Published online Jun 18, 2017. doi: 10.4254/wjh.v9.i17.791
Peer-review started: December 29, 2016
First decision: February 4, 2017
Revised: March 6, 2017
Accepted: April 23, 2017
Article in press: April 24, 2017
Published online: June 18, 2017
Processing time: 167 Days and 18.8 Hours
To evaluate the performance of aspartate aminotransferase to platelet ratio (APRI) score against FibroScan in predicting the presence of fibrosis.
Data of patients who concurrently had APRI score, FibroScan and liver biopsy to assess their hepatitis C virus (HCV) and hepatitis B virus (HBV) over 6 years were retrospectively reviewed and details of their disease characteristics and demographics were recorded. Advanced fibrosis was defined as ≥ F3.
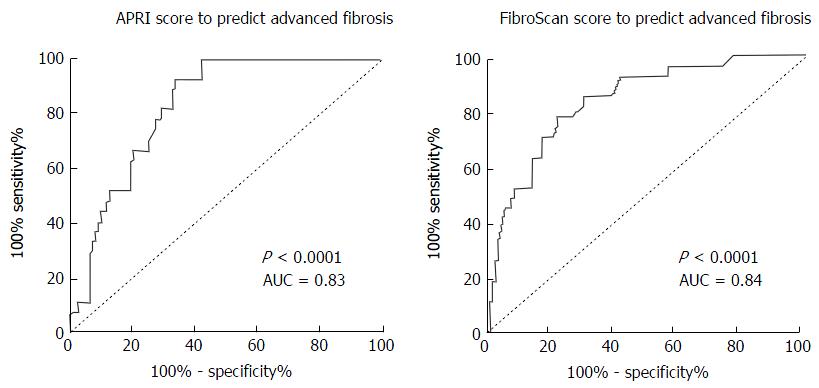
Of the 3619 patients (47.5 ± 11.3 years, 97M:36F) who had FibroScans and APRI for HCV and HBV, 133 had concurrent liver biopsy. Advanced liver fibrosis was found in 27/133 (20%, F3 = 21 and F4 = 6) patients. Although APRI score (P < 0.001, AUC = 0.83) and FibroScan (P < 0.001, AUC = 0.84) predicted the presence of advanced fibrosis, the sensitivities and specificities were only modest (APRI score: 51.9% sensitivity, 84.9% specificity; FibroScan: 63% sensitivity, 84% specificity). Whilst 13/27 (48%) patients with advanced fibrosis had APRI ≤ 1.0, no patients with APRI ≤ 0.5 had advanced fibrosis, with 100% sensitivity. The use of APRI ≤ 0.5 would avoid the need for FibroScan in 43% of patients.
APRI score and FibroScan performed equally well in predicting advanced fibrosis. A proposed APRI cut-off score of 0.5 could be used as a screening tool for FibroScan, as cut-off score of 1.0 will miss up to 48% of patients with advanced fibrosis. Further prospective validation studies are required to confirm this finding.
Core tip: This is the first study to show that an aspartate aminotransferase to platelet ratio (APRI) score of 0.5 could potentially be used as a screening tool to predict the need for FibroScan in patients with hepatitis C or hepatitis B. Our study showed that an APRI score of 0.5 could reduce the need for FibroScan in 43% of the study cohort with high sensitivity.
- Citation: Wong S, Huynh D, Zhang F, Nguyen NQ. Use of aspartate aminotransferase to platelet ratio to reduce the need for FibroScan in the evaluation of liver fibrosis. World J Hepatol 2017; 9(17): 791-796
- URL: https://www.wjgnet.com/1948-5182/full/v9/i17/791.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i17.791
Chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) are among the most common causes of liver fibrosis[1]. A determination of the degree of liver fibrosis in these patients is essential to guide management as well as for prognostication[2-6]. Liver biopsy has long been considered the gold standard for assessment of liver fibrosis[2,7,8]. However, liver biopsy is an invasive procedure that carries a 0.3%-0.6% overall risk for complications and a 0.05% mortality rate[8,9]. Several contraindications also exist which may preclude patients from having a liver biopsy, namely coagulopathy[4]. As a liver biopsy only samples approximately 1/50000 of the liver, there have been concerns with sampling errors despite an adequate number of portal tracts and sample size[7,10,11]. Intra- and inter-observer variation in histological interpretation has also been reported[12,13]. Given these limitations, much research has been dedicated to evaluating non-invasive methods to determine liver fibrosis[5,9]. Of these, the FibroScan and aspartate aminotransferase (AST) to platelet ratio (APRI) are commonly used in our hospital.
FibroScan is a novel non-invasive method that measures liver stiffness using both ultrasound and low-frequency elastic waves[14]. A recent meta-analysis showed that FibroScan had a good sensitivity, specificity and high accuracy for detecting liver cirrhosis[15]. However, invalid assessments rates have been quoted to range between 2.4% and 9.4%, mainly due to high body mass index[13].
In 2003, Wai et al[2] proposed a novel index APRI with an area under the receiver operating curve (AUROC) for predicting significant fibrosis and cirrhosis 0.80 and 0.89 respectively. A recent meta-analysis showed that an APRI score greater than 1.0 is able to predict cirrhosis with a sensitivity of 76% and a specificity of 72%[16]. This suggests that and APRI score of 1.0 or more would not be an ideal screening tool given it could miss a proportion of patients with cirrhosis. The aim of this study was thus, to evaluate and compare the performance of APRI score against FibroScan in predicting the presence of liver fibrosis and to determine the best APRI cut-off score which can predict the likelihood of fibrosis and the need for further assessment with FibroScan.
A retrospective analysis was performed of all the patients with HCV or HBV, who had been referred for FibroScan to the Department of Gastroenterology and Hepatology in the Royal Adelaide Hospital, the largest tertiary referral hospital in South Australia, between January 2010 and June 2016. Inclusion criteria were infection with either HCV or HBV, a valid FibroScan assessment, a liver biopsy within 12 mo of the FibroScan and an APRI score within 6 mo of the liver biopsy. HCV was defined as a positive HCV RNA and HBV was defined as a positive hepatitis B surface antigen and HBV DNA. Exclusion criteria were the use of the XL probe, current interferon-based treatment, co-infection with human immunodeficiency virus, other causes of chronic liver disease, hepatocellular carcinoma, prior liver transplantation, blood results more than 6 mo before or after the liver biopsy, incomplete FibroScan reports and invalid FibroScan assessments. An invalid FibroScan was defined as an interquartile range of more than 30% and a success rate of less than 60%. The project was approved by The Royal Adelaide Hospital Research Ethics Committee, and all patient data were de-identified (RAH protocol approval number: R20160616).
Detailed data was collected from FibroScan reports and electronic medical records which included age, gender, HCV or HBV, FibroScan results, FibroScan success rate, FibroScan interquartile range, AST level, platelet count and Scheuer fibrosis scores on liver biopsy reports.
The APRI score was calculated using the proposed formula:
APRI = [(AST level/ULN)/platelet count (109/L)] × 100 (2)
The reference value for AST used was 45 IU, which is the upper limit of normal in our laboratory. The FibroScan cut-offs used to define cirrhosis were a median of 14kPa and 12.9 kPa for HCV and HBV respectively. Liver fibrosis based on the Scheuer fibrosis system was either no fibrosis (F0), enlarged, fibrotic portal tracts (F1), periportal or portal-portal septa but intact architecture (F2), fibrosis with architectural distortion but no obvious cirrhosis (F3) or probable or definite cirrhosis (F4)[17,18]. Advanced fibrosis was defined as F3 and F4.
Patient characteristics were expressed as mean ± SD or n (%). Diagnostic performances for FibroScan and APRI score were analysed separately according to sensitivity (Se), specificity (Sp), negative predictive values (NPV), positive predictive values (PPV) and AUROC.
Of the 3619 patients (47.5 ± 11.3 years, 97M:36F) who had FibroScans performed, 133 (3.7%) had either HCV or HBV with concurrent APRI score and liver biopsy assessment. The mean FibroScan score was 11.5 kPa and the mean APRI score was 0.75. The baseline characteristics of the 133 patients are summarized in Table 1. Histological analysis revealed that 25 (18.8%) patients were F0, 42 (31.6%) were F1, 39 (29.3%) were F2, 21 (15.8%) were F3 and 6 (4.5%) were F4. Therefore, advanced fibrosis was found in 27/133 (20%) patients.
| Characteristic | Value |
| Gender | 97M/36F |
| Age (yr) | 47.5 ± 11.3 |
| Indication for FibroScan | |
| HCV | 79 |
| HBV | 54 |
| Mean FibroScan score (kPa) | 11.5 |
| Mean IQR (kPa) | 2.17 |
| Mean success rate | 95.6% |
| Mean APRI score | 0.75 |
| Mean AST level (U/L) | 65.5 |
| Mean platelet count (× 109/L) | 214 |
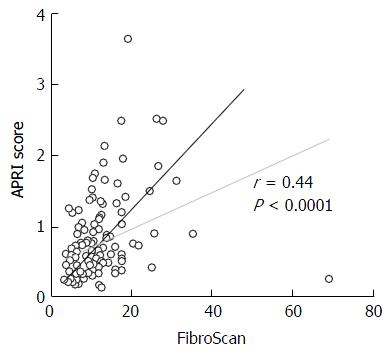
Although both APRI (P < 0.001, AUC = 0.83) and FibroScan (P < 0.001, AUC = 0.84) assessments were able to predict the presence advanced fibrosis (Figure 1), the Se, Sp, NPV and PPV of both APRI and FibroScan were only modest (Table 2). Overall, there was good correlation between the APRI score and FibroScan score (Figure 2).
| APRI | FibroScan | |
| Sensitivity | 51.9% | 63.0% |
| Specificity | 84.9% | 84.0% |
| PPV | 46.7% | 50.0% |
| NPV | 87.4% | 89.9% |
| Accuracy | 78.2% | 79.7% |
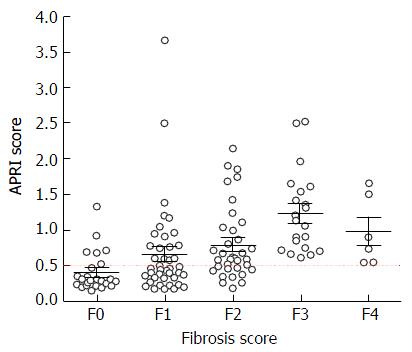
Based on liver biopsy 9/39 (23%) patients with F2, 12/21 (57%) patients with F3 and 2/6 (33%) patients with F4 had an APRI score of 1.0 or more. Thus, the use of APRI score of 1.0 or more to screen for the need for FibroScan would have missed 13/27 (48%) patients with advanced fibrosis (F3 and F4).
In contrast, based on our plot chart (Figure 3), none of the patients with APRI score of 0.5 or less had F3 or F4 on liver biopsy. Using a lower cut-off APRI score of 0.5 would increase the sensitivity to 100%, but reduce the specificity to 59%. More importantly, the use of APRI score of 0.5 or less would avoid the need of FibroScan assessment in 43% of patients with HCV or HBV who were referred for the procedure.
Early and accurate assessment of the degree of liver fibrosis is essential in the management and prognostication of patients with HCV and HBV[2-6]. Given the issues associated with liver biopsy, much research has been dedicated to evaluating non-invasive methods to determine liver fibrosis[5,9]. This study focused on the performance of FibroScan as well as APRI to detect liver fibrosis as these are commonly used in our hospital.
In regards to FibroScan, the AUROC for advanced fibrosis in our study was 0.84. This is comparable to previous studies where the AUROC has ranged between 0.85 to 0.91[3,5,8]. Similarly, the AUROC of 0.83 obtained in the study for APRI was in concordance with previous reports of approximately 0.83 to 0.89[2,3,16]. Overall, our study showed that there was good correlation between FibroScan and APRI in predicting the presence of fibrosis and this is in keeping with results from previous studies[3,8].
There has been an increasing use of FibroScan in our hospital as evident by the growing number done over the past few years; 472 FibroScans in 2013, 612 in 2014 and 761 in 2015. FibroScan is painless, easy to perform and has good patient acceptance[13]. The diagnostic performance is however, influenced by high body mass index[8,13,19]. Thus, the study design excluded patients who required the use of the XL probe.
A recent systematic review looking at the cost-effectiveness of FibroScan compared to liver biopsy showed that FibroScan is economically attractive, but does incur added cost of approximately $1250 to $2922[1]. Apart from the cost, the accessibility of FibroScan may be an issue in the primary health care and resource limited setting. Thus, it would be ideal to have a less expensive, non-invasive method to screen for patients who would need a FibroScan. The APRI score is an appealing tool, particularly in rural areas, given its ease of use and routine availability of the components of the score in all laboratories.
We evaluated the use of APRI score for this purpose and found that: (1) the use of the currently suggested APRI cut-off score of 1.0 or more to screen for FibroScan would have missed 13/27 (48%) patients with advanced fibrosis; and (2) the use of a lower APRI cut-off score of 0.5 will prevent this problem and avoid the need for FibroScan assessment in 43% of patients with HCV or HBV. A recent study detected the F4 cut-off value for APRI to be 0.7[20]. This cut-off would have missed 2/6 (33.3%) F4 patients in our study.
Although the proposed APRI cut-off of 0.5 would miss approximately one-third of patients with significant fibrosis (F2), this proportion would be even higher if the cut-off value of 1.0 is used. With the recent evolution in the treatment of HCV, sustained virological response is achievable in the vast majority of patients. Current guidelines recommend anti-viral therapy for all patients except those with limited life expectancy or clear contraindications[21]. The decision for treatment initiation is no longer guided by fibrosis stage except in situations where there are limitations to universal treatment of all patients, and for guiding the duration of treatment in patients with established cirrhosis. Fibrosis staging, however, remains relevant for prognostication. While the new suggested cut-off may miss patients with F2 fibrosis, it is more critical to identify patients with F3 and F4 patients who require ongoing hepatocellular cancer surveillance and screening/surveillance for varices[21]. Current guidelines do not recommend routine follow- up of patients with F0-F2 fibrosis following successful treatment of HCV, although this decision would be dependent on clinical judgement especially in patients with confounding risk factors for fibrosis progression (obesity, alcohol, etc).
In regards to HBV, the decision to initiate treatment is based on the disease phase (immune tolerant, immune active, immune control or immune escape) and risk of disease progression or liver related complications. This is mostly guided by ALT and HBV DNA level[22]. Liver biopsy or FibroScan is not required for make treatment decision but may be useful in patients who have elevated DNA levels but normal ALT levels[22]. As the nature of chronic hepatitis B is dynamic, it is recommended that all patients undergo serial monitoring. Given the indices for the APRI score are routine laboratory test and will change with disease progression, this should prompt recalculation of the APRI score and re-staging of the disease by FibroScan or liver biopsy if deemed necessary.
The weakness of this study is the relatively small sample size of patients with liver biopsies. While liver biopsy has historically been considered the “gold standard” for assessment of liver fibrosis, it is imperfect with concerns with of sampling error due to patchy distribution of fibrosis, risk of complications and expense. It has now largely been replaced by non-invasive measures of fibrosis as first line/standard of care for fibrosis assessment. Consequently, the volume of liver biopsies performed in our centre and across most centres has fallen dramatically and it would no longer be considered to perform routine liver biopsies in patients with viral hepatitis. In this study, we only included patient with hepatitis B and C and the finding cannot be generalised to patients with other aetiology for their liver disease. Furthermore, differences exist between patients with hepatitis B and hepatitis C which may impact on their APRI score or FibroScan readings. High ALT levels in hepatitis B may lead to overestimation of fibrosis by FibroScan, whilst HCV-associated immune thrombocytopenia may falsely elevate the APRI score[23]. We also acknowledge that this is a retrospective study from a single centre. Intra- and inter-observer variation in histological interpretation was avoided with the use of a single pathologist who specializes in gastrointestinal pathology.
We, therefore, propose that the use of a new cut-off APRI score of 0.5 could potentially be used to predict the need for FibroScan in the evaluation of patients with viral hepatitis, which would result in significant reduction in health care cost and resources.
In the evaluation of patients with HCV or HBV, APRI score and FibroScan performed equally well in predicting advanced fibrosis. The use of APRI ≥ 1.0 to predict the need for FibroScan would miss 48% of patients with advanced fibrosis. In the current study, we found that an APRI cut off score of 0.5 is more reliable than 1.0, and able to predict the presence of advanced fibrosis in 100%. More importantly, the use of APRI score of 0.5 or more as a screening tool for advanced fibrosis can reduce the need for FibroScan in 43%. Larger prospective validation studies are warranted to confirm this finding.
FibroScan is a novel non-invasive method that identifies significant liver fibrosis and cirrhosis. Consequently, its use has greatly increased, posing a demand to the health care system. Aspartate aminotransferase (AST) to platelet ratio index (APRI) is a cheap, blood-test based scoring system that can predict liver fibrosis. Previous study suggested that a score of 1.0 has modest sensitivity and specificity in predicting cirrhosis. This study examined the relationship between the APRI and F-score in predicting advanced fibrosis related to viral hepatitis, and whether it can be used to predict the need of FibroScan.
The focus of this study is to examine the use of APRI score to predict the need for FibroScan assessment, thus, allowing a better stratification of need and demand of FibroScan in a busy hepatology centres.
Using liver biopsy as gold standard, APRI score and FibroScan performed equally well in predicting advanced fibrosis. More important, the current study found that an APRI cut-off score of 0.5 can be used as a screening tool for FibroScan, as the previously proposed cut-off score of 1.0 missed up to 48% of patients with advanced fibrosis. The use of the newer APRI cut-off score of 0.5 resulted in the avoidance of needs for FibroScan assessment in 43% of referred patients.
APRI, therefore, should be routinely used in clinical practice and can be used a guide to perform FibroScan. This practice is likely to be cost-effective and improve the work flow of the FibroScan service.
FibroScan is a novel non-invasive method that measures liver stiffness using both ultrasound and low-frequency elastic waves. AST to platelet ratio index (APRI) {calculated by [(AST level/ULN)/Platelet count (109/L)] × 100} is a scoring system that can predict the presence of advanced fibrosis with good sensitivity and specificity.
The manuscript is a retrospective study evaluated the performance of APRI score against FibroScan in predicting the presence of fibrosis and proposed a new-cut off score of APRI as a screening tool. This study provides a good concept and enhances utilization of APRI score.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Cabibi D, El-Shabrawi MHF, Kanda T S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | van Katwyk S, Coyle D, Cooper C, Pussegoda K, Cameron C, Skidmore B, Brener S, Moher D, Thavorn K. Transient elastography for the diagnosis of liver fibrosis: a systematic review of economic evaluations. Liver Int. 2016;37:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3242] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 3. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 4. | Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep. 2004;6:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 6. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 8. | Mendes LC, Ferreira PA, Miotto N, Zanaga L, Gonçales E, Lazarini MS, Gonçales FL, Stucchi RS, Vigani AG. Transient elastography and APRI score: looking at false positives and false negatives. Diagnostic performance and association to fibrosis staging in chronic hepatitis C. Braz J Med Biol Res. 2016;49:e5432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Abdollahi M, Pouri A, Ghojazadeh M, Estakhri R, Somi M. Non-invasive serum fibrosis markers: A study in chronic hepatitis. Bioimpacts. 2015;5:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 11. | Borsoi Viana MS, Takei K, Collarile Yamaguti DC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann Hepatol. 2009;8:26-31. [PubMed] |
| 12. | Seo YS, Kim MY, Kim SU, Hyun BS, Jang JY, Lee JW, Lee JI, Suh SJ, Park SY, Park H. Accuracy of transient elastography in assessing liver fibrosis in chronic viral hepatitis: A multicentre, retrospective study. Liver Int. 2015;35:2246-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1070] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 14. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 15. | Geng XX, Huang RG, Lin JM, Jiang N, Yang XX. Transient elastography in clinical detection of liver cirrhosis: A systematic review and meta-analysis. Saudi J Gastroenterol. 2016;22:294-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 791] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 17. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2507] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 18. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1197] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 19. | Xu SH, Li Q, Hu YP, Ying L. Development of a model based on biochemical, realtime tissue elastography and ultrasound data for the staging of liver fibrosis and cirrhosis in patients with chronic hepatitis B. Mol Med Rep. 2016;14:3609-3619. [PubMed] |
| 20. | Orasan OH, Iancu M, Sava M, Saplontai-Pop A, Cozma A, Sarlea ST, Lungoci C, Ungureanu MI, Negrean V, Sampelean D. Non-invasive assessment of liver fibrosis in chronic viral hepatitis. Eur J Clin Invest. 2015;45:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 990] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 22. | Martin P, Lau DT, Nguyen MH, Janssen HL, Dieterich DT, Peters MG, Jacobson IM. A Treatment Algorithm for the Management of Chronic Hepatitis B Virus Infection in the United States: 2015 Update. Clin Gastroenterol Hepatol. 2015;13:20712-2087.e16. [PubMed] |
| 23. | Kim SU, Kim DY, Park JY, Lee JH, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Choi EH. How can we enhance the performance of liver stiffness measurement using FibroScan in diagnosing liver cirrhosis in patients with chronic hepatitis B? J Clin Gastroenterol. 2010;44:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |