Peer-review started: August 10, 2016
First decision: September 12, 2016
Revised: September 30, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: January 18, 2017
Processing time: 159 Days and 12.5 Hours
To investigate predictors for fibrosis specifically in a high risk population of morbidly obese patients, including detailed evaluation of lifestyle.
We conducted a cross-sectional study among morbidly obese patients attending the bariatric clinic at the Tel-Aviv Medical Center between the years 2013-2014 with body mass index (BMI) above 40 or above 35 with co-morbidity. Patients with serum hepatitis B surface antigen or anti-hepatitis C virus antibodies, genetic liver diseases, autoimmune disease or high alcohol intake (≥ 30 g/d in men or ≥ 20 g/d in women) were excluded from the study. Liver fibrosis was estimated by transient elastography (FibroScan®), using the ‘‘XL’’ probe. We collected data on age and gender, education, smoking status and amount, medical history, nutrition and lifestyle habits. All these data were collected using structured and validated questionnaires. Fasting blood test were available for a subsample.
Fibroscan was performed on a total of 91 patients, of which 77 had a valid examination according to the accepted criteria. Of those, 21% had significant fibrosis (F2) and 39% had advanced or severe fibrosis (F3 or F4). In multivariate analysis, male gender and BMI had a positive association with advanced fibrosis; the OR for fibrosis F ≥ 2 was 7.93 (95%CI: 2.36-26.64, P = 0.001) for male gender and 1.33 (1.11-1.60 kg/m2, P = 0.002) for BMI. The OR for fibrosis F ≥ 3 was 2.92 (1.08-7.91, P = 0.035) for male gender and 1.17 (1.03-1.33, P = 0.018) for BMI. Subjects were categorized to subgroups based on the combination of male gender and BMI of 40 and above. A significant dose response association with stiffness level was noted across these categories, with the highest stiffness among men with a higher BMI (P = 0.001). In addition, a significant positive correlation between pack-years cigarette smoking and liver stiffness was demonstrated among men (r = 0.54, P = 0.012).
In the morbidly obese population, a higher BMI, male gender and degree of smoking in men bears a greater risk for advanced nonalcoholic fatty liver disease.
Core tip: The presented results indicate that male gender and a higher body mass index (BMI) are risk factors for advanced fibrosis in morbidly obese patients. There is also a positive correlation between cigarette smoking and liver stiffness in men. Our study highlights the fact that even in the upper BMI ranges, higher BMI bears greater risk for advanced disease. Therefore, in the morbidly obese population it may seem useful to emphasize the importance of weight reduction, even within the range of obesity.
- Citation: Zelber-Sagi S, Shoham D, Zvibel I, Abu-Abeid S, Shibolet O, Fishman S. Predictors for advanced fibrosis in morbidly obese non-alcoholic fatty liver patients. World J Hepatol 2017; 9(2): 91-98
- URL: https://www.wjgnet.com/1948-5182/full/v9/i2/91.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i2.91
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in the developed[1] and developing countries[2,3] with estimated prevalence of 20%-40%, and is predicted to become the leading indication for liver transplantation in the United States[4]. NAFLD is tightly associated with the metabolic syndrome and its complications. Obesity is the most important risk factor for NAFLD, which affects as much as 74% of obese individuals[5]. Two large electronic databases have demonstrated a clear association between a higher body mass index (BMI), diabetes and male gender and the risk for NAFLD[6]. Furthermore, among morbidly obese patients, who are candidates for bariatric surgeries, the prevalence is even higher and reaches 96%[7-10].
NAFLD encompasses a wide spectrum of histological and clinical manifestations, ranging from simple steatosis to steatohepatitis, fibrosis and cirrhosis[11]. It is estimated that approximately 6%-13% of patients with simple steatosis progress to steatohepatitis, of which approximately 10%-29% reach liver cirrhosis within 10 years[12]. Moreover, non-alcoholic steatohepatitis cirrhosis is a known risk factor for hepatocellular carcinoma[4]. Given the relative high prevalence of severe fibrosis (12%) in morbidly obese patients[7-10], this population is especially prone to a detrimental course. Therefore, it is of upmost importance to identify those patients with high likelihood for advanced fibrosis who may later develop cirrhosis and hepatocellular carcinoma.
Several studies have aimed to find predictors and risk factors for advanced fibrosis, although most of them did not focus on morbidly obese population. In a study of 103 NAFLD patients who underwent serial liver biopsies to follow fibrosis progression rate[13], only type-2 diabetes mellitus (T2DM), BMI and initial stage of fibrosis were associated with a higher rate of disease progression. Of note, in this study only 68% of patients were obese. Sub-analysis of 3041 subjects from the Rotterdam study[14] revealed that liver stiffness above 8 kilopascals (kPa), as measured by FibroScan, was strongly associated with steatosis and T2DM. In this cohort the average BMI was 27, thus not representing a morbidly obese population. With respect to lifestyle and other co-morbidities, smoking and obstructive sleep-apnea were demonstrated to be positively associated with liver fibrosis[15,16]. Once again, most of the patients were not morbidly obese with an average BMI of 34 and 28 respectively.
Given the scarce data regarding risk factors for advanced fibrosis in morbidly obese population, the aim of the present study was to investigate predictors for fibrosis, specifically in this high risk population, including detailed evaluation of lifestyle. To none invasively assess liver fibrosis we used transient elastography (FibroScan®), which is a validated tool to determine liver stiffness[17], and was demonstrated to be one of the most accurate tests for the non-invasive evaluation of liver fibrosis in NAFLD with a clinical prognostic value[18].
We conducted a cross-sectional study among morbidly obese patients attending the bariatric clinic at the Tel-Aviv Medical Center between the years 2013-2014 with BMI above 40 or above 35 plus at least one co-morbidity (i.e., hypertension, type 2 diabetes, cardiovascular disease, lung disease and respiratory disorders), according to the Israeli Health Ministry indications published on 2013 (http://www.health.gov.il/hozer/mr33_2013.pdf). Patients with serum HBsAg or anti-hepatitis C virus antibodies, genetic liver diseases, autoimmune disease or high alcohol intake (≥ 30 g/d in men or ≥ 20 g/d in women)[19,20] were excluded from the study. All procedures performed in this study were approved by the institutional research committee of the Tel-Aviv Medical Center and in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Liver stiffness measurement (LSM) was measured using the ‘‘XL’’ probe. LSM were considered representative only if they had at least 10 valid acquisitions with a success rate > 60%[21]. All measurements were taken by the same operator (experience, > 10000 measurements) which was blinded to other parameters of the patients. As previously described[22], the examination was performed with the patient lying down in the dorsal decubitus position with the right arm in maximal abduction. The tip of the probe transducer was placed on the skin, between the ribs at the level of the right lobe of the liver. The results were expressed in kPa and each LSM corresponds to the median of 10 validated measurements. The cut-off values for fibrosis stage were according to the suggested best cutoffs to distinguish between fibrosis levels among NAFLD patients[23,24]: < 7.1; F0-F1: 7.1-9.5 kPa; F2: 9.6-11.5; F3: > 11.5; and F4: Significant fibrosis was defined as F ≥ 2, and advanced fibrosis was defined as F ≥ 3[25-27].
We collected data on age and gender, education, smoking status and amount, medical history including diabetes and medical treatment, nutrition, lifestyle habits and health status. All these data were collected using a structured and uniform questionnaire completed by all participants, tailored for the current study based on validated questionnaires used in national Israeli surveys[28]. Fasting blood test were available for a subsample of 40-48 patients.
Statistical analyses were performed using SPSS version 22 (SPSS Inc., Chicago, IL, United States) software. Continuous variables are presented as means ± SD. To test differences in continuous variables between the two groups the independent samples t-test or the Mann-Whitney U test were performed. Associations between nominal variables were performed with the Pearson χ2 test. The Pearson correlation was used for the evaluation of the correlation between liver stiffness and other measurements. A multivariate logistic regression analysis was performed to test the adjusted association between significant liver stiffness and potential predictors. Using a receiver operating characteristic curve, the best BMI cutoff point to predict significant fibrosis was 40, which represents grade-3 obesity. To test the combined effect of male gender and BMI of 40 and above (high BMI), we created a new variable with three categories: Lower BMI plus female gender, either male gender or high BMI, both male gender and high BMI. One way ANOVA of variance was used to test the difference in the distribution of liver stiffness between the categories with a P for trend test. Pearson χ2 test was used to test the association between these categories and the categories of fibrosis severity with a P for trend test. P < 0.05 was considered statistically significant for all analyses.
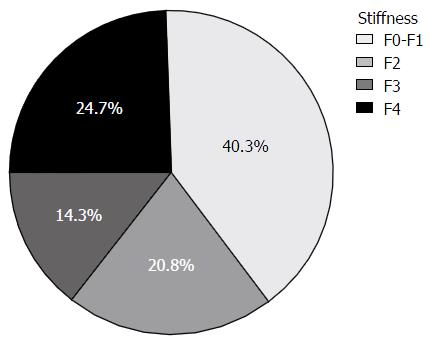
A total of 201 consecutive patients were recruited, of which 91 agreed to undergo Fibroscan exam, and 77 patients had a valid examination according to the accepted criteria[21]. As depicted in Table 1, the average BMI was 41.71 ± 4.68 kg/m2, with a range between 32.25 to 56.36 kg/m2, 48 had a BMI of 40 and above. Alcohol consumption was very low and no patient had to be excluded due to excessive consumption. The available blood tests indicated impaired fasting glucose and mildly elevated triglycerides and ALT levels. Most patients (60%) had some level of fibrosis (F2 and above) according to the Fibroscan examination. Of note, 39% of the patients had advanced or severe fibrosis (F3 or F4), 21% had significant fibrosis (F2) and only 40% of the patients had minimal or no fibrosis (F0-F1) (Figure 1).
| Variable | n1 | Mean ± SD |
| Age (yr) | 77 | 42.4 ± 12.98 |
| Gender (male) % | 77 | 46.8 |
| BMI (kg/m2) | 77 | 41.71 ± 4.68 |
| Glucose (mg/dL) (< 100) | 48 | 116.81 ± 35.08 |
| Total cholesterol (mg/dL) (< 200) | 42 | 191.97 ± 34.46 |
| LDL (mg/dL) | 42 | 112.80 ± 33.05 |
| HDL (mg/dL) | 40 | 44.19 ± 11.87 |
| TG (mg/dL) (< 150) | 42 | 182.38 ± 56.36 |
| ALT (U/L) (5-39) | 47 | 44.28 ± 44.49 |
| AST (U/L) (7-40) | 47 | 28.91 ± 20.11 |
| Success rate % | 77 | 84.31 ± 13.55 |
| Stiffness | 77 | 10.24 ± 6.27 |
| Sedentary time2 (min/d) | 77 | 263.84 ± 176.56 |
| Daily activity (score3) | 77 | 20.56 ± 3.47 |
| Alcohol servings/week | 77 | 0.66 ± 1.67 |
| Current or past smokers % | 77 | 48.1 |
Male gender was significantly more prevalent in subjects with fibrosis level of F2 and above or F3 and above, as compared to subjects with minimal or no fibrosis (63% vs 22.6%, P < 0.001 and 63.3% vs 36.2%, P = 0.020, respectively) (Tables 2 and 3). In addition, BMI was significantly higher in subjects with fibrosis level of F2 and above or F3 and above as compared to subjects with minimal or no fibrosis (42.99 ± 5.11 kg/m2vs 39.82 ± 3.16 kg/m2, P < 0.001 and 42.32 ± 5.3 kg/m2vs 40.68 ± 3.96 kg/m2, P = 0.023, respectively) (Tables 2 and 3). No other significant differences were noted between groups in nutritional, physical activity and biochemical parameters (Tables 2 and 3). Of note, there was no correlation between ALT and fibrosis (correlation r = 0.17, P = 0.253).
| Variable | F < 2 (n = 31) | F ≥ 2 (n = 46) | P |
| Gender (men) % | 22.6 | 63 | < 0.001 |
| Age (yr) | 42.32 ± 13.89 | 42.96 ± 12.29 | 0.838 |
| Education (yr) | 13.13 ± 2.05 | 13.78 ± 2.9 | 0.281 |
| BMI (kg/m2) | 39.82 ± 3.16 | 42.99 ± 5.11 | < 0.001 |
| Type 2 diabetes drugs (%) | 19.4 | 17.4 | 0.827 |
| All sugared soft drinks (cups/d) | 1.76 ± 2.74 | 1.38 ± 2.24 | 0.502 |
| Carbonated sugared drinks intake (cups/d) | 0.99 ± 1.52 | 0.89 ± 1.98 | 0.816 |
| Diet carbonated drinks intake (cups/d) | 0.46 ± 0.98 | 0.88 ± 1.6 | 0.196 |
| Coffee intake (cups/d) | 2.22 ± 1.87 | 1.77 ± 1.74 | 0.287 |
| Alcohol servings/week | 0.97 ± 1.80 | 0.46 ± 1.56 | 0.189 |
| Current or past smokers (%) | 48.4 | 47.8 | 0.961 |
| Fruits intake (portions/d) | 1.71 ± 1.35 | 1.74 ± 1.31 | 0.924 |
| Vegetables intake (portions/d) | 2.77 ± 2.38 | 2.64 ± 1.93 | 0.788 |
| Fried food intake (portions/d) | 1.18 ± 1.25 | 1.08 ± 0.77 | 0.690 |
| Leisure time physical activity (min/wk) | 189 ± 83.06 | 237.69 ± 437.89 | 0.733 |
| Sedentary time1 (min/d) | 264.19 ± 171.7 | 263.61 ± 181.65 | 0.989 |
| Daily activity (score2) | 20.19 ± 2.83 | 20.8 ± 3.85 | 0.452 |
| Glucose (mg/dL) | 113.72 ± 42.35 | 119.42 ± 28.13 | 0.580 |
| Total cholesterol (mg/dL) | 202.68 ± 37.79 | 182.24 ± 28.6 | 0.054 |
| LDL (mg/dL) | 121.52 ± 38.77 | 104.04 ± 23.97 | 0.088 |
| HDL (mg/dL) | 46.55 ± 11.42 | 41.83 ± 12.13 | 0.213 |
| Triglycerides (mg/dL) | 170.73 ± 60.25 | 192 ± 52.3 | 0.228 |
| ALT (U/L) | 34.89 ± 37.77 | 50.66 ± 48.13 | 0.237 |
| AST (U/L) | 26.4 ± 24.47 | 30.78 ± 16.43 | 0.467 |
| GGT (U/L) | 22.52 ± 12.01 | 43.06 ± 23.53 | 0.090 |
| Variable | F < 3 (n = 47) | F ≥ 3 (n = 30) | P |
| Gender (male) % | 36.2 | 63.3 | 0.020 |
| Age (yr) | 42.47 ± 14 | 43.07 ± 11.09 | 0.836 |
| Education (yr) | 13.19 ± 2.59 | 14.03 ± 2.55 | 0.166 |
| BMI (kg/m2) | 40.68 ± 3.96 | 42.32 ± 5.3 | 0.023 |
| Type 2 diabetes drugs (%) | 17 | 20 | 0.741 |
| All sugared soft drinks (cups/d) | 1.64 ± 2.45 | 1.37 ± 2.46 | 0.635 |
| Carbonated sugared drinks intake (cups/d) | 0.9 ± 1.55 | 0.98 ± 2.16 | 0.850 |
| Diet carbonated drinks intake (cups/d) | 0.73 ± 1.44 | 0.68 ± 1.33 | 0.890 |
| Coffee intake (cups/d) | 1.96 ± 1.88 | 1.93 ± 1.69 | 0.951 |
| Alcohol (servings/wk) | 0.87 ± 1.76 | 0.33 ± 1.47 | 0.152 |
| Current or past smokers (%) | 48.9 | 46.7 | 0.846 |
| Fruits intake (portions/d) | 1.57 ± 1.25 | 1.97 ± 1.4 | 0.204 |
| Vegetables intake (portions/d) | 2.43 ± 2.11 | 3.12 ± 2.07 | 0.162 |
| Fried food intake (portions/d) | 1.12 ± 1.12 | 1.12 ± 0.75 | 0.999 |
| Leisure time physical activity (min/wk) | 155.62 ± 89.96 | 355.71 ± 585.97 | 0.403 |
| Sedentary time1 (min/d) | 265.66 ± 178.79 | 261.00 ± 176.01 | 0.911 |
| Daily activity (score2) | 20.32 ± 3.49 | 20.93 ± 3.45 | 0.452 |
| Glucose (mg/dL) | 117 ± 39.93 | 116.53 ± 27.08 | 0.964 |
| Total cholesterol (mg/dL) | 197.75 ± 37.39 | 180.41 ± 25 | 0.083 |
| LDL (mg/dL) | 117.81 ± 35.94 | 101.61 ± 22.00 | 0.087 |
| HDL (mg/dL) | 44.85 ± 11.16 | 42.82 ± 13.6 | 0.619 |
| Triglycerides (mg/dL) | 176.28 ± 55.14 | 194.57 ± 58.84 | 0.328 |
| ALT (U/L) | 36.5 ± 33.26 | 55.76 ± 56.26 | 0.147 |
| AST (U/L) | 26.89 ± 21.99 | 31.89 ± 17.11 | 0.409 |
| GGT (U/L) | 29.89 ± 26.25 | 43.4 ± 16.96 | 0.241 |
In multivariate analysis, including age, gender and BMI, the positive association between male gender (OR = 7.93, 95%CI: 2.36-26.64, P = 0.001) and BMI (OR = 1.33, 95%CI: 1.11-1.60, P = 0.002) and fibrosis F ≥ 2 was maintained. Similarly, the positive association between male gender (OR = 2.92, 95%CI: 1.08-7.91, P = 0.035) and BMI (OR = 1.17, 95%CI: 1.03-1.33, P = 0.018) and fibrosis F ≥ 3 was maintained (Table 4).
| Variable | Model 1: F ≥ 2 | Model 2: F ≥ 3 | ||
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (yr) | 1.03 (0.99-1.08) | 0.153 | 1.03 (0.98-1.07) | 0.246 |
| Gender (male) | 7.93 (2.36-26.64) | 0.001 | 2.92 (1.08-7.91) | 0.035 |
| BMI (kg/m2) | 1.33 (1.11-1.60) | 0.002 | 1.17 (1.03-1.33) | 0.018 |
Subjects were categorized to subgroups based on the combination of gender and BMI of 40 and above: Subgroup (1) women and BMI below 40; subgroup (2) either men or BMI of 40 and above; subgroup (3) both men and BMI of 40 and above. A significant dose response association was noted across these categories for stiffness as a continuous variable (P for trend = 0.001) (Figure 2A), for the rate of subjects with F ≥ 2 (P for trend < 0.001) (Figure 2B) and for the rate of subjects with F ≥ 3 (P for trend = 0.011) (Figure 2C). The highest stiffness or prevalence of significant/advanced fibrosis was among men with a higher BMI.
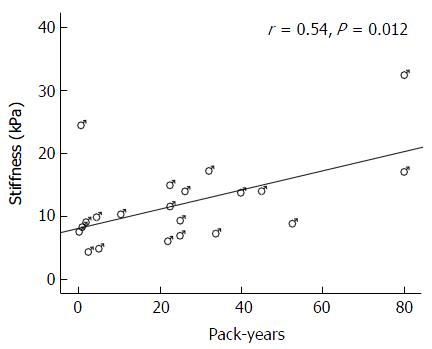
Twenty one of the men and 16 of the women were current or past smokers. Among them, there was a significant positive correlation between cigarette smoking measured by pack-years (number of packs multiplied with the number of years of smoking) and liver stiffness (r = 0.37, P = 0.025). However, stratification by gender reveled that this association existed among men (r = 0.54, P = 0.012) (Figure 3), but not among women (r = -0.10, P = 0.716).
The presented results indicate that male gender and a higher BMI are risk factors for advanced fibrosis in morbidly obese patients. Notably, there is also a positive correlation between cigarette smoking measured by pack-years and liver stiffness in men. Our data corroborate previous publications[5,7-10], demonstrating high rate of NAFLD with significant percent of severe (F4) fibrosis in morbidly obese patients, which in our population reached approximately 25% of the patients. Given the magnitude of the disease in the morbidly obese patients, only few attempts have been performed to stratify the risk for advanced fibrosis in this unique population, mostly among patients undergoing bariatric surgery. Ong et al[29] have found in 212 consecutive morbidly obese patients in whom liver biopsy was taken during bariatric surgery, that waist to hip ratio and AST levels were independently associated with severe fibrosis. In our cohort, liver enzymes were not predictive of advanced fibrosis. In another study[7] which obtained liver biopsy from 181 patients undergoing bariatric surgery, only age was significantly associated with advanced disease (moderate and severe fibrosis), in contrast to our results which did not show such an association (Tables 2 and 3). In line with our results, Dixon et al[8] have found in 105 consecutive biopsied bariatric patients that advanced fibrosis was associated with male gender and not with the presence of T2DM. In contrast, Beymer et al[9] has found T2DM as the only predictor to advanced fibrosis. This discrepancy may be explained by systemic insulin resistance characterizing most of the patients in the aforementioned cohorts who have not developed overt diabetes yet. This assumption is supported by the higher C-peptide level found in patients with advance fibrosis in Dixon’s cohort[8]. Interestingly, a recent cohort analyzing 134 South Indian patients have found arterial hypertension as a sole independent risk factor for fibrosis[30].
In our study we have extended the search for advanced liver fibrosis predictors toward diet elements, eating behavior and lifestyle variables, all of which have shown an association with obesity in general[31] and some of them with NAFLD in particular[32]. Whereas the association between active and passive smoking and NAFLD has been already demonstrated[33], we succeeded to show a significant positive correlation between cigarette smoking measured by pack-years and liver stiffness in men (Figure 2). Our findings are corroborated by the data of the Multicenter Nonalcoholic Steatohepatitis Clinical Research Network generated from 1081 patients[16], in which multivariate analysis has demonstrated 1.6 fold increased odds for advanced fibrosis among those with a smoking history of ≥ 10 pack-years. Among non-diabetics, a history of ≥ 10 pack-years was associated with even a higher chance of 2.5 fold for advanced fibrosis. Multiple mechanisms may be involved in smoking injury, such as insulin resistance, oxidative stress and hypoxia[16]. Further studies are needed to confirm this finding among morbid obese patients and to elucidate this phenomenon.
We failed to find an association between liver stiffness and dietary parameters or eating patterns in this study. This lack of association may stem from potential sources of bias that need to be considered. First, recall and reporting bias may exist, especially on lifestyle habits and partially because of social desirability among obese population[34]. The bias was minimized by the use of structured and validated questionnaires. Also, no information about the purpose and the hypotheses of the study was provided to the participants in order to minimize the report bias. In addition, the interview was performed before receiving the results of the Fibroscan and thus the information bias is expected to be non-differential. Second, a measurement of liver stiffness is not the gold standard for the assessment of liver fibrosis. However, the Fibroscan test with the XL transducer is adjusted for patients with obesity and morbid obesity and compared with the standard transducer leads to lower rates of test failure (1.1% vs 16%) and an established validity[17,21]. However, among obese subjects, unreliable results may still be observed with the XL probe[35]. Nevertheless, despite this disadvantage, Fibroscan was demonstrated to be one of the most accurate tests for the non-invasive diagnosis of liver fibrosis in NAFLD[18].
In conclusion, our study highlights the fact in even in the upper BMI ranges, higher BMI bears greater risk for advanced disease. In addition, male gender may a risk factor for advanced disease in the morbidly obese population. The suggested association with the degree of smoking in men will have to be confirmed in further studies with a larger sample size.
We would like to thank Ms. Stella Levit for performing the Fibroscan examinations.
Given the high prevalence of severe fibrosis (12%) in morbidly obese patients, this population is especially prone to a detrimental course of nonalcoholic fatty liver disease (NAFLD). Therefore, it is of upmost importance to identify those patients with high likelihood for advanced fibrosis who may later develop cirrhosis and hepatocellular carcinoma.
The presented results indicate that male gender and a higher body mass index (BMI) are risk factors for advanced fibrosis in morbidly obese patients. There is also a positive correlation between cigarette smoking and liver stiffness in men.
Their study highlights the fact in even in the upper BMI ranges, higher BMI bears greater risk for advanced disease. In addition, male gender may be a risk factor for advanced disease in the morbidly obese population.
This study may indicate that weight reduction, even a modest one within the morbid obesity range, may be helpful in prevention of advanced fibrosis in morbid obese patients. Men may need more closer monitoring of fibrosis, and if supported by larger studies, may be advised to undergo smoking cessation.
NAFLD encompasses a wide spectrum of histological and clinical manifestations, ranging from simple steatosis to steatohepatitis, fibrosis and cirrhosis.
This is an interesting and well-organized study. The results are clearly presented.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Julie NL, Sipos F, Tziomalos K, Zeng Z S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1330] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 4. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1659] [Article Influence: 118.5] [Reference Citation Analysis (2)] |
| 5. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 302] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 6. | Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, Desai J, Gill JM, Welsh P, Waterworth D. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab. 2016;101:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Crespo J, Fernández-Gil P, Hernández-Guerra M, Cayón A, Mayorga M, Domínguez-Diez A, Fernández-Escalante JC, Pons-Romero F. Are there predictive factors of severe liver fibrosis in morbidly obese patients with non-alcoholic steatohepatitis? Obes Surg. 2001;11:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 9. | Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg. 2003;138:1240-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Gholam PM, Kotler DP, Flancbaum LJ. Liver pathology in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2002;12:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 12. | Hsu CS, Kao JH. Non-alcoholic fatty liver disease: an emerging liver disease in Taiwan. J Formos Med Assoc. 2012;111:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 659] [Article Influence: 33.0] [Reference Citation Analysis (1)] |
| 14. | Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, Leebeek FW, Hofman A, Stricker BH, Castera L. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 15. | Agrawal S, Duseja A, Aggarwal A, Das A, Mehta M, Dhiman RK, Chawla Y. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 16. | Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Naveau S, Lamouri K, Pourcher G, Njiké-Nakseu M, Ferretti S, Courie R, Tranchart H, Ghinoiu M, Balian A, Prévot S. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes Surg. 2014;24:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 19. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 20. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 789] [Article Influence: 52.6] [Reference Citation Analysis (1)] |
| 21. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 22. | Zelber-Sagi S, Yeshua H, Shlomai A, Blendis L, Leshno M, Levit S, Halpern Z, Oren R. Sampling variability of transient elastography according to probe location. Eur J Gastroenterol Hepatol. 2011;23:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 24. | Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Schwabl P, Bota S, Salzl P, Mandorfer M, Payer BA, Ferlitsch A, Stift J, Wrba F, Trauner M, Peck-Radosavljevic M. New reliability criteria for transient elastography increase the number of accurate measurements for screening of cirrhosis and portal hypertension. Liver Int. 2015;35:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 954] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 27. | de Lédinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, Le Bail B, Choi PC, Chermak F, Yiu KK. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Nitzan Kaluski D, Goldsmith R, Chinitz A, Ben Arie-Magled O, Mayer C, Green M. First National Health and Nutrition Survey. Israel Center for Disease Control. 2004;. |
| 29. | Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, Goodman Z, Younossi ZM. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Praveenraj P, Gomes RM, Kumar S, Karthikeyan P, Shankar A, Parthasarathi R, Senthilnathan P, Rajapandian S, Palanivelu C. Prevalence and Predictors of Non-Alcoholic Fatty Liver Disease in Morbidly Obese South Indian Patients Undergoing Bariatric Surgery. Obes Surg. 2015;25:2078-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102-S138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1717] [Cited by in RCA: 2036] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 32. | Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377-3389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 210] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 33. | Liu Y, Dai M, Bi Y, Xu M, Xu Y, Li M, Wang T, Huang F, Xu B, Zhang J. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. 2013;23:115-121. [PubMed] |
| 34. | Lissner L. Measuring food intake in studies of obesity. Public Health Nutr. 2002;5:889-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |