Published online Jul 8, 2017. doi: 10.4254/wjh.v9.i19.850
Peer-review started: March 15, 2017
First decision: April 14, 2017
Revised: May 8, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: July 8, 2017
Processing time: 112 Days and 9.2 Hours
To establish if serial Hepascore tests (referred to as delta Hepascore) in those with chronic hepatitis C (CHC) correlate with the increase and/or decrease in risk of liver related complications.
Three hundred and forty-six CHC patients who had two Hepascore tests performed were studied. During 1944 patient years follow-up 28 (8.1%) reached an endpoint. The Hepascore is a serum test that provides clinically useful data regarding the stage of liver fibrosis and subsequent clinical outcomes in chronic liver disease.
Patients with a baseline Hepascore > 0.75 had a significantly increased rate of reaching a composite endpoint consisting of hepatocellular carcinoma, liver death, and/or decompensation (P < 0.001). In those with an initial Hepascore > 0.75, a subsequent improved Hepascore showed a significantly decreased risk for the composite endpoint (P = 0.004). There were no negative outcomes in those with a stable or improved delta Hepascore. The minimum time between tests that was found to give a statically significant result was in those greater than one year (P = 0.03).
In conclusion, Hepascore is an accurate predictor of liver related mortality and liver related morbidity in CHC patients. Of note, we have found that there is a decreased risk of mortality and morbidity in CHC patients when the patient has an improving delta Hepascore. Repeat Hepascore tests, when performed at a minimum one-year interval, may be of value in routine clinical practice to predict liver related clinical outcomes and to guide patient management.
Core tip: The growing burden of hepatitis C is well recognized. The use of serum fibrosis markers such as Hepascore to monitor change in clinical risk in hepatitis C has a significant potential benefit to optimise the management in these patients. However, there is no information on the value of serial serum fibrosis tests and their improvement over time in determining changes in liver related clinical outcomes. We have found that there is a decreased risk of mortality and morbidity in chronic hepatitis C patients when the patient has an improving delta Hepascore, and serial tests may be of use in clinical practice.
- Citation: Jeffrey AW, Huang Y, de Boer WB, Adams LA, MacQuillan G, Speers D, Joseph J, Jeffrey GP. Improved Hepascore in hepatitis C predicts reversal in risk of adverse outcome. World J Hepatol 2017; 9(19): 850-856
- URL: https://www.wjgnet.com/1948-5182/full/v9/i19/850.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i19.850
The use of direct acting antivirals (DAA) therapy in chronic hepatitis C (CHC) treatment has resulted in up to 99% eradication of hepatitis C virus (HCV) in patients receiving treatment, depending on the genotype and type of DAA used[1,2]. The increased efficacy and minimal side effects of newer DAA’s means that many more patients will access therapy, if financially able. To this end, in March 2016 the Pharmaceutical Benefits Scheme (PBS) in Australia listed sofosbuvir, ledipasvir/sofosbuvir and daclatasvir for the treatment with CHC (4) which will provide access to treatment for all Australians. It is estimated that there will be a 93% reduction in advanced liver disease cases due to the new DAA therapies compared to current regimens or no treatment[3]. HCV eradication has been shown to reduce liver fibrosis and liver related complications but the time required for this reversal is not known[2,4,5]. In addition, other co-factors such as NAFLD and alcohol use may be present and prevent or impair reversal of hepatic fibrosis. Therefore the problem remains that CHC patients with significant or advanced liver fibrosis at the time of successful HCV eradication may require long term monitoring for liver related complications for an uncertain period of time[6].
Fibrosis severity is currently measured non-invasively using serum fibrosis markers or transient elastography (Fibroscan®). The histopathological staging of fibrosis using liver biopsy has historically been the best predictor of liver related mortality and liver related morbidity associated with CHC[7]. However liver biopsy is now rarely used to stage CHC patients due to the risk of serious complications and issues with sampling error[8]. Several non-invasive serum fibrosis markers have been developed and are currently used as non-invasive alternatives to liver biopsy. Recent advances have now demonstrated that some serum fibrosis markers are able to directly predict adverse liver related outcomes rather than just provide a surrogate marker of liver fibrosis[9]. Hepascore is one of these markers, and it is used to predict liver related complications in patients with CHC. Hepascore has also been shown to be comparable to liver biopsy[10-12]. The Hepascore result itself ranges from 0 to 1.0 with a lower value indicating less severe or absent liver fibrosis and consequently better liver related clinical outcomes[10]. Measurement of the change in severity of liver fibrosis over time is also a strong prognostic tool in CHC[7]. The use of non-invasive serum fibrosis markers to monitor regression/progression of fibrosis in CHC has a significant potential benefit to optimise the clinical management in these patients. However, there is no information on the value of serial serum fibrosis tests and their change over time in determining changes in liver related clinical outcomes.
This aim of this study is to establish if serial Hepascore tests (referred to as delta Hepascore) in those with CHC correlate with the increase and/or decrease in risk of liver related complications.
Hepatitis C patients who presented to Sir Charles Gairdner Hospital (SCGH) based in Western Australia from 1992 to 2012 and who also had two Hepascore tests performed were studied. We defined our inclusion criteria as all patients with hepatitis C, both treated and untreated. We also included patients regardless of if they achieved a sustained virological response (SVR). Our exclusion criteria consisted those with co-existing hepatitis B infection, human immunodeficiency virus as well as any other liver diseases. We also excluded patients who had received a previous liver transplantation. We received ethics approval for this study from the Department of Health Ethics Committee and the SCGH Ethics Committee.
Baseline and second Hepascore test dates and results were collected for each patient. The WA based Data Linkage System, called WADLS was used to collect long term patient morbidity and mortality figures[13]. This is a wide scale population based linkage system that has been used extensively in the past and validated in previous population and cohort studies[14,15]. The WADLS contains records of cancer registrations as well as in-patient hospital morbidity and death records of the Western Australian population, from 1966 to the present. For this study, the events collected were all-cause mortality, liver related mortality, liver related morbidity and cancer registration. The WADLS database has previously been used as part of published and validated studies on liver fibrosis assessment and use of other non-invasive markers including Hepascore[10,11].
The primary endpoint for this study was liver related death (LRD) or liver transplantation. Secondary endpoints included onset of hepatocellular carcinoma (HCC) and liver decompensation (LD) of all causes. A composite endpoint included all of these endpoints but patients were only included once. The follow-up time used for the analysis of the baseline Hepascore test was from the time of the test until a primary or secondary endpoint or the conclusion of the study. The follow-up time used for the analysis of delta Hepascore was from the time of the second Hepascore test until an end point or end of study was reached. Delta Hepascore was calculated as the second Hepascore minus the baseline Hepascore. Patients who reached an endpoint before the second Hepascore test were excluded from delta Hepascore analysis. Hepascore is a serum marker that incorporates gamma glutamyl transpeptidase, hyaluronic acid and alpha 2 macroglobulin.
Statistics were undertaken using the SPSS Statistics software package and Kaplan-Meier survival analysis. Multivariate cox regression was used to assess the prognostic significance of an initial Hepascore, second Hepascore, and delta Hepascore to predict LRD, HCC or LD. Significance was defined as P < 0.05. Patients were placed into groups based on the baseline Hepascore value (0-0.25, 0.26-0.5, 0.51-0.75, 0.76-1.0) and the delta Hepascore (delta < -0.1, -0.1 ≤ delta ≤ 0.1, delta > 0.1) for the analysis. Survival probabilities for using baseline Hepascore values and delta Hepascore values were then calculated using Kaplan-Meier curves with significance calculated using the log rank test.
Area under Receiver Operating Characteristic (AUROC) curves were calculated to assess the capacity of baseline Hepascore and delta Hepascore values to predict liver related outcomes. The optimal time interval between Hepascore tests was assessed by Kaplan-Meier analysis according to the time between tests: < 1 year and ≥ 1 year.
A total of 346 patients met the inclusion criteria and were followed for a mean of 5.5 years, during which 28 (8.1%) had a LRD, developed LD and/or HCC (Table 1). The mean age of the cohort was 53.6 years and 220 (63.6%) were male. Of the total cohort, 8 (2.3%) had a LRD, 15 (4.6%) developed LD and 16 (4.3%) developed HCC. The mean baseline and second Hepascore values were 0.48 (SD ± 0.34) and 0.57 (SD ± 0.34) respectively and the mean delta Hepascore was 0.09 (SD ± 0.23). The time between Hepascore tests ranged from 0.03 and 12.5 years, with a mean of 3.3 and the mean follow-up time after the second Hepascore was 2.4 years. Multivariate cox regression showed that baseline Hepascore and delta Hepascore were independently predictive of reaching a composite clinical endpoint (LRD, HCC or LD), with P values of 0.02 and 0.013 respectively (Table 2).
| Characteristic | All patients | Patients with first Hepascore > 0.75 | All patients | Patients with first Hepascore > 0.75 | ||||
| Number | Percent | Number | Percent | mean | Range | mean | Range | |
| Number | 346 | - | 100 | - | - | - | - | - |
| Gender (male) | 220 | 63.6 | 76 | 76 | - | - | - | - |
| SVR | 38 | 11.0 | 16 | 16 | - | - | - | - |
| Composite endpoint | 28 | 8.1 | 21 | 21 | - | - | - | - |
| LRD | 8 | 2.3 | 8 | 8 | - | - | - | - |
| LD | 16 | 4.6 | 12 | 12 | - | - | - | - |
| HCC | 15 | 4.3 | 12 | 12 | - | - | - | - |
| Result | - | - | - | - | ||||
| Bilirubin (μmol/L)1 | - | - | - | - | 9.0 | 1.0-200 | 12 | 2.3-200 |
| GGT (U/L)1 | - | - | - | - | 55.0 | 8.0-1005 | 93.5 | 17-713 |
| HA (μg/L)1 | - | - | - | - | 30.3 | 1.0-1211 | 124.5 | 16-1211 |
| A2M (μg/mL)1 | - | - | - | - | 2.5 | 0.6-6 | 3.6 | 1.5-6.0 |
| Age (yr) | - | - | - | - | 53.6 | 30-80 | 58.3 | 36-80 |
| Baseline Hepascore | - | - | - | - | 0.48 | 0.02-1.0 | 0.93 | 0.77-1.0 |
| Second Hepascore | - | - | - | - | 0.57 | 0.04-1.0 | 0.87 | 0.13-1.0 |
| Delta Hepascore | - | - | - | - | 0.09 | -0.80-0.94 | -0.06 | -0.8-0.23 |
| Time between baseline and second Hepascore (yr) | - | - | - | - | 3.3 | 0.03-12.5 | 2.8 | 0.03-10.3 |
| Follow-up after second Hepascore (yr) | - | - | - | - | 2.2 | 0.01-7.3 | 1.9 | 0.01-5.7 |
| Variable | Follow-up from the baseline Hepascore | Follow-up from the second Hepascore | ||
| P | Hazard ratio (95%CI) | P | Hazard ratio (95%CI) | |
| Baseline Hepascore | < 0.001 | 5.85 (2.25-15.18) | 0.020 | 12.86 (1.49-111.17) |
| Second Hepascore | - | - | 0.891 | 3288.82 (0.0-4.6E + 53) |
| Delta Hepascore | - | - | 0.013 | 4.77 (1.35-16.45) |
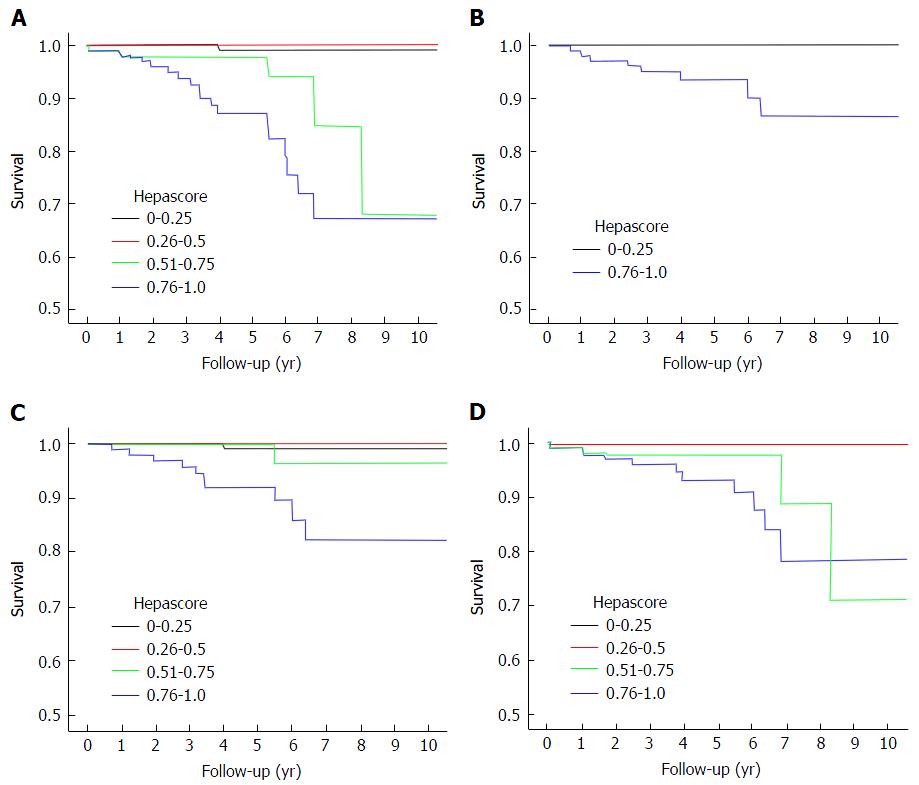
Patients were grouped into 4 categories according to their baseline Hepascore (0-0.25, 0.26-0.5, 0.51-0.75 and 0.76-1.0). One hundred and twenty-nine (37%) had a Hepascore ≤ 0.25, 73 (21%) had a Hepascore from 0.26 to 0.5, 43 (12%) had a Hepascore from 0.51 to 0.75 and 100 (29%) had a Hepascore > 0.75. Kaplan-Meier survival curve analysis found that those patients with a baseline Hepascore > 0.75 had a significantly increased rate of LRD (n ≤ 0.001), HCC (n ≤ 0.001), LD (n ≤ 0.001) and composite endpoint (P < 0.001) (Table 3 and Figure 1). Hazard ratios could not be calculated because of the lack of adverse liver related outcomes in the other three lower value Hepascore groups.
| Test | End point | P value (log rank) | Cohort size |
| Baseline Hepascore alone | Composite Endpoint | < 0.001 | 346 |
| LRD | < 0.001 | 352 | |
| LD | < 0.001 | 348 | |
| HCC | < 0.001 | 350 | |
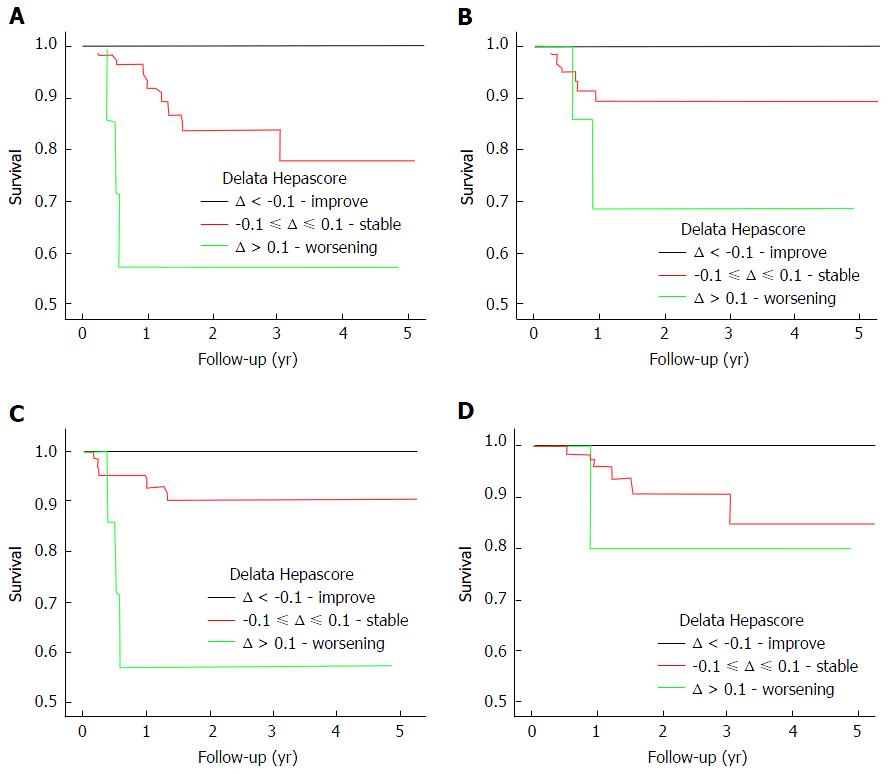
| Delta Hepascore | Composite Endpoint | 0.004 | 96 |
| LRD | 0.048 | 105 | |
| LD | 0.001 | 101 | |
| HCC | 0.178 | 100 |
Patients with a baseline Hepascore > 0.75 were then analysed using the delta Hepascore value. The delta Hepascore values were divided into those with an improved Hepascore (delta < -0.1), a stable Hepascore (-0.1 ≤ delta ≤ 0.1) and a worsened Hepascore (delta > 0.1). Survival curve analysis found that in those with an improved Hepascore there was a significantly decreased risk of LRD, LD and a composite endpoint (P = 0.048, P = 0.001, P = 0.004 respectively) as shown in Figure 2. Twelve (17%) patients with a stable or worsened Hepascore reached a composite end point in contrast with those patients who had an improved Hepascore, who had no negative outcomes. Comparison between those patients with a stable Hepascore and those with a worse Hepascore was not possible as 19.5% of patients had a baseline Hepascore value > 0.9 (the maximum Hepascore value is limited to 1.0). Thirty-eight (11%) patients had anti-viral treatment and reached a SVR. Of those achieving SVR only 4 patients reached an endpoint. Excluding these patients from the analysis made no difference to the results.
AUROC analysis was performed using the baseline Hepascore alone and with a combination of the baseline Hepascore and delta Hepascore (Table 4). There was a marked improvement in the AUROC for the combined baseline and delta Hepascore values compared to baseline Hepascore values alone with an AUROC for LRD of 0.95 and 0.89, for LD of 0.77 and 0.75 and for HCC of 0.93 and 0.87, respectively (Table 4).
| Test | End point | AUROC |
| Baseline Hepascore alone | Composite endpoint | 0.80 |
| LRD | 0.89 | |
| LD | 0.75 | |
| HCC | 0.87 | |
| Baseline Hepascore > 0.75 and Delta Hepascore | Composite endpoint | 0.84 |
| LRD | 0.95 | |
| LD | 0.77 | |
| HCC | 0.93 |
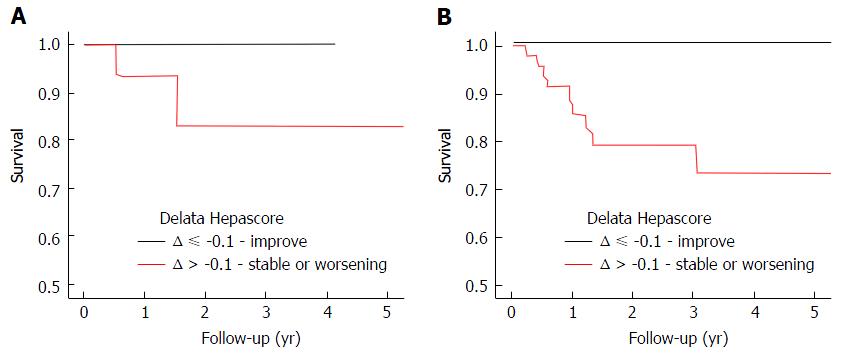
Sub-group analysis was then completed to determine the minimum time required between Hepascore tests to determine delta Hepascore. Survival curve analysis found that in those patients with a baseline Hepascore > 0.75, delta Hepascore is only predictive of a composite endpoint if the time between Hepascore tests is more than one year (P = 0.03) (Figure 3).
No previous studies have reported the use of repeated non-invasive serum fibrosis markers to predict improved liver related clinical outcomes. In this well documented cohort of CHC patients with a long follow-up period, 8.1% had an adverse liver related outcome after a mean of 5.5 years of follow-up. Cox regression found that a high (> 0.75) baseline Hepascore value was independently associated with increased rates of adverse liver related outcomes (P < 0.001), consistent with previous reports[11,12]. Importantly the delta Hepascore was also independently associated with predicting a composite clinical endpoint (LRD, HCC, LD) (P = 0.004). The AUROC for predicting the composite end point using the initial Hepascore and delta Hepascore was 0.84, which was increased compared to the AUROC using Hepascore alone (0.80).
Patients with an initial Hepascore value greater than 0.75 had an increased risk of developing an adverse liver related end point and this equated to a 5-year risk of 10% and a 10-year risk of 35%. CHC patients with an initial Hepascore less than or equal to 0.75 had a negligible (%) risk for developing these complications over 10 years. Further analysis found that in patients with a baseline Hepascore greater than 0.75 and who had a subsequent improvement in their second Hepascore of more than 0.1 (delta < -0.1), no adverse liver related end points occurred after a mean of 2.5 years. In contrast, those CHC patients with an initial Hepascore greater than 0.75 and who had a stable or worsened delta Hepascore there was an increased risk of experiencing an adverse liver related outcome. Hepascore has a range of values from 0 to 1.0, therefore only those patients with a baseline Hepascore below 0.9 could have an increased delta Hepascore (delta > 0.1) on subsequent testing. This limited the value of sub-group analysis comparing worsening (delta > 0.1) or stable (-0.1 ≤ delta ≤ 0.1) delta Hepascore values in those with an initial Hepascore greater than 0.75.
The minimum time interval between Hepascore tests that resulted in useful clinical information was one year. Only when the Hepascore test interval was one year or more was there a significant association between delta Hepascore and the risk of adverse liver related outcomes (P = 0.03). Our findings show that there is a reduced risk of negative outcome in CHC patients who have an initial Hepascore over 0.75, but have an improved delta Hepascore, and will potentially allow a change in clinical management whereby the need for surveillance for varices and hepatocellular cancer may be reduced.
This study has some limitations. Firstly, due to the retrospective nature of this study, the second Hepascore test was not performed after a fixed time period. This time period was sufficient to demonstrate variation in delta Hepascore, however a fixed follow-up period could be established for future research. Secondly, the data linkage system, which has allowed the collection of comprehensive data from a central source did not include information on alcohol consumption, diet and exercise. However, we believe that this data would not impact on the results of this study.
In conclusion, Hepascore is an accurate predictor of liver-related mortality and morbidity in CHC patients. Of note, we have found that there is a decreased risk of mortality and morbidity in CHC patients when the patient has an improving delta Hepascore. Repeat Hepascore tests, when performed at a minimum one-year interval, may be of value in routine clinical practice to predict liver related clinical outcomes and to guide patient management.
Several non-invasive serum fibrosis markers have been developed and are currently used as non-invasive alternatives to liver biopsy. Hepascore is one such marker that is able to predict severity of fibrosis, comparable to liver biopsy. Recent advances have now demonstrated that serum fibrosis markers such as Hepascore are able to directly predict adverse liver related outcomes rather than just provide a surrogate marker of liver fibrosis. Hepascore can also be used to monitor regression/progression of fibrosis in chronic hepatitis C (CHC).
The use of Hepascore to monitor regression/progression of fibrosis in CHC has a significant potential benefit to optimise the clinical management in these patients. However, there is no information on the value of serial serum fibrosis tests and their change over time in determining changes in liver related clinical outcomes.
Hepascore was found to an accurate predictor of liver-related mortality and morbidity in CHC patients. Of note, the authors have found that there is a decreased risk of mortality and morbidity in CHC patients when the patient has an improving delta Hepascore. Repeat Hepascore tests, when performed at a minimum one-year interval, are of value in routine clinical practice to predict liver related clinical outcomes and to guide patient management.
Repeat Hepascore tests, when performed at a minimum one-year interval, may be of value in routine clinical practice to predict liver related clinical outcomes and to guide patient management.
SVR: Sustained viral response. SVR is specific to hepatitis C and is the absence of HCV RNA for 24 wk after the cessation of treatment.
Current study was from the group who originally described Hepascore as a non-invasive marker of fibrosis in patients with chronic hepatitis C. In the current study, the authors use “baseline” Hepascore as a prognostic indicator. In addition, the authors also found the change in Hepascore over time (“Delta Hepascore”) was also a predictor of liver related events or death.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheung R, Hwang SG S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: A meta-analysis. World J Hepatol. 2015;7:806-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis. 2015;15:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2015;41:544-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Akhtar E, Manne V, Saab S. Cirrhosis regression in hepatitis C patients with sustained virological response after antiviral therapy: a meta-analysis. Liver Int. 2015;35:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Arif A, Levine RA, Sanderson SO, Bank L, Velu RP, Shah A, Mahl TC, Gregory DH. Regression of Fibrosis in Chronic Hepatitis C After Therapy with Interferon and Ribavirin. Dig Dis Sci. 2003;48:1425-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Bunchorntavakul C, Reddy KR. Treat chronic hepatitis C virus infection in decompensated cirrhosis - pre- or post-liver transplantation? the ironic conundrum in the era of effective and well-tolerated therapy. J Viral Hepat. 2016;23:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The Lancet. 1997;349:825-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 8. | Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Huang Y, Adams LA, MacQuillan G, Speers D, Joseph J, Bulsara MK, Jeffrey GP. Serum models accurately predict liver-related clinical outcomes in chronic hepatitis C. J Gastroenterol Hepatol. 2016;31:1736-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Chinnaratha MA, Jeffrey GP, MacQuillan G, Rossi E, de Boer BW, Speers DJ, Adams LA. Prediction of morbidity and mortality in patients with chronic hepatitis C by non-invasive liver fibrosis models. Liver Int. 2014;34:720-727. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 12. | Huang Y, Adams LA, Joseph J, Bulsara MK, Jeffrey GP. The ability of Hepascore to predict liver fibrosis in chronic liver disease: a meta-analysis. Liver Int. 2016;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Holman CD, Bass AJ, Rouse IL, Hobbs MS. Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust N Z J Public Health. 1999;23:453-459. [PubMed] |
| 14. | Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346:f2539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 579] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Holman CD, Price SD, Sanfilippo FM, Preen DB, Bulsara MK. Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: retrospective cohort study. BMJ. 2009;338:a2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |