Published online Jul 18, 2017. doi: 10.4254/wjh.v9.i20.896
Peer-review started: February 14, 2017
First decision: April 17, 2017
Revised: May 8, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: July 18, 2017
Processing time: 159 Days and 4.4 Hours
To determine risk factors, causative organisms and antimicrobial resistance of bacterial infections following living-donor liver transplantation (LDLT) in cirrhotic patients.
This prospective study included 45 patients with hepatitis C virus-related end-stage liver disease who underwent LDLT at Ain Shams Center for Organ Transplant, Cairo, Egypt from January 2014 to November 2015. Patients were followed-up for the first 3 mo after LDLT for detection of bacterial infections. All patients were examined for the possible risk factors suggestive of acquiring infection pre-, intra- and post-operatively. Positive cultures based on clinical suspicion and patterns of antimicrobial resistance were identified.
Thirty-three patients (73.3%) suffered from bacterial infections; 21 of them had a single infection episode, and 12 had repeated infection episodes. Bile was the most common site for both single and repeated episodes of infection (28.6% and 27.8%, respectively). The most common isolated organisms were gram-negative bacteria. Acinetobacter baumannii was the most common organism isolated from both single and repeated infection episodes (19% and 33.3%, respectively), followed by Escherichia coli for repeated infections (11.1%), and Pseudomonas aeruginosa for single infections (19%). Levofloxacin showed high sensitivity against repeated infection episodes (P = 0.03). Klebsiella, Acinetobacter and Pseudomonas were multi-drug resistant (MDR). Pre-transplant hepatocellular carcinoma (HCC) and duration of drain insertion (in days) were independent risk factors for the occurrence of repeated infection episodes (P = 0.024).
MDR gram-negative bacterial infections are common post-LDLT. Pre-transplant HCC and duration of drain insertion were independent risk factors for the occurrence of repeated infection episodes.
Core tip: We evaluated 45 patients with hepatitis C virus-related end-stage liver disease for the occurrence of bacterial infections during the first 3 mo post-living-donor liver transplantation. Thirty-three patients (73.3%) suffered from bacterial infections; 21 of them had a single infection episode, and 12 had repeated infection episodes. Bile was the most common site for both single and repeated episodes of infection (28.6% and 27.8%, respectively). Multi-drug resistant gram-negative bacteria, especially Klebsiella, Acinetobacter and Pseudomonas, were the most commonly isolated bacteria. Pre-transplant hepatocellular carcinoma and duration of drain insertion were independent risk factors for occurrence of repeated infection episodes.
- Citation: Montasser MF, Abdelkader NA, Abdelhakam SM, Dabbous H, Montasser IF, Massoud YM, Abdelmoaty W, Saleh SA, Bahaa M, Said H, El-Meteini M. Bacterial infections post-living-donor liver transplantation in Egyptian hepatitis C virus-cirrhotic patients: A single-center study. World J Hepatol 2017; 9(20): 896-904
- URL: https://www.wjgnet.com/1948-5182/full/v9/i20/896.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i20.896
Infection following living-donor liver transplantation (LDLT) is a serious problem with a high mortality rate reaching 50%. Many factors were associated with high risks of acquiring infection following LDLT, including the difficulty of surgery, the poor patient’s condition, and the immunosuppressive drugs[1].
Nearly 80% of recipients develop one infection episode during the first year, predominantly during the first three months post-transplant. Bacterial infections account for 50%-75% of infections post-LDLT and commonly occur in the first month post-transplant[2].
Patients may become infected with antimicrobial-resistant bacteria, especially methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecalis, Clostridium difficile, and gram-negative bacteria[3]. Currently, multidrug-resistant (MDR) organisms are the most common cause of nosocomial infections in liver transplant recipients[1].
Multiple organism infection is common as well as concurrent infections caused by different infectious agents[4]. Infections are usually difficult to diagnose because the usual manifestations of infection, such as fever and leukocytosis, may be absent and because of the need to exclude an acute rejection episode[5].
The aim of the present study was to determine risk factors, causative organisms and antimicrobial resistance patterns of bacterial infections following LDLT in Egyptian cirrhotic patients.
Forty-five adult patients with hepatitis C virus (HCV)-related end-stage liver disease (ESLD) who were eligible for and underwent LDLT at Ain Shams Center for Organ Transplant, Cairo, Egypt, during the period from January 2014 to November 2015, were included in the current prospective study. They were followed-up for the first 3 mo post-LDLT for detection of bacterial infections.
Patients with other etiologies for ESLD (hepatitis B virus, primary biliary cirrhosis, and others) and patients with pre-operative infections, infections within 48 h after transplantation or early post-operative death were excluded.
Each patient provided an informed written consent prior to enrollment. The study protocol was accepted by the Research Ethical Committee of the Faculty of Medicine-Ain Shams University. This was in accordance to the ethical guidelines of the 1975 Declaration of Helsinki.
Immediately following liver transplantation (LT), we used triple-therapy of immunosuppressive drugs which was comprised of a steroid, a calcineurin inhibitor: Cyclosporine or tacrolimus, and mycophenolate mofetil. In patients with renal dysfunction, immunosuppression with monoclonal antibodies to T-cells was used. In patients with hepatocellular carcinoma (HCC), tacrolimus monotherapy was used to decrease the incidence of HCC recurrence.
Piperacillin/tazobactam 4.5 mg/d was used post-operatively for 5 d. A polymerase chain reaction (PCR) assay for cytomegalovirus (CMV) was done every two weeks until patient’s discharge. Ganciclovir for prevention of CMV disease was used if the PCR assay was positive.
All patients were checked for the following parameters: (1) pre-operatively: Demographic data, other co-morbidities, presence of HCC, any bridging techniques, Child and MELD scores, CBC with differential cell count, liver profile, C-reactive protein, serum ferritin, documented or suspected SBP and third generation cephalosporin administration, renal impairment, and positive cultures; (2) intra-operatively: Total operative period, cold and warm ischemia time, amount of transfused blood or blood products and type of biliary anastomosis; and (3) post-operatively: Intensive care unit stay, ventilator duration, duration of central venous line and catheter insertion, duration of abdominal drain placement, dialysis post-transplant and immunosuppressive drugs.
Post-operative infection was defined as any positive culture, based on clinical suspicion, within 3 mo following LDLT, according to the Centers for Disease Control and Prevention’s definition of a nosocomial infection and as described in liver transplant recipients[6,7]. The diagnosis of wound infection was established by the presence of redness/induration and the presence of pus on exploration and/or positive wound culture. The diagnosis of urinary tract infection was based upon the following criteria: The patient has at least one of the following symptoms or signs with no other identified cause: fever (> 38 °C), dysuria, frequency, urgency, suprapubic or costovertebral angle pain or tenderness, as well as a positive urine culture, that is, ≥ 105 CFU/mL of urine with no more than 2 species of microorganisms. The diagnosis of pneumonia was based upon the presence of pulmonary infiltrates together with clinical symptoms indicating lower respiratory tract infection, the identification of a relevant etiologic microorganism, and the absence of another possible diagnosis during the follow-up. Bloodstream infection was diagnosed when microorganisms were isolated from one blood culture. Ascitic fluid cultures were performed for all patients with manifestations of bacterial peritonitis or who were suspected of having bacterial peritonitis. Samples were collected before the start of any antimicrobial treatment. Bile samples were withdrawn for those suspected of having a biliary tract infection. In cases of suspected sepsis-induced cholestasis, cultures from blood, the biliary tube, abdominal drains, urine, and sputum were collected, and culture based-treatment was started accordingly.
The term multidrug-resistant (MDR) was used to refer to pathogens resistant to three or more classes of the following antibiotics: extended-spectrum penicillins, 3rd generation cephalosporins, quinolones, carbapenems, and aminoglycosides[8].
Recruited patients were divided into two groups. Group 1 included patients who had a single episode of post-operative bacterial infection, and Group 2 included those patients who had more than one episode of a bacterial infection.
Statistical analyses were performed using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, United States). Continuous numerical variables were shown as the mean and standard deviation, and differences between groups were compared using the unpaired t test. Discrete numerical variables were shown as the median and interquartile range, and the Mann-Whitney test was used to compare intergroup differences. Categorical data were shown as ratios or as the number and percentage, and differences between groups were compared using Pearson’s χ2 test or Fisher’s exact test. Variables shown to be significantly associated with the occurrence of repeated infection episodes by univariate analysis were entered in multivariate binary logistic regression analysis to identify independent predictors of this outcome. Time-to-event analysis was done using the Kaplan-Meier method, and the log-rank test was used to compare individual Kaplan-Meier curves. A P-value < 0.05 was considered significant.
The statistical methods for this study were performed by Sameh M. Hakim, Diploma of Medical Biostatistics, Faculty of Medicine of Ain Shams University, Cairo, Egypt.
The present study enrolled forty-five adult patients with HCV-related ESLD, and each patient was followed-up for 3 mo post-LDLT for the occurrence of bacterial infections. Thirty-three patients (73.3%) suffered from bacterial infections post-transplant and fulfilled the inclusion criteria. They were further subdivided into two groups. Group 1 included 21 patients who developed a single episode of infection (19 males and 2 females), and Group 2 included 12 patients (all of them were males) who developed recurrent episodes of infection (total number of attacks = 36) throughout the follow-up period.
Table 1 shows the comparison between patients who developed a single episode of infection post-LDLT and those who developed repeated episodes of infection regarding pre-operative parameters. The presence of pre-transplant hepatocellular carcinoma (HCC) showed a statistically significant increased risk of developing repeated episodes of infection post-LDLT (P = 0.033).
| Single episode of infection (n = 21) | Repeated episodes of infection (n = 12) | P value | |
| Recipient's age (yr, mean ± SD) | 51.2 ± 8.3 | 52.08 ± 8.7 | 0.767 |
| Donor's age (yr, mean ± SD) | 26.9 ± 6.3 | 32.3 ± 6.1 | 0.021 |
| Recipient's gender (male/female) | 19/2 | 12/0 | 0.523 |
| Donor’s gender (male/female) | 19/2 | 10/2 | 0.610 |
| Hepatocellular carcinoma | 6 (28.6) | 8 (66.7) | 0.033 |
| History of bridging procedures1 | 4 (19.0) | 6 (50.0) | 0.114 |
| History of SBP | 10 (47.6) | 1 (8.3) | 0.052 |
| History of paracentesis | 11 (52.4) | 4 (33.3) | 0.290 |
| Diabetes mellitus | 8 (38.1) | 5 (41.7) | 1.000 |
| Child-Pugh class (B/C) | 10/11 | 5/7 | 0.741 |
| MELD score (median, interquartile range) | 14 (12-16) | 16 (15-18) | 0.136 |
| Thrombocytopenia2 | 20 (95.2) | 12 (100.0) | 1.000 |
| Leucopenia3 | 9 (42.9) | 5 (41.7) | 0.947 |
| Renal impairment | 2 (9.5) | 1 (8.3) | 1.000 |
| High serum ferritin4 | 13 (61.9) | 5 (41.7) | 0.261 |
| High C-reactive protein5 | 14 (66.7) | 11 (91.7) | 0.206 |
There was no significant difference between patients who developed a single episode and those who developed repeated episodes of infection regarding the operative details (P > 0.05) (Table 2).
| Single episode of infection (n = 21) | Repeated episodes of infection (n = 12) | P value | |
| CIT (min), mean ± SD | 43.6 ± 17.3 | 50.8 ± 17.7 | 0.259 |
| WIT (min), mean ± SD | 45.7 ± 13.4 | 50.8 ± 12.4 | 0.288 |
| Recipient's operative time (h), mean ± SD | 10.3 ± 1.1 | 10.5 ± 1.5 | 0.704 |
| Packed red cell transfusion (U), (median, interquartile range) | 2 (2-4) | 3 (2-6) | 0.493 |
Table 3 shows that the duration of drain insertion revealed a statistically significant increased risk for the development of repeated episodes of infection (P = 0.002).
| Single episode of infection (n = 21) | Repeated episodes of infection (n = 12) | P value | |
| Length of ICU stay (d) | 6 (5-7) | 7 (5-7) | 0.969 |
| Days on mechanical ventilator | 1 (1-1) | 1 (1-1) | 0.176 |
| Days with CVC | 6 (5-7) | 6 (5-7) | 0.770 |
| Days with urinary catheter | 6 (5-7) | 7 (6-8) | 0.467 |
| Days with drains | 17 (15-20) | 25 (21-30) | 0.002 |
| Time-to-infection (d) | 14 (12-17) | 9 (6-19) | 0.189 |
Table 4 shows that bile was found to be the most common site for both single and repeated episodes of infection (28.6% and 27.8%, respectively), followed by the bloodstream for repeated infection episodes (22.2%) and drains for a single infection episode (23.8%).
| Single episode (n = 21) | Repeated episodes (n = 36)1 | P value | |
| Site of organism isolation | 0.896 | ||
| Bile | 6 (28.6) | 10 (27.8) | |
| Wound | 1 (4.8) | 2 (5.6) | |
| Sputum | 3 (14.3) | 7 (19.4) | |
| Drains | 5 (23.8) | 7 (19.4) | |
| Blood | 3 (14.3) | 8 (22.2) | |
| Urine | 2 (9.5) | 2 (5.6) | |
| Ascitic fluid | 1 (4.8) | 0 (0.0) | |
| Oragnism isolated | 0.456 | ||
| Goagulase (-) Staph. aureus | 3 (14.3) | 1 (2.8) | |
| Staph. aureus | 0 (0.0) | 1 (2.8) | |
| MRSA | 3 (14.3) | 1 (2.8) | |
| E. coli | 2 (9.5) | 4 (11.1) | |
| Klebsiella species | 2 (9.5) | 3 (8.3) | |
| Pseudomonas aeruginosa | 4 (19.0) | 3 (8.3) | |
| Acinetobacter baumannii | 4 (19.0) | 12 (33.3) | |
| Proteus | 0 (0.0) | 2 (5.6) | |
| Enterobacteriaceae | 1 (4.8) | 1 (2.8) | |
| Enterococci | 1 (4.8) | 2 (5.6) | |
| Bacillus species | 0 (0.0) | 2 (5.6) | |
| Pseudomonas + Acinetobacter | 1 (4.8) | 0 (0.0) | |
| Pseudomonas + Klebsiella | 0 (0.0) | 2 (5.6) | |
| Acinetobacter + Klebsiella | 0 (0.0) | 1 (2.8) | |
| Acinetobacter + coagulase (-) Staph. aureus | 0 (0.0) | 1 (2.8) |
The most common isolated organisms were gram-negative bacteria for both single and repeated episodes of infections. Acinetobacter baumannii was found solely to be the most common organism isolated from both single and repeated infection episodes (19% and 33.3%, respectively), followed by Escherichia coli (E. coli) for repeated infections (11.1%), and Pseudomonas aeruginosa for a single infection (19%). Additionally, Acinetobacter baumannii was found in combination with other organisms in three cultures.
Table 5 shows the antimicrobial sensitivity pattern in patients who suffered from single vs repeated episodes of infection. The sensitivity of levofloxacin was found to be statistically significant against repeated episodes of infection (P = 0.03). Repeated episodes of infection showed 100% resistance to penicillins. Single episodes of infection were 100% resistant to ciprofloxacin and co-trimoxazole. Both single and repeated episodes of infections were 100% resistant to cefotaxime and aztreonam.
| Antimicrobial | All episodes of infection (n = 57) | Single episode of infection (n = 21) | Repeated pisodes of infection (n = 36) | P value | |
| Levofloxacin | S | 11 (52.4) | 2 (22.2) | 9 (75.0) | 0.030 |
| R | 10 (47.6) | 7 (77.8) | 3 (25.0) | ||
| Ciprofloxacin | S | 5 (38.5) | 0 (0.0) | 5 (45.5) | 0.487 |
| R | 8 (61.5) | 2 (100.0) | 6 (54.5) | ||
| Co-trimoxazole | S | 1 (7.1) | 0 (0.0) | 1 (10.0) | 1.000 |
| R | 13 (92.9) | 4 (100.0) | 9 (90.0) | ||
| Penicillin | S | 1 (11.1) | 1 (14.3) | 0 (0.0) | 1.000 |
| R | 8 (88.9) | 6 (85.7) | 2 (100.0) | ||
| Doxycycline | S | 14 (77.8) | 5 (100.0) | 9 (69.2) | 0.278 |
| R | 4 (22.2) | 0 (0.0) | 4 (30.8) | ||
| Vancomycin | S | 8 (88.9) | 4 (100.0) | 4 (80.0) | 1.000 |
| R | 1 (11.1) | 0 (0.0) | 1 (20.0) | ||
| Piperacillin-tazobactam | S | 8 (72.7) | 3 (75.0) | 5 (71.4) | 1.000 |
| R | 3 (27.3) | 1 (25.0) | 2 (28.6) | ||
| Aminoglycosides | S | 9 (75.0) | 1 (50.0) | 8 (80.0) | 0.455 |
| R | 3 (25.0) | 1 (50.0) | 2 (20.0) | ||
| Imipenem | S | 20 (69.0) | 8 (88.9) | 12 (60.0) | 0.201 |
| R | 9 (31.0) | 1 (11.1) | 8 (40.0) | ||
| Ceftrioxone | S | 7 (38.9) | 1 (16.7) | 6 (50.0) | 0.316 |
| R | 11 (61.1) | 5 (83.3) | 6 (50.0) | ||
| Cefotaxime | R | 8 (100.0) | 7 (100.0) | 1 (100.0) | - |
| Aztreonam | R | 6 (100.0) | 1 (100.0) | 5 (100.0) | - |
Regarding the pattern of resistance of isolated organisms to the major antibiotic groups, most of the isolated gram-negative organisms were found to be resistant to several groups of antibiotics; especially Klebsiella species, Acinetobacter baumannii and Pseudomonas aeruginosa, all of which were proven to be MDR.
The detailed antibiotic-resistance pattern was as follows: For Klebsiella species, 100% of the isolates showed resistance to each of the quinolones and aminoglycosides, 87.5% showed resistance to cephalosporins, 80% to carbapenems, and 25% showed resistance to piperacillin-tazobactam. For Acinetobacter baumannii, 100% of the isolates showed resistance to aminoglycosides, 60% to carbapenems, 46.5% to quinolones, 42% to cephalosporins, and 33.3% showed resistance to piperacillin-tazobactam. For Pseudomonas aeruginosa, 100% of the isolates showed resistance to quinolones, and 83.3% showed resistance to cephalosporins. Meanwhile, 100% of them were sensitive to aminoglycosides, piperacillin-tazobactam and carbapenems. For E. coli, 70% of the isolates showed resistance to cephalosporins, 50% to quinolones, and 25% showed resistance to aminoglycosides. Moreover, 100% of them were sensitive to piperacillin-tazobactam and carbapenems.
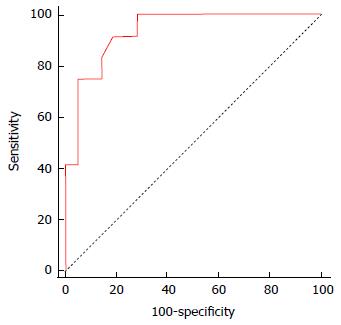
Table 6 and Figure 1 show that the two variables identified by multivariate analysis as independent risk factors for the occurrence of repeated episodes of infection were HCC and the duration of drain insertion (in days) (P = 0.024 and odds ratio = 25.44 and 1.38, respectively).
| Regression coefficient | SE | Odds ratio | 95%CI | P value | |
| Donor’s age (yr) | 0.05 | 0.08 | 1.05 | 0.90-1.23 | 0.552 |
| Hepatocellular carcinoma (HCC = 1, no HCC = 0) | 3.24 | 1.43 | 25.44 | 1.53-422.21 | 0.024 |
| Duration of drain insertion (d) | 0.32 | 0.14 | 1.38 | 1.04-1.83 | 0.024 |
| Constant | -10.28 | ||||
| Model diagnostics | |||||
| -2 Log Likelihood test | P value, < 0.001 | ||||
| Hosmer and Lemeshow test | P value, 0.369 | ||||
| Correct classification rate | 87.88% | ||||
| ROC curve analysis | |||||
| AUC | 0.935 (95%CI: 0.791-0.991; P value < 0.0001) | ||||
| Sensitivity, % | 91.7 (95%CI: 61.5-99.8) | ||||
| Specificity, % | 81.0 (95%CI: 58.1-94.6) | ||||
| PPV, % | 73.3 (95%CI: 43.8-92.7) | ||||
| NPV, % | 94.4 (95%CI: 72.7-99.9) | ||||
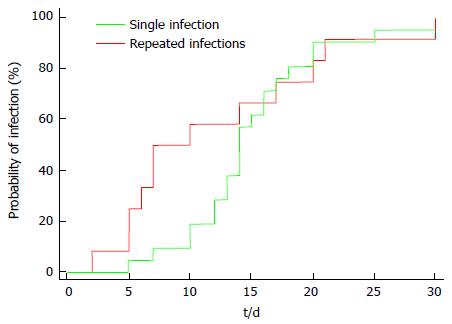
The median time-to-infection was 14 d in the single infection episode group and 8.5 d in the repeated infection episodes group, with no significant difference observed between groups (P = 0.647) (Table 7 and Figure 2).
| Single infection episode (n = 21) | Repeated infection episodes (n = 12) | |
| Median time to infection (d) | 14 (95%CI: 13-16) | 8.5 (95%CI: 6-17) |
| Hazard ratio | 1.16 (95%CI: 56-2.40) | |
| Log-rank test | P value = 0.647 |
Infectious complications have become the most common sources of mortality and morbidity following LT. Multiple organism infection is common. The occurrence of infection following LT is due to the dysfunction of the patient’s defensive mechanisms, as a result of liver cirrhosis and the use of immunosuppressant drugs[4].
The current study included 45 patients with HCV-related ESLD who were eligible for and underwent LDLT at Ain Shams Center for Organ Transplant, Cairo, Egypt during the period from January 2014 to November 2015. They were followed-up for the first 3 mo post-LDLT for the detection of bacterial infections.
In the current study, 73.3% of included patients developed a nosocomial bacterial infection in the first 3 mo post-LDLT. This finding is in agreement with previous reports, which denoted a high incidence of bacterial infections post-LDLT ranging from 50% to 75%[1,2].
In the current study, the presence of pre-transplant HCC was an independent risk factor for the occurrence of repeated episodes of bacterial infection in the recipients during the early post-transplant period. HCC patients are more susceptible to infection due to poor long-term nutrition, poor physical condition and weak immune system[1].
In the present study, the duration of time for abdominal drain placement was considered an independent risk factor for the development of repeated episodes of bacterial infection as confirmed by the multivariate binary logistic regression model. Patients with prolonged drain insertion time had an increased risk of developing recurrent episodes of infection compared to patients who had less drain insertion time.
Results in our study revealed that the major sites of bacterial infections in patients who experienced a single infection episode were as follows: Bile (28.6%), followed by the drains (23.8%), sputum (14.3%), bloodstream infections (14.3%), urine (9.5%) and lastly wound and ascitic fluid infection (4.8% each). These results were in accordance with another Egyptian multicenter study performed by Mukhtar et al[1]. In contrast, Kim et al[9] and Iida et al[10] revealed that the most dominant bacterial infection was bacteremia, which was catheter-related. El-Araby et al[11] showed that the main sites of infection were the chest (24.4%), followed by the bile duct or cholangitis (17.1%), and lastly the bloodstream (12.2%). However, Kawecki et al[12] revealed that the urinary tract was the main site of infection after LDLT. The discrepancies between the major sites of post-transplant infection between the different centers are most likely related to the variability of the hygienic measures, infection control programs, as well as the peri-, intra- and post-operative disparities.
In the current study, the most common isolated organisms were the gram-negative bacteria for both single and repeated episodes of infections, and these results were consistent with El-Araby et al[11] and Linares et al[13]. Shi et al[14] reported the same results and explained that the prevalence of gram-negative bacteria may be because these bacteria are inhabitants of the digestive tract. In the current study, Acinetobacter baumannii and Pseudomonas aeruginosa were found to be the most common organisms in the single infection episode group (19% each), followed by methicillin resistant Staphylococcus aureus (MRSA) and coagulase-negative Staphylococcus aureus (14.3% each), and Klebsiella species and E. coli (9.5% each). These results were in accordance with Zhong et al[15]. However, Sganga et al[5] and Iida et al[10] concluded that Pseudomonas aeruginosa was the most common isolated organism.
At present, MDR organisms are the most common causes of nosocomial infections in post-LDLT patients. Zhong et al[15] found that MDR gram-negative bacilli were isolated in 56% of patients with gram-negative infection, which was in accordance with Shi et al[14], who stated that the three most common pathogens of MDR gram-negative bacilli were Acinetobacter baumannii, E. coli and Klebsiella species. This finding is not fully consistent with a previous report by Pappas et al[16] who found that the four most common MDR gram-negative bacilli were E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii. The difference in the findings between the studies was related to differences in patients’ underlying diseases and nosocomial infections.
Our results are consistent to some extent with that of Mukhtar et al[1] in their retrospective multicenter Egyptian study on bacterial infections post-LDLT. The authors reported that Pseudomonas aeruginosa was the most commonly isolated species (26%), followed by Klebsiella (19%), E. coli (16%), Acinetobacter baumannii (8%), and MRSA (7.7%). In their study, 75% of the gram-negative bacteria were MDR, including 90% of Acinetobacter baumannii isolates, 76% of Pseudomonas aeruginosa isolates, 57% of Klebsiella species isolates, and 53% of E. coli isolates.
Our study revealed that most of the gram-negative organisms were found to be resistant to several groups of antibiotics, especially Klebsiella species, Acinetobacter baumannii and Pseudomonas aeruginosa, which proved to be MDR. In the study of Zhong et al[15], Acinetobacter baumannii displayed resistance to all antibiotic groups, including β-lactams, quinolones, and aminoglycosides and even showed high resistance to carbapenems, including 100% resistance to meropenem and imipenem. E. coli was found to be sensitive to aminoglycosides, carbapenems and piperacillin-tazobactam but showed a pattern of resistance to cephalosporins. Among all the antibiotics used in the current cohort, levofloxacin was found to be of statistical significance regarding its sensitivity in the treatment of repeated episodes of infection.
It is worth mentioning that all infection episodes in our study occurred in the first month post-operative and by applying Kaplan-Meier analysis for time-to-infection. The median time-to-infection was 14 d in the single infection episode group and 8.5 d in the repeated infection episodes group. Similarly, previous studies have reported that the majority of bacterial infections occurred during the first month following LT[1,17].
In conclusion, MDR gram-negative bacterial infections are common post-LDLT. Pre-transplant HCC and duration of drain insertion are independent risk factors for the occurrence of repeated infection episodes.
The authors gratefully acknowledge members of the Ain Shams Center for Organ Transplant, Cairo, Egypt for their support.
Bacterial infections are common following living-donor liver transplantation (LDLT), especially multiple-organism infections. The occurrence of infection following liver transplantation is due to the dysfunction of the patient’s defensive mechanisms, as a result of liver cirrhosis and the use of immunosuppressant drugs.
The authors assessed 45 patients with hepatitis C virus-related end-stage liver disease for the occurrence of bacterial infections during the first 3 mo post-LDLT. Thirty-three patients (73.3%) suffered from bacterial infections; 21 patients experienced a single episode of infection, and 12 patients experienced repeated episodes of infection. Bile was the most common site for both single and repeated episodes of infection (28.6% and 27.8%, respectively). Multi-drug resistant (MDR) gram-negative bacteria, especially Klebsiella, Acinetobacter and Pseudomonas, were the most commonly isolated bacteria. Pre-transplant hepatocellular carcinoma and duration of drain insertion were independent risk factors for the occurrence of repeated infection episodes.
This study is a single-center Egyptian study that addresses risk factors, causative organisms and antimicrobial resistance of bacterial infections following LDLT in cirrhotic patients.
The findings in this study may help in determining the proper antimicrobial prophylaxis for cirrhotic patients pre-LDLT.
MDR was used to refer to pathogens resistant to three or more classes of the following antibiotics: Extended-spectrum penicillins, 3rd generation cephalosporins, quinolones, carbapenems, and aminoglycosides.
Acceptance of this manuscript for publication is recommended.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aljumah AA S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Mukhtar A, Abdelaal A, Hussein M, Dabous H, Fawzy I, Obayah G, Hasanin A, Adel N, Ghaith D, Bahaa M. Infection complications and pattern of bacterial resistance in living-donor liver transplantation: a multicenter epidemiologic study in Egypt. Transplant Proc. 2014;46:1444-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Saner FH, Olde Damink SW, Pavlakovic G, van den Broek MA, Rath PM, Sotiropoulos GC, Radtke A, Canbay A, Paul A, Nadalin S. Pulmonary and blood stream infections in adult living donor and cadaveric liver transplant patients. Transplantation. 2008;85:1564-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | del Pozo JL. Update and actual trends on bacterial infections following liver transplantation. World J Gastroenterol. 2008;14:4977-4983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Fagiuoli S, Colli A, Bruno R, Burra P, Craxì A, Gaeta GB, Grossi P, Mondelli MU, Puoti M, Sagnelli E. Management of infections in cirrhotic patients: report of a consensus conference. Dig Liver Dis. 2014;46:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Sganga G, Bianco G, Fiori B, Nure E, Spanu T, Lirosi MC, Frongillo F, Agnes S. Surveillance of bacterial and fungal infections in the postoperative period following liver transplantation: a series from 2005-2011. Transplant Proc. 2013;45:2718-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128-140. [PubMed] |
| 7. | CDC definitions for nosocomial infections, 1988. Am Rev Respir Dis. 1989;139:1058-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8748] [Article Influence: 624.9] [Reference Citation Analysis (0)] |
| 9. | Kim SI, Kim YJ, Jun YH, Wie SH, Kim YR, Choi JY, Yoon SK, Moon IS, Kim DG, Lee MD. Epidemiology and risk factors for bacteremia in 144 consecutive living-donor liver transplant recipients. Yonsei Med J. 2009;50:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Iida T, Kaido T, Yagi S, Yoshizawa A, Hata K, Mizumoto M, Mori A, Ogura Y, Oike F, Uemoto S. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transpl. 2010;16:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | El-Araby H, Ghoneim EM, Abd Elaziz AM, Ibrahim TM. Early infections after Living Donor Liver Transplantation in Egyptian Children (Single center experience). EJMM. 2010;19:67-75 Available from: https://www.yumpu.com/en/document/view/41683533/amal-a-wafy-md-kamal-m-hanna-md-ayman-salem-md-/9. |
| 12. | Kawecki D, Pacholczyk M, Łagiewska B, Adadyński L, Lisik W, Sawicka-Grzelak A, Durlik M, Paczek L, Chmura A, Mlynarczyk G. Urinary tract infections in the early posttransplant period after liver transplantation: etiologic agents and their susceptibility. Transplant Proc. 2011;43:3052-3054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Linares L, García-Goez JF, Cervera C, Almela M, Sanclemente G, Cofán F, Ricart MJ, Navasa M, Moreno A. Early bacteremia after solid organ transplantation. Transplant Proc. 2009;41:2262-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Shi SH, Kong HS, Xu J, Zhang WJ, Jia CK, Wang WL, Shen Y, Zhang M, Zheng SS. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients. Transpl Infect Dis. 2009;11:405-412. [PubMed] |
| 15. | Zhong L, Men TY, Li H, Peng ZH, Gu Y, Ding X, Xing TH, Fan JW. Multidrug-resistant gram-negative bacterial infections after liver transplantation-spectrum and risk factors. J Infect. 2012;64:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1860] [Cited by in RCA: 2217] [Article Influence: 246.3] [Reference Citation Analysis (1)] |
| 17. | Romero FA, Razonable RR. Infections in liver transplant recipients. World J Hepatol. 2011;3:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (2)] |