Published online Jul 8, 2017. doi: 10.4254/wjh.v9.i19.857
Peer-review started: September 24, 2016
First decision: November 21, 2016
Revised: March 20, 2017
Accepted: April 18, 2017
Article in press: April 20, 2017
Published online: July 8, 2017
Processing time: 287 Days and 12.3 Hours
To investigate the association between hepatic steatosis and change in left ventricular mass index (LVMI) over five years, and examine whether systolic and diastolic blood pressures are mediators of the association between hepatic steatosis and LVMI using a general population sample.
We analyzed data from the Study of Health in Pomerania. The study population comprised 1298 individuals aged 45 to 81 years. Hepatic steatosis was defined as the presence of a hyperechogenic pattern of the liver together with elevated serum alanine transferase levels. Left ventricular mass was determined echocardiographically and indexed to height2.7. Path analyses were conducted to differentiate direct and indirect paths from hepatic steatosis to LVMI encompassing systolic and diastolic blood pressure as potential mediating variables.
Hepatic steatosis was a significant predictor for all measured echocardiographic characteristics at baseline. Path analyses revealed that the association of hepatic steatosis with LVMI change after five years was negligibly small (β = -0.12, s.e. = 0.21, P = 0.55). Systolic blood pressure at baseline was inversely associated with LVMI change (β = -0.09, s.e. = 0.03, P < 0.01), while no association between diastolic blood pressure at baseline and LVMI change was evident (β = 0.03, s.e. = 0.05, P = 0.56). The effect of the indirect path from hepatic steatosis to LVMI via systolic baseline blood pressure was small (β = -0.20, s.e. = 0.10, P = 0.07). No indirect effect was observed for the path via diastolic baseline blood pressure (β = 0.03, s.e. = 0.06, P = 0.60). Similar associations were observed in the subgroup of individuals not receiving beta-blockers, calcium channel blockers, or drugs acting on the renin-angiotensin system.
Baseline associations between hepatic steatosis and LVMI do not extend to associations with LVMI change after five years. More studies are needed to study the longitudinal effects of hepatic steatosis on LVMI.
Core tip: Data regarding the association between hepatic steatosis and left ventricular remodeling are limited and previous studies revealed conflicting results. In the present study, hepatic steatosis as defined by liver hyperechogenicity and increased alanine transferase levels was a significant predictor for all measured echocardiographic characteristics at baseline. In contrast, hepatic steatosis was not a predictor of relevance for left ventricular mass index (LVMI) change. Systolic and diastolic blood pressures did not mediate the association between hepatic steatosis and LVMI.
- Citation: Piontek K, Schmidt CO, Baumeister SE, Lerch MM, Mayerle J, Dörr M, Felix SB, Völzke H. Is hepatic steatosis associated with left ventricular mass index increase in the general population? World J Hepatol 2017; 9(19): 857-866
- URL: https://www.wjgnet.com/1948-5182/full/v9/i19/857.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i19.857
Hepatic steatosis is highly prevalent in Western countries and regarded as the hepatic manifestation of the metabolic syndrome[1]. Results from previous studies indicate that the metabolic syndrome and its components such as overweight and hypertension are associated with an increase in left ventricular mass (LVM)[2,3]. Data on the association between hepatic steatosis and LVM are limited; only four cross-sectional studies of small sample size exist addressing this relationship. The first study investigated the effect of hepatic steatosis on left ventricular geometry and function in normotensive, nondiabetic patients and demonstrated that patients with hepatic steatosis had mildly altered left ventricular geometry and early signs of left ventricular diastolic dysfunction compared to controls[1]. The second study analyzed the relationship between left ventricular morphology, metabolic parameters and hepatic steatosis in patients with hypertension and revealed that individuals with hepatic steatosis had a similar prevalence of left ventricular hypertrophy (LVH) compared to individuals without hepatic steatosis[4]. The third study using data from hypertensive, diabetic patients revealed that the frequency of LVH was higher in individuals with hepatic steatosis compared to individuals without hepatic steatosis. This study further showed that individuals with hepatic steatosis yielded 6-fold higher odds ratios for LVH than individuals without hepatic steatosis[5]. The fourth study was of case-control design and demonstrated that hepatic steatosis was significantly associated with left ventricular dysfunction in diabetic patients[6]. Due to the design of the aforementioned studies, inferences about effect directions between hepatic steatosis and left ventricular remodelling cannot be made. In particular, there is no differentiation between direct paths from hepatic steatosis to LVM progression or indirect effects via mediators. However, the evaluation of potential mediators is important for a better understanding of the mechanisms underlying a putative association between hepatic steatosis and LVM. We hypothesize that blood pressure is a potential key mediator on the path from hepatic steatosis to LVM as LVH is known to be the major cardiac sequel of hypertension[7,8]. Thus, blood pressure should be adequately considered in studies aimed to investigate the association between hepatic steatosis and LVM.
To our knowledge, there is no previous research providing data on the association between hepatic steatosis and left ventricular mass index (LVMI) encompassing the following criteria: (1) using a general population sample; (2) using longitudinal data to improve inferences on the direction of effects; and (3) using methods to differentiate between direct and indirect pathways of hepatic steatosis on LVMI via blood pressure. The two major aims of the present study were, first, to investigate the association between hepatic steatosis and LVMI in a general population sample with prospective 5-year follow-up examination and, second, to analyze the mediating role of systolic and diastolic blood pressure on the pathway from hepatic steatosis to LVMI.
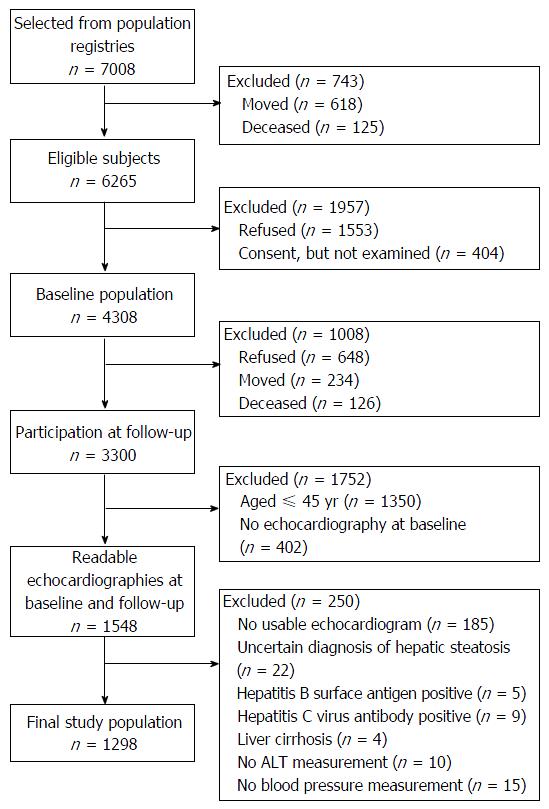
The Study of Health in Pomerania (SHIP) is a population-based cohort study conducted in West Pomerania, the northeastern area of Germany[9]. The sample recruitment procedure is displayed in Figure 1. At baseline, a sample of 7008 individuals aged 20 to 79 years was drawn from population registries. Only individuals with German citizenship and main residency in the study area were included. The net sample (without migrated or deceased persons) comprised 6265 eligible individuals. Each individual received a maximum of three postal invitation letters. In case of non-response, letters were followed by a phone call or by home visits. The SHIP population finally comprised 4308 participants (response 68.8%). Baseline examinations were conducted between 1997 and 2001. Between 2002 and 2006, all participants were re-invited for an examination follow-up, in which 3300 individuals (83.5% of eligible persons) took part[10]. Follow-up examinations were conducted on average 5.3 years after baseline (median: 5.0, 25th percentile: 5.0, 75th percentile: 5.3). All participants gave informed written consent. The study protocol was consistent with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the University of Greifswald. The study was monitored by a review board of independent scientists.
Among the 3300 participants with follow-up data, only those aged 45 years and older underwent echocardiographic examination at baseline (n = 1950). Of these, 1548 participants received a second echocardiography at follow-up. Readable echocardiograms from both examinations were available for 1538 individuals. Among these, 185 echocardiograms were not evaluable, 22 individuals had an uncertain diagnosis of hepatic steatosis, five were tested positive for hepatitis B surface antigen, nine were tested positive for anti-hepatitis C virus antibody, and four had a self-reported history of liver cirrhosis. Furthermore, ten participants had missing data on serum alanine transferase (ALT), and 15 participants lacked blood pressure measurements. Exclusion of these participants resulted in a final study population of 1298 individuals for the present analyses.
Baseline assessments included data on demographics, behavioural risk factors, the individual’s medical history and medication as well as data from somatometric, sonographic, echocardiographic and laboratory examinations.
Data on demographics, behavioral risk factors such as physical activity, alcohol consumption, and smoking status were collected using computer-assisted personal interviews. The following demographic variables were assessed: Gender, age and school educational attainment (in years of schooling completed). Individuals who participated in physical training during summer or winter for at least one hour a week were classified as being physically active. Alcohol consumption was assessed using a beverage-specific quantity-frequency measure: Number of days with alcohol consumption (beer, wine, spirits), and the quantity of alcohol consumed on such a day over the last month. Average daily consumption (in grams of pure ethanol) was calculated by multiplying frequency and amount, using beverage specific standard ethanol contents[11]. According to smoking habits, individuals were categorized into current, former, and never-smokers. Data on diabetes mellitus were obtained by self-reported physician’s diagnosis of the disease.
The somatometric measures included body weight and height as well as waist circumference (WC). Height and weight were measured for the calculation of the body mass index [BMI, weight (kg)/height2 (m2)]. WC was measured to the nearest 0.1 cm using an inelastic tape midway between the lower rib margin and the iliac crest in the horizontal plane, with the subject standing comfortably with weight distributed evenly on both feet.
Systolic and diastolic blood pressure were measured between 8 am and 7 pm three times after an initial five minute rest period at the right arm of seated individuals using a digital blood pressure monitor (HEM-705CP, Omron Corporation, Tokyo, Japan). Each reading was followed by a further rest period of three minutes. One of two differently sized cuffs was applied according to the circumference of the participant’s arm. The mean of the second and third measurement was calculated and used for the present analyses. Pulse pressure was defined as the difference between mean systolic and diastolic pressures. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medication.
For the laboratory examinations, non-fasting blood samples were drawn from the cubital vein in the supine position. The laboratory takes part quarterly in the official national German external proficiency testing programs. In addition, internal quality controls were analyzed daily. Hepatitis B surface antigen and anti-hepatitis C virus antibodies were determined by enzyme-linked immunosorbent assays (AxSym HBSAG and AxSym HCV, Abbot, Abbot Park, IL, United States). Serum ALT levels were measured photometrically (Hitachi 704; Roche, Mannheim, Germany) and expressed as μmol/L × s, which corresponds to (μmol/L × s) × 60 = IU/L.
Sonographic examinations were performed by physicians using a 5 MHz transducer and a high resolution instrument (Vingmed VST Gateway, Santa Clara, CA, United States). The sonographers were unaware of the participants’ clinical and laboratory characteristics. In SHIP, ultrasound examinations and readings underlie strict quality standards[12]. Hepatic steatosis was defined as the presence of a hyperechogenic liver pattern, with evident density differences between hepatic and renal parenchyma[13-15] together with increased serum ALT levels (> 75th percentile)[16].
Two-dimensional and M-mode echocardiography was performed by trained physicians using a Vingmed CFM 800A system (GE Medical Systems, Waukesha, WI, United States). All data and measurements were stored digitally. M-mode images of the left ventricle were recorded at the papillary level. Left ventricular dimensions [interventricular septum thickness (IVS), posterior wall thickness (LVPW), and left ventricular end-diastolic diameter (LVDD)] were measured off-line using the leading edge convention. LVMI was calculated as follows: LVMI = 0.80 × {1.04 × [(LVDD + IVS + LVPW)3 – LVDD3]} + 0.60/height2.7[17,18]. LVH was defined as a LVMI of > 48 g/m2.7 in men and > 44 g/m2.7 in women[19]. Comparisons of intra-reader, intra-observer, inter-reader, and inter-observer LVMI measurements revealed Spearman coefficients of > 0.85 and differences in mean (± 2 SD) of < 5% (< 25%).
The study population was divided into two groups based on the presence or absence of liver hyperechogenicity and increased ALT levels at baseline: Category 1 comprised individuals without hyperechogenic liver pattern and without increased serum ALT levels and individuals fulfilling only one of the named criteria. Category 2 comprised individuals with hepatic steatosis as defined by both liver hyperechogenicity and increased serum ALT levels.
Using analyses of variance and χ2-statistics, differences in baseline characteristics between individuals with and without hepatic steatosis regarding demographics, behavioural risk factors, and clinical characteristics were analyzed. Changes in echocardiographic parameters and blood pressure are depicted using absolute numbers and percentages. Bivariate correlations were calculated based on Pearson correlation coefficients.
We conducted path analyses to evaluate direct effects of hepatic steatosis on LVMI and the indirect effects via systolic and diastolic blood pressure. Standardized regression coefficients for systolic and diastolic blood pressure as well as LVMI are presented in the figures. The χ2-value, comparative fit index (CFI), and the root mean square error (RMSEA) are provided as indicators of model fit. CFI is an incremental fit index comparing the fit of the model of interest with the independence model with values ranging from zero to one. RMSEA is a descriptive approximate estimation of the overall fit of the model in the population. Values have a lower bound of zero. A CFI > 0.96 and a RMSEA < 0.05 are commonly regarded as indicative of a satisfactory model fit[20,21]. Parameter estimates were obtained based on a robust weighted least square approach (WLSMV), which is suitable to handle categorical and non-normal data[21]. Age and sex were considered as independent predictors for all variables in the models. In addition, baseline body weight was included. LVMI was not regressed on body weight since body weight is part of the calculation of LVMI. The time of the day of blood pressure measurement was controlled for all indicators of blood pressure.
To evaluate possible bias due to missing data, we applied statistical inverse probability weights accounting for known individual characteristics of the study participants related to missing data on the echocardiographic examination at follow-up. These inverse probability weights were derived from logistic regression analyses with age, sex, body weight, waist circumference, alcohol intake, smoking, and a summative comorbidity index as predictors.
We repeated our analyses in the subgroup of individuals not receiving medication with possible influence on LVM [beta-blockers, anatomical-therapeutical (ATC) codes C07; calcium channel blockers, ATC codes C08; and drugs acting on the renin-angiotensin system, ATC codes C09] as sensitivity analysis.
P values were estimated for two-sided tests. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed using STATA 10.2 (Stata Corporation, College Station, TX, United States) to conduct descriptive statistics. MPLUS 5.1 (Muthén and Muthén, Los Angeles, CA, United States) was used for path analyses. Data analyses were performed by Carsten O Schmidt who is an expert in the field of biomedical statistics.
At baseline, 1106 (85.1%) individuals fulfilled no or one criterion for hepatic steatosis, while 192 (14.9%) individuals had hepatic steatosis as defined by the combined presence of hyperechogenic liver pattern and increased serum ALT levels. LVH was present in 48.3% of the study population. The mean LVMI was 49.8 g/m2.7 (SD = 14.7). General characteristics of the study population at baseline are presented in Table 1.
| No/one criterion for hepatic steatosis | US+ and ALT+ | P-value | |
| n = 1106 | n = 192 | ||
| Age (yr), M (SD) | 59.6 (8.8) | 57.2 (7.8) | P < 0.01 |
| Male gender | 442 (40.0) | 139 (72.4) | P < 0.001 |
| School education | n.s. | ||
| < 10 yr | 570 (51.5) | 102 (53.1) | |
| 10 yr | 358 (32.4) | 67 (34.9) | |
| > 10 yr | 178 (16.1) | 23 (12.0) | |
| Waist circumference (cm), M (SD) | 89.0 (11.5) | 100.8 (10.9) | P < 0.001 |
| Body weight (kg), M (SD) | 75.6 (12.8) | 88.6 (13.9) | P < 0.001 |
| BMI, (kg/m2), M (SD) | 27.4 (4.3) | 30.5 (4.6) | P < 0.001 |
| Smoking | P < 0.001 | ||
| Never-smoker | 516 (46.6) | 57 (29.7) | |
| Ex-smoker | 382 (34.5) | 99 (51.7) | |
| Current smoker | 208 (18.8) | 36 (18.8) | |
| Alcohol consumption (g/d), M (SD) | 9.1 (14.5) | 15.6 (19.5) | P < 0.001 |
| Diabetes mellitus | 100 (9.0) | 26 (13.5) | P < 0.001 |
| Systolic blood pressure (mmHg), M (SD) | 139.3 (20.2) | 148.5 (17.4) | P < 0.001 |
| Diastolic blood pressure (mmHg), M (SD) | 84.7 (10.8) | 89.9 (10.4) | P < 0.001 |
| Pulse pressure (mmHg), M (SD) | 54.6 (14.7) | 58.6 (13.4) | P < 0.01 |
| Hypertension | 660 (59.7) | 163 (84.9) | P < 0.001 |
| Intake of drugs with ATC07 | 239 (21.6) | 39 (20.3) | n.s. |
| Intake of drugs with ATC08 | 140 (12.7) | 28 (14.6) | n.s. |
| Intake of drugs with ATC09 | 198 (17.9) | 58 (30.2) | P < 0.001 |
| IVS, M (SD) | 9.7 (2.2) | 10.9 (2.5) | P < 0.001 |
| LVEDD, M (SD) | 50.9 (5.6) | 52.4 (5.9) | P < 0.01 |
| PWD, M (SD) | 9.6 (1.9) | 10.4 (2.0) | P < 0.001 |
| LVM (g), M (SD) | 181.8 (53.5) | 215.8 (61.3) | P < 0.001 |
| LVMI (g/m2.7), M (SD) | 46.2 (13.3) | 51.0 (13.7) | P < 0.001 |
| LVH | 499 (45.1) | 114 (59.4) | P < 0.001 |
Compared to individuals fulfilling no or one criterion for hepatic steatosis, individuals with hepatic steatosis were more often male, had lower educational attainment, a higher WC, a higher body weight, a higher BMI, were less often never-smokers and reported a higher average daily alcohol consumption. Moreover, individuals with hepatic steatosis reported more often diabetes mellitus, had higher systolic and diastolic blood pressure, higher pulse pressure and were more often hypertensive compared to individuals fulfilling no or one criterion for hepatic steatosis. Individuals with hepatic steatosis reported more often the intake of drugs acting on the renin-angiotensin system compared to the reference group. Regarding echocardiographic characteristics, individuals with hepatic steatosis showed a higher interventricular septum thickness, a higher posterior wall thickness, a higher left ventricular end-diastolic diameter, a higher left ventricular mass, a higher left ventricular mass index and more often left ventricular hypertrophy than the reference group.
There was an increase in echocardiographic parameters from baseline to follow-up with higher values in individuals with hepatic steatosis compared to individuals fulfilling no or one criterion (Table 2). Blood pressure decreased from baseline to follow-up in both groups, while the proportion of hypertensive individuals slightly increased in the reference group and decreased in individuals with hepatic steatosis.
| Baseline | Follow-up | P-value | |
| M (SD) | M (SD) | ||
| IVS, M (SD) | |||
| No/one criterion for hepatic steatosis | 9.7 (2.2) | 11.2 (2.7) | P < 0.001 |
| US+ and ALT+ | 10.9 (2.5) | 12.0 (2.9) | P < 0.001 |
| LVEDD, M (SD) | |||
| No/one criterion for hepatic steatosis | 50.9 (5.6) | 48.8 (5.5) | P < 0.001 |
| US+ and ALT+ | 52.4 (5.9) | 50.6 (5.3) | P < 0.001 |
| PWD, M (SD) | |||
| No/one criterion for hepatic steatosis | 9.6 (1.9) | 9.9 (1.9) | P < 0.001 |
| US+ and ALT+ | 10.4 (2.0) | 10.9 (2.1) | P < 0.01 |
| LVM (g), M (SD) | |||
| No/one criterion for hepatic steatosis | 181.8 (53.5) | 192.2 (56.8) | P < 0.001 |
| US+ and ALT+ | 215.8 (61.3) | 226.1 (62.4) | P < 0.01 |
| LVMI (g/m2.7), M(SD) | |||
| No/one criterion for hepatic steatosis | 46.2 (13.3) | 49.2 (14.6) | P < 0.001 |
| US+ and ALT+ | 51.0 (13.7) | 53.7 (14.4) | P < 0.01 |
| SBP (mmHg), M (SD) | |||
| No/one criterion for hepatic steatosis | 139.3 (20.2) | 136.3 (19.2) | P < 0.001 |
| US+ and ALT+ | 148.5 (17.4) | 142.8 (19.0) | P < 0.001 |
| DBP (mmHg), M (SD) | |||
| No/one criterion for hepatic steatosis | 84.7 (10.8) | 81.2 (10.3) | P < 0.001 |
| US+ and ALT+ | 89.9 (10.4) | 85.0 (11.1) | P < 0.001 |
| LVH | |||
| No/one criterion for hepatic steatosis | 499 (45.1) | 597 (54.0) | P < 0.001 |
| US+ and ALT+ | 114 (59.4) | 128 (66.7) | P < 0.001 |
| Hypertension | |||
| No/one criterion for hepatic steatosis | 660 (59.7) | 686 (62.0) | P < 0.001 |
| US+ and ALT+ | 163 (84.9) | 154 (80.2) | P < 0.001 |
Hepatic steatosis was significantly correlated with all variables in the path models, but effect sizes were small (standardized coefficients ranging from 0.11 to 0.17, Table 3). Baseline measures of LVMI and blood pressure were most closely related to their respective counterparts at follow-up. Systolic blood pressure was consistently more closely associated to LVMI than diastolic blood pressure.
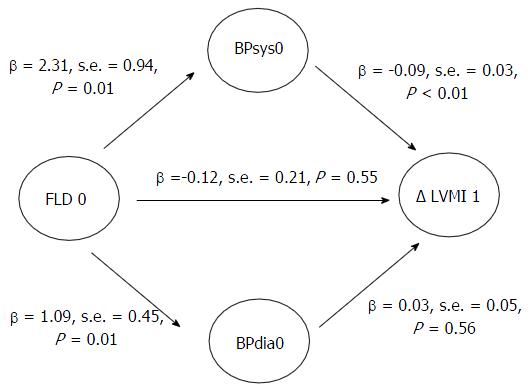
Figure 2 depicts the results of path analyses in the whole study population with systolic and diastolic blood pressure as potential mediators. The model fit was very good. Analyses revealed a very small, non-significant direct effect of baseline hepatic steatosis on LVMI change (β = -0.12, s.e. = 0.21, P = 0.55) and a negligible indirect effect via diastolic blood pressure (β = 0.03, s.e. = 0.06, P = 0.60, respectively). The moderate indirect effect via systolic blood pressure was borderline significant (β = -0.20, s.e. = 0.10, P = 0.07). Systolic blood pressure at baseline was inversely associated with LVMI change (β = -0.09, s.e. = 0.03, P < 0.01), while no association between diastolic blood pressure at baseline and LVMI change was evident (β = 0.03, s.e. = 0.05, P = 0.56).
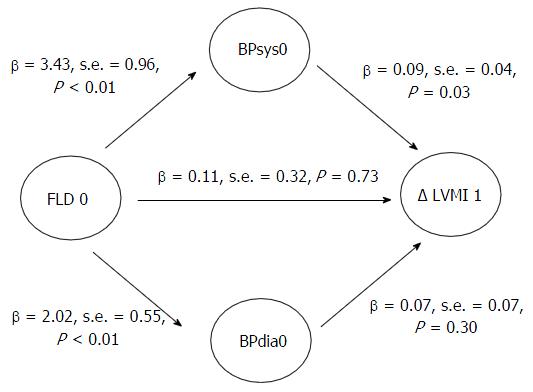
Repeating our analyses after excluding individuals not receiving beta-blockers, calcium channel blockers or drugs acting on the renin-angiotensin system revealed similar results (Figure 3).
We further repeated our analyses after excluding 30 individuals with high risk drinking according to the recommendations of the World Health Organization (consumption levels of 40 g/d in women and > 60 g/d in men). Analyses revealed almost identical results (direct effect of baseline hepatic steatosis on LVMI change: β = -0.13, s.e. = 0.21, P = 0.54).
To the best of our knowledge, the present study is the first to investigate the association between hepatic steatosis and change in LVMI and the mediating role of systolic and diastolic blood pressure in this association using data from a prospective population-based cohort. While we observed relevant baseline associations between hepatic steatosis, blood pressure and LVMI, these associations were not relevant in the prediction of LVMI change. Our analyses suggest that hepatic steatosis is no predictor of relevance for LVMI change over time.
Previously, only four studies addressed the association between hepatic steatosis and left ventricular morphology[1,4-6]. These studies were of cross-sectional design, used data from small and inhomogeneous samples of patients and yielded conflicting results. The findings of the present study are in good agreement with results from the case-control study by Goland et al[1] demonstrating normotensive patients with hepatic steatosis to have larger intraventricular septum and posterior wall thickness and larger LVM than controls. In our study, LVM at baseline was 181.8 g in individuals fulfilling no or one criterion for hepatic steatosis and 215.8 g in individuals with hepatic steatosis. LVH was present in 45.1% of the individuals fulfilling no or only one criterion for hepatic steatosis and in 59.4% of the individuals with hepatic steatosis. Larger differences were found in the study by Mantovani et al[5] analyzing data from hypertensive, diabetic patients with hepatic steatosis. In that study, 82% of the patients with hepatic steatosis had LVH, while the proportion was 18% in patients without hepatic steatosis. Furthermore, patients with hepatic steatosis yielded 6-fold higher odds ratios for LVH compared to patients without hepatic steatosis. In contrast to the cross-sectional findings of our study, Bonapace et al[6] demonstrated no significant differences between patients with hepatic steatosis and patients without hepatic steatosis regarding left ventricular mass. Fallo et al[4] reported a comparable prevalence of LVH in patients with and without hepatic steatosis. However, that study was performed in hypertensive inpatients, in which a high prevalence of both FLD and LVH has been reported[4,22,23]. Therefore, the reported results cannot be directly compared with results from a general population sample.
Regarding longitudinal associations, we only found negligible direct effects of baseline hepatic steatosis on LVMI change. We hypothesized that blood pressure is a mediating factor involved in the pathway from hepatic steatosis to LVMI as blood pressure has been found to be a major risk factor for left ventricular remodelling[24,25]. Yet, we failed to demonstrate indirect effects from hepatic steatosis on LVMI change via systolic and diastolic blood pressure. Interestingly, we observed an inverse association between systolic blood pressure at baseline and change in LVMI after five years. This finding is in contrast to previous studies revealing that both systolic and diastolic blood pressure are important correlates of LVM, whereas systolic blood pressure has been found to be more closely related to LVM than diastolic blood pressure[26]. Our data showed a drop in systolic and diastolic blood pressure from baseline to follow-up in the study sample, whereas this drop was more pronounced in individuals with hepatic steatosis than in individuals fulfilling no or one criterion for hepatic steatosis. We suppose that information on high blood pressure given by study physicians after baseline examination may have led to lifestyle modification or a rise in health consciousness in the study participants including the intake of blood pressure-lowering medication resulting in lower blood pressure at follow-up examination.
Regarding pharmacological interventions, treatment with antihypertensive drugs is indicated in the management of patients with cardiac hypertrophy, whereas the validity of data regarding the effects of antihypertensive medication on LVH regression is limited due to methodological weaknesses of existing studies[27]. Drugs acting on the renin-angiotensin system, beta blockers, and calcium channel blockers have been shown to diminish left ventricular mass with different efficacy[28]. In the present study population, 20.3% of the individuals with hepatic steatosis reported the intake of beta blockers, 14.6% the intake of calcium channel blockers and 20.3% the intake of drugs acting on the renin-angiotensin system. In addition to blood pressure lowering effects, these drugs may lead to LVMI regression[29]. It might be assumed that the observed decrease in blood pressure in the present sample was attended by LVMI regression covering a potentially present association between hepatic steatosis and LVMI. Repeating our analyses after excluding individuals taking beta blockers, calcium channel blockers, and drugs acting on the renin-angiotensin system confirmed our results in general. This finding indicates that the use of the respective medication did not have an influence on the association between hepatic steatosis and LVMI in the entire population as these drugs may prevent further increase of LVM or support regression of LVH[30,31].
Besides pharmacological treatment, lifestyle modification including weight loss and a reduction of alcohol and salt intake may contribute to LVH regression[29]. The role of physical activity remains controversial. It has been demonstrated that regular physical activity is associated with lower blood pressure and reduced cardiac remodeling, while exercise can also lead to the development of LVH[32]. In hypertensive individuals, exercise may have a positive effect on cardiac remodelling with regression or prevention of LVH[32].
With respect to alcohol consumption, analyses after excluding participants with high risk drinking did not change the results of our study. We therefore assume that alcohol consumption had no major role in the association between hepatic steatosis and LVMI. However, it needs to be considered that the number of individuals with high risk drinking was low and drinking above recommended levels is a risk factor for both hepatic steatosis and changes in cardiac structure.
In the present general population sample, both hepatic steatosis and LVH were highly prevalent stressing the public health relevance of these disease conditions in the general population.
Our study has several strengths, but also potential limitations that should be considered. Major strengths encompass the population-based longitudinal design, the large sample size and the high prevalence of hepatic steatosis and LVH in the study region[13,33]. Further strengths encompass the ultrasound and laboratory methods to detect hepatic steatosis and the strict quality management by standardized protocols and certified staff[9]. Limitations may arise from the inability to perform liver biopsy due to ethical concerns although known as the gold standard in the diagnosis of hepatic steatosis. Regarding methodological issues, path analyses allow for a useful differentiation of direct and indirect effects and therefore improve the interpretation of relationships among multiple variables. Limitations comprise potential selection bias due to selective drop out and initial non-response. However, previous analyses do not suggest a major effect on the outcomes under study[10,34]. More measurement points covering a larger time interval might be needed to improve our inferences on direct and indirect effects. Limitations may further arise from the inability to perform liver biopsy due to ethical concerns although known as the gold standard in the diagnosis of hepatic steatosis.
We conclude that hepatic steatosis as defined by liver hyperechogenity and increased ALT levels was not a predictor of relevance for LVMI change after five years in the present population-based cohort of individuals aged 45 to 81 years. Nevertheless, both hepatic steatosis and LVH were highly prevalent in the present indicating the importance of both disease conditions in the general population and the necessity for risk factor reduction to avoid subsequent morbidity and mortality.
Hepatic steatosis is highly prevalent in Western countries and regarded as the hepatic manifestation of the metabolic syndrome. The metabolic syndrome and its components such as overweight and hypertension are associated with an increase in left ventricular mass (LVM). Data on the association between hepatic steatosis and LVM are limited; only four cross-sectional studies of small sample size exist addressing this relationship. Due to the design of the aforementioned studies, inferences about effect directions between hepatic steatosis and left ventricular remodelling cannot be made. In particular, there is no differentiation between direct paths from hepatic steatosis to LVM progression or indirect effects via mediators.
There is no previous research providing data on the association between hepatic steatosis and left ventricular mass index (LVMI) encompassing the following criteria: (1) using a general population sample; (2) using longitudinal data to improve inferences on the direction of effects; and (3) using methods to differentiate between direct and indirect pathways of hepatic steatosis on LVMI via blood pressure.
The present study is the first to investigate the association between hepatic steatosis and change in LVMI and the mediating role of systolic and diastolic blood pressure in this association using data from a prospective population-based cohort.
The authors conclude that hepatic steatosis as defined by liver hyperechogenity and increased ALT levels was not a predictor of relevance for LVMI change after five years in the present population-based cohort of individuals aged 45 to 81 years. Nevertheless, both hepatic steatosis and LVH were highly prevalent in the present study population indicating the importance of both disease conditions in the general population and the necessity for risk factor reduction to avoid subsequent morbidity and mortality.
This is an interesting and well-written manuscript. This study investigated the association between hepatic steatosis and change in LVMI over 5 years in a study population of 1298 individuals aged 45 to 81 years. Hepatic steatosis was demonstrated to be a significant predictor for all measured echocardiographic characteristics at baseline but not for LVMI change.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Chuang WL, Losano G, Marzuillo P, Vespasiani-Gentilucci U S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Goland S, Shimoni S, Zornitzki T, Knobler H, Azoulai O, Lutaty G, Melzer E, Orr A, Caspi A, Malnick S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol. 2006;40:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Kotsis V, Stabouli S, Toumanidis S, Tsivgoulis G, Rizos Z, Trakateli C, Zakopoulos N, Sion M. Obesity and daytime pulse pressure are predictors of left ventricular hypertrophy in true normotensive individuals. J Hypertens. 2010;28:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Fallo F, Dalla Pozza A, Sonino N, Lupia M, Tona F, Federspil G, Ermani M, Catena C, Soardo G, Di Piazza L. Non-alcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in essential hypertension. Nutr Metab Cardiovasc Dis. 2009;19:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Mantovani A, Zoppini G, Targher G, Golia G, Bonora E. Non-alcoholic fatty liver disease is independently associated with left ventricular hypertrophy in hypertensive Type 2 diabetic individuals. J Endocrinol Invest. 2012;35:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 511] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4052] [Cited by in RCA: 3988] [Article Influence: 113.9] [Reference Citation Analysis (1)] |
| 9. | Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40:294-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 771] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 10. | Haring R, Alte D, Völzke H, Sauer S, Wallaschofski H, John U, Schmidt CO. Extended recruitment efforts minimize attrition but not necessarily bias. J Clin Epidemiol. 2009;62:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Bühringer G, Augustin R, Bergmann E, Bloomfield K, Funk W, B . J. Alkoholkonsum und Alkoholbezogene Störungen in Deutschland [Alcohol consumption and alcoholrelated problems in Germany]. Baden-Baden: Nomos Verlagsgesellschaft 2000; . |
| 12. | Lüdemann J, Piek M, Wood WG, Meyer S, Greiner B, John U, Hense HW. [Methods for quality assurance of medical examination in epidemiological field studies: the "Study of Health in Pomerania" (SHIP)]. Gesundheitswesen. 2000;62:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848-1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 174] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (11)] |
| 14. | Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol. 2001;35:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Völzke H, Schwarz S, Baumeister SE, Wallaschofski H, Schwahn C, Grabe HJ, Kohlmann T, John U, Dören M. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Baumeister SE, Völzke H, Marschall P, John U, Schmidt CO, Flessa S, Alte D. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4462] [Cited by in RCA: 4708] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 18. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8282] [Cited by in RCA: 8800] [Article Influence: 463.2] [Reference Citation Analysis (0)] |
| 19. | Abs R, Bengtsson BA, Hernberg-Stâhl E, Monson JP, Tauber JP, Wilton P, Wüster C. GH replacement in 1034 growth hormone deficient hypopituitary adults: demographic and clinical characteristics, dosing and safety. Clin Endocrinol (Oxf). 1999;50:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 165] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Hu L-tB, Peter M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Structural Equation Modeling. 1999;6:1-55. [DOI] [Full Text] |
| 21. | Yu CY. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models With Binary and Continuous Outcomes. Doctoral Thesis. Los Angeles: University of California 2002; . |
| 22. | Donati G, Stagni B, Piscaglia F, Venturoli N, Morselli-Labate AM, Rasciti L, Bolondi L. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut. 2004;53:1020-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Korner PI, Jennings GL. Assessment of prevalence of left ventricular hypertrophy in hypertension. J Hypertens. 1998;16:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 565] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Schirmer H, Lunde P, Rasmussen K. Prevalence of left ventricular hypertrophy in a general population; The Tromsø Study. Eur Heart J. 1999;20:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Verdecchia P, Angeli F, Gattobigio R, Guerrieri M, Benemio G, Porcellati C. Does the reduction in systolic blood pressure alone explain the regression of left ventricular hypertrophy? J Hum Hypertens. 2004;18 Suppl 2:S23-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Cuspidi C, Esposito A, Negri F, Sala C, Masaidi M, Giudici V, Zanchetti A, Mancia G. Studies on left ventricular hypertrophy regression in arterial hypertension: a clear message for the clinician? Am J Hypertens. 2008;21:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Frohlich ED, González A, Díez J. Hypertensive left ventricular hypertrophy risk: beyond adaptive cardiomyocytic hypertrophy. J Hypertens. 2011;29:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Sheridan DJ, Kingsbury MP, Flores NA. Regression of left ventricular hypertrophy; what are appropriate therapeutic objectives? Br J Clin Pharmacol. 1999;47:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Ruggenenti P, Iliev I, Costa GM, Parvanova A, Perna A, Giuliano GA, Motterlini N, Ene-Iordache B, Remuzzi G. Preventing left ventricular hypertrophy by ACE inhibition in hypertensive patients with type 2 diabetes: a prespecified analysis of the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT). Diabetes Care. 2008;31:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Trimarco B, De Luca N, Cuocolo A, Ricciardelli B, Rosiello G, Lembo G, Volpe M. Beta blockers and left ventricular hypertrophy in hypertension. Am Heart J. 1987;114:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Hegde SM, Solomon SD. Influence of Physical Activity on Hypertension and Cardiac Structure and Function. Curr Hypertens Rep. 2015;17:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Völzke H, Stritzke J, Kuch B, Schmidt CO, Lüdemann J, Döring A, Schunkert H, Hense HW. Regional differences in the prevalence of left ventricular hypertrophy within Germany. Eur J Cardiovasc Prev Rehabil. 2009;16:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Schmidt CO, Alte D, Völzke H, Sauer S, Friedrich N, Valliant R. Partial misspecification of survey design features sufficed to severely bias estimates of health-relateiid outcomes. J Clin Epidemiol. 2011;64:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |