | HBV | TACE resection | Pectineal muscle | 96 | Unknown | Multiple intrahepatic HCC | Unknown |
| [32] | 2008 | 54/M | HBV | Resection | Rectus femoris muscle | 60 | Sorafenib | Multiple pulmonary metastasis | Sorafenib |
| [33] | 2008 | 52/M | HBV | Liver transplant | Chest wall | 60 | Resection | None | None |
| [34] | 2007 | 63/M | Unknown | Resection | Gastrocnemius muscle | 18 | Resection | None | None |
| [35] | 2007 | 53/M | HCV | None | Gluteus maximus muscle | 0 | Resection | None | None |
| [36] | 2006 | 50/M | HBV | Resection | Psoas muscle | 12 | Resection | None | None |
| [37] | 2005 | 39/F | HBV | Resection | Chest wall | 11 | Resection | None | None |
Previous studies have described the importance of controlling viral status to prevent HCC recurrence and improve survival after curative treatment for HBV-related HCC[38,39]. Huang et al[38] reported that preoperative antiviral treatment decreased viral reactivation rate, and pre- plus postoperative antiviral treatment achieved a better 5-year overall survival rate than postoperative antiviral treatment alone by decreasing HBV-related HCC recurrence. On the other hand, only one study described a correlation between HBV status and EHM. Sasaki et al[40] reported that HBV infection was an independent predictor for the occurrence of EHM in patients with large HCC tumors. In addition, the authors posit that HBV infection might promote the establishment of EHM through modulation of the adhesion-de-adhesion balance of HCC cells[40]. In our case, although the patient’s HBV status was well-controlled by entecavir after hepatectomy, the patient did not receive any anti-viral treatment preoperatively despite a high viral load. No previous case reports of muscle recurrence included patient HBV status or antiviral treatments. Although the relationship between HBV infection and skeletal muscle recurrence has not been clarified, we consider controlling HBV viral load through antiviral treatment prior to surgical intervention important due to the high incidence of HBV infection among patients with HCC with EHM recurrence.
We report the first case of HCC with EHM to the paravertebral muscle. Though this is a single case, it raises interest in detecting EHM at an earlier stage and initiating therapy if the patient’s overall health permits. A study of surgical and non-surgical treatment with systemic vs loco-regional therapy may shed further light on this topic.
We acknowledgements Transplant and Hepatobiliary Surgery Henry Ford Hospital, 2790 West Grand Boulevard, Detroit, MI 48202, United States.
A 55-year-old male with a history of hepatitis B virus (HBV) induced liver cirrhosis complained of back pain two years after removal of hepatocellular carcinoma (HCC).
Computed tomography (CT) scan showed a mass at the left paravertebral muscle, biopsy of which was consistent with moderate to poorly differentiated HCC.
Rhabdomyosarcoma, fibromatoses, hemangioma, or metastatic tumor of HCC.
A mild elevation of the alpha-fetoprotein level (15.1 ng/mL). HBV DNA counts of 12.7 × 106 copies/mL.
CT scan showed an enhancing lesion 3.7 cm in size at the left paravertebral muscle.
A biopsy of the mass showed moderate to poorly differentiated HCC, consistent with metastatic HCC.
The tumor was treated with four sessions of stereotactic hypo-fractionated image guided radiation therapy at 10 Gy per fraction with a total dose of 40 Gy.
There were only 17 cases of the skeletal muscle metastasis of HCC. These were at various locations from the skeletal muscles of body trunk to peripheral muscles.
Extrahepatic metastases (EHM) of HCC to the skeletal muscle tissue are extremely rare. Median survival times for patients with HCC who have EHM are 4.9-7.0 mo. Currently, there is no standardized treatment for HCC patients with EHM.
This case invites interest in detecting EHM at an earlier phase and the initiation of therapy if the patient’s health and overall assessment permits. A study of surgical and non-surgical treatment with systemic vs loco-regional therapy may shed further light on this situation.
The paper is well-written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lazar C, Silva LD, Squadrito G, Tarazov PG S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 2. | Brito AF, Abrantes AM, Tralhão JG, Botelho MF. Targeting Hepatocellular Carcinoma: What did we Discover so Far? Oncol Rev. 2016;10:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Wang M, Xi D, Ning Q. Virus-induced hepatocellular carcinoma with special emphasis on HBV. Hepatol Int. 2017;11:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Crocetti L, Bargellini I, Cioni R. Loco-regional treatment of HCC: current status. Clin Radiol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Gong XL, Qin SK. Progress in systemic therapy of advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:6582-6594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 515] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 7. | Natsuizaka M, Omura T, Akaike T, Kuwata Y, Yamazaki K, Sato T, Karino Y, Toyota J, Suga T, Asaka M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 9. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 10. | Granito A, Marinelli S, Terzi E, Piscaglia F, Renzulli M, Venerandi L, Benevento F, Bolondi L. Metronomic capecitabine as second-line treatment in hepatocellular carcinoma after sorafenib failure. Dig Liver Dis. 2015;47:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Casadei Gardini A, Foca F, Scartozzi M, Silvestris N, Tamburini E, Faloppi L, Brunetti O, Rudnas B, Pisconti S, Valgiusti M. Metronomic capecitabine versus best supportive care as second-line treatment in hepatocellular carcinoma: a retrospective study. Sci Rep. 2017;7:42499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Xu L, Zhu Y, Shao J, Chen M, Yan H, Li G, Zhu Y, Xu Z, Yang B, Luo P. Dasatinib synergises with irinotecan to suppress hepatocellular carcinoma via inhibiting the protein synthesis of PLK1. Br J Cancer. 2017;116:1027-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Chang AY, Wang M. Molecular mechanisms of action and potential biomarkers of growth inhibition of dasatinib (BMS-354825) on hepatocellular carcinoma cells. BMC Cancer. 2013;13:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Dasatinib in treating patients with advanced liver cancer that cannot be removed by surgery. 2015. |
| 15. | Woo HY, Yoo SY, Heo J. New chemical treatment options in second-line hepatocellular carcinoma: what to do when sorafenib fails? Expert Opin Pharmacother. 2017;18:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Connell LC, Harding JJ, Abou-Alfa GK. Advanced Hepatocellular Cancer: the Current State of Future Research. Curr Treat Options Oncol. 2016;17:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Jo S, Shim HK. A patient who has survived for a long period with repeated radiotherapies for multifocal extrahepatic metastases from hepatocellular carcinoma. Radiat Oncol J. 2013;31:267-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, Goto T, Omata M, Yoshida H, Koike K. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475-4483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 19. | Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Nakachi K, Yoshino M. Extrahepatic spread from hepatocellular carcinoma: who are candidates for aggressive anti-cancer treatment? Jpn J Clin Oncol. 2004;34:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Berger Y, Spivack JH, Heskel M, Aycart SN, Labow DM, Sarpel U. Extrahepatic metastasectomy for hepatocellular carcinoma: Predictors of long-term survival. J Surg Oncol. 2016;114:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Takemura N, Hasegawa K, Aoki T, Sakamoto Y, Sugawara Y, Makuuchi M, Kokudo N. Surgical resection of peritoneal or thoracoabdominal wall implants from hepatocellular carcinoma. Br J Surg. 2014;101:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Bar-Yehuda S, Barer F, Volfsson L, Fishman P. Resistance of muscle to tumor metastases: a role for a3 adenosine receptor agonists. Neoplasia. 2001;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Shah M, Chauhan K, Patel T, Gami A, Saha M, Dhariaya C. Hepatocellular carcinoma- manifesting as chest wall metastasis: Report of two cases. Guja Med J. 2014;69:107-108. |
| 24. | Traficante D, Assalone P, Tomei F, Calista F, Falleti J, Caranci E, Di Lullo L. A case report of HCC cutaneous metastasis. J Gastrointest Oncol. 2014;5:E65-E67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Subramaniam N, Hiremath B, Pujar A. Metastasis of diffuse hepatocellular carcinoma to an extremely unusual site. BMJ Case Rep. 2013;2013:pii: bcr2013200437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Furumoto K, Miura K, Nagashima D, Kojima H, Mori T, Ito D, Kajimura K, Kogire M. Solitary metastasis to the intercostal muscle from hepatocellular carcinoma: A case report. Int J Surg Case Rep. 2012;3:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Yu S, Estess A, Harris W, Dillon J. A rare occurrence of hepatocellular carcinoma metastasis to the mandible: report of a case and review of the literature. J Oral Maxillofac Surg. 2012;70:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Jiang H, Wang Z, Xian J, Ai L. Bilateral multiple extraocular muscle metastasis from hepatocellular carcinoma. Acta Radiol Short Rep. 2012;1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Michalaki V, Zygogianni A, Kouloulias V, Balafouta M, Vlachodimitropoulos D, Gennatas CG. Muscle metastasis from hepatocellular carcinoma. J Cancer Res Ther. 2011;7:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Sano T, Izuishi K, Takebayashi R, Kushida Y, Masaki T, Suzuki Y. Education and imaging. Hepatobiliary and pancreatic: isolated diaphragmatic metastasis from hepatocellular carcinoma. J Gastroenterol Hepatol. 2009;24:1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Sirigu D, Loi L, Mura R, Migaleddu V, Campisi G. Muscle metastasis from hepatocellular carcinoma in a patient treated with TACE. J Ultrasound. 2009;12:45-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Yau T, Wong H, Chan P, To M, Poon RT. Intramuscular recurrence in a hepatocellular carcinoma patient with indolent disease course. World J Surg Oncol. 2008;6:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Onen A, Sanli A, Karacam V, Karapolat S, Gokcen B, Acikel U. Chest-wall metastasis in a patient who underwent liver transplantation due to hepatocellular carcinoma. Heart Lung Circ. 2008;17:156-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Masannat YA, Achuthan R, Munot K, Merchant W, Meaney J, McMahon MJ, Horgan KJ. Solitary subcutaneous metastatic deposit from hepatocellular carcinoma. N Z Med J. 2007;120:U2837. [PubMed] |
| 35. | Young C, Munk P. Hepatocelllar carcinoma presenting as musculoskeletal metastases: a report of two cases. Euro J Rad Ext. 2007;62:25-29. |
| 36. | Wu MH, Wu YM, Lee PH. The psoas muscle as an unusual site for metastasis of hepatocellular carcinoma: report of a case. Surg Today. 2006;36:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Verhoef C, Holman FA, Hussain SM, de Man RA, de Wilt JH, IJzermans JN. Resection of extrahepatic hepatocellular carcinoma metastasis can result in long-term survival. Acta Chir Belg. 2005;105:533-536. [PubMed] |
| 38. | Huang S, Xia Y, Lei Z, Zou Q, Li J, Yang T, Wang K, Yan Z, Wan X, Shen F. Antiviral Therapy Inhibits Viral Reactivation and Improves Survival after Repeat Hepatectomy for Hepatitis B Virus-Related Recurrent Hepatocellular Carcinoma. J Am Coll Surg. 2017;224:283-293.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Kubo S, Takemura S, Tanaka S, Shinkawa H, Nishioka T, Nozawa A, Kinoshita M, Hamano G, Ito T, Urata Y. Management of hepatitis B virus infection during treatment for hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2015;21:8249-8255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Hepatitis B virus infection predicts extrahepatic metastasis after hepatic resection in patients with large hepatocellular carcinoma. Ann Surg Oncol. 2007;14:3181-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |