Published online Jun 18, 2017. doi: 10.4254/wjh.v9.i17.781
Peer-review started: December 19, 2016
First decision: March 28, 2017
Revised: April 28, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: June 18, 2017
Processing time: 181 Days and 2.7 Hours
To study hepatic vasoconstriction and glucose release induced by angiotensin (Ang)II or Epi in rats with pharmacological hypertension and spontaneously hypertensive rat (SHR).
Isolated liver perfusion was performed following portal vein and vena cava cannulation; AngII or epinephrine (Epi) was injected in bolus and portal pressure monitored; glucose release was measured in perfusate aliquots.
The portal hypertensive response (PHR) and the glucose release induced by AngII of L-NAME were similar to normal rats (WIS). On the other hand, the PHR induced by Epi in L-NAME was higher whereas the glucose release was lower compared to WIS. Despite the similar glycogen content, glucose release induced by AngII was lower in SHR compared to Wistar-Kyoto rats although both PHR and glucose release induced by Epi in were similar.
AngII and Epi responses are altered in different ways in these hypertension models. Our results suggest that inhibition of NO production seems to be involved in the hepatic effects induced by Epi but not by AngII; the diminished glucose release induced by AngII in SHR is not related to glycogen content.
Core tip: Angiotensin (Ang)II and epinephrine (Epi) induce hemodynamic and metabolic responses in a normal liver. These responses are altered in different ways in two models of hypertension. We observed that inhibition of NO production seems to be involved in the hepatic hemodynamic and metabolic effects induced by Epi but not by AngII. Furthermore, diminished glucose release induced by AngII in spontaneously hypertensive rat is not related to glycogen content, but might be due to the glycogen phosphorylase activation by AngII.
- Citation: Kimura DC, Nagaoka MR, Borges DR, Kouyoumdjian M. Angiotensin II or epinephrine hemodynamic and metabolic responses in the liver of L-NAME induced hypertension and spontaneous hypertensive rats. World J Hepatol 2017; 9(17): 781-790
- URL: https://www.wjgnet.com/1948-5182/full/v9/i17/781.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i17.781
The renin-angiotensin-aldosterone system (RAAS) regulates blood pressure homeostasis and vascular injury and repair responses. This system has been associated with diverse physiological functions, but also with inflammation, fibrosis, and target-organ damage. Local forms of the RAAS have been described in many tissues[1-5]. The importance of RAAS in the pathophysiology of hypertension has been observed in brain, heart, adrenal glands, vasculature, and kidney[6-9].
Several components of RAAS are present in the liver, which synthesizes angiotensinogen, a glycoprotein that contains the sequence of angiotensin in its amino-terminal portion. Angiotensin converting enzyme (ACE) is a carboxypeptidase present primarily in the perivenous region. Besides converting angiotensin (Ang) I in AngII, it is the major kininase involved in bradykinin degradation in the liver[10]. In 1976, Borges et al[11] showed that both AngI and AngII infused into the portal vein of a rat induced hypertensive effect, and they also demonstrated for the first time the conversion of AngI into AngII by the rat liver. This hypertensive response induced by AngII is mediated by AT1 receptor because when losartan was co-infused with AngII into the liver portal vein it abolished the hypertension response[12]. Captopril infusion prevented pressor action of AngI, thus the PHR previously attributed to AngI is actually a result of its conversion to AngII by hepatic ACE. This conversion is rapid, but the portal hypertensive action after AngI in bolus injection is significantly delayed compared to AngII injection[13]. Metabolic effects induced by AngII, such as glucose release and O2 consumption, are only diminished in the presence of losartan, which demonstrates that these effects are partially dissociated on bivascular liver perfusion. Therefore, another receptor besides AT1R might also be involved on these AngII hepatic effects[12,14].
ACE inhibition or blockade of angiotensin receptors are widely used in clinical medicine in the treatment of hypertension. The role of the hepatic RAAS has been associated with fibrosis and cirrhosis, and its resulting portal hypertension. Up-regulation of hepatic ACE, ACE2 and AT1R was observed in animal models of fibrosis and cirrhosis by bile duct ligation or carbon tetrachloride induction[15-17]. AngII, via AT1R, stimulates activation of quiescent stellate cells, activates myofibroblasts proliferation, and promotes the release of inflammatory cytokines, as well as the excessive deposition of extracellular matrix components[18].
The catecholaminergic sympathetic nervous system is another common system with metabolic (glucose and lactate release as well as oxygen consumption increase) and hemodynamic (vasoconstriction) effects. This system plays a key role in blood pressure homeostasis and normal metabolism and participates in the pathophysiology of many diseases. The liver contains abundant sympathetic innervation derived from the hepatic nerve plexus, and circulating catecholamines regulate liver tone[19]. The presence of the α1- and β-adrenergic receptors on hepatocytes was demonstrated in various species like catfish, goldfish, and rats[20-22]. In fed state, epinephrine (Epi) promotes hepatic glucose production by activation of glycogenolysis and, in fasted state, Epi accelerates gluconeogenesis[23].
In patients with essential hypertension, plasma levels of norepinephrine are significantly elevated and the increased sympathetic activity is accompanied by diastolic and systolic pressure increases. Neuroadrenergic factors may contribute to the maintenance and progression of hypertensive state as well as its development[24]. A correlation between the RAAS and the sympathetic nervous system has also been described. The latter is activated by AngIIand plays a fundamental role in the homeostasis of blood pressure control[25]. The multifactorial etiology of hypertension has led researchers to postulate, over time, various experimental models, each one involving one or more mechanisms, contributing to the assembly of a human essential hypertension “mosaic”. A pharmacological hypertension model is the blockade of nitric oxide synthesis. Biancardi et al[26] showed that vasoconstriction in response to L-NAME by the sympathetic tone plays an important role in the initiation and maintenance of hypertension. The RAAS also contributes to high blood pressure in animals chronically treated with L-NAME. Chronic treatment with ACE inhibitors or AT1 blockers is able to prevent the onset of, or reverse, a hypertension and renal injury already established, indicating a involvement of RAAS in the genesis and maintenance of this hypertension[27]. A spontaneously hypertensive rat (SHR) is the widely used genetic hypertension model that presents elevated sympathetic activity[28]. Although these animals are generally considered to be characterized by a low activity of circulating RAAS[29], some studies indicate that treatment with ACE inhibitors or AT1 receptor blockers or both reduces cardiac or renal dysfunction or both of these dysfunctions in SHRs[30-32].
Although the liver is not a target organ in physiopathology of hypertension, the presence of AT1 receptor and ACE may still indicate unknown specific roles. Sympathetic hyperactivity was described in most models of hypertension[28] but little is known about the consequences of this hyperactivity in the liver. Therefore, the aim of this work was to evaluate the hepatic response to AngII and Epi in hypertension models. Using the isolated rat liver perfusion, we studied the vasoconstrictor hepatic effect as well as metabolic (glucose release) effect of AngII and Epi in two different hypertension experimental models: One genetic (SHR) and one pharmacological (systemic inhibition of NO synthase).
Adult male Wistar EPM-1 rats (WIS), SHRs (bred by the Central Animal House of the Federal University of São Paulo - UNIFESP), and Wistar Kyoto (WKY) rats (bred by Central House of the University of de São Paulo - USP) aged 12-16 wk were used. The animals were housed in a conditioned environment and were fed a standard laboratory diet (Purina) and water ad libitum. This study was conducted according to the International Guiding Principles for Biomedical Research Involving Animals[33] and was approved by the Ethics in Research Committee of UNIFESP (CEP 1455/09).
After one week of acclimatization, two experimental groups were studied: (1) L-NAME, pharmacologic induced model of hypertension: Wistar EPM-1 rats received NG-nitro-L-arginine methylester (0.5 mg/mL) in drinking water for 10 d and were compared to healthy, Wistar EPM-1 rats; and (2) SHRs were compared to WKY rats.
Body weight and tail indirect systolic blood pressure (SBP) were recorded weekly. SBP was measured by tail-cuff plethysmography (NIBP Controller, ADInstruments, Australia) in unanesthetized rats that were placed in a warm cupboard (45 °C) for 15 min. SBP values for individual rats were obtained from the average of 3-4 consecutive measurements and were considered valid only when these readings did not differ by more than 5 mmHg. Procedure was performed at least 48 h before the perfusion experiments to minimize the influence of animal stress on our results. Upon confirmation of animal hypertension, perfusion of rat liver in situ was conduct as previously described[34].
Blood samples were collected from the abdominal aorta before portal vein cannulation. They were centrifuged at 3000 rpm to remove red cells, and serum was stored at -20 °C. Glucose was determined by enzymatic method (Glucose PAP kit, Labtest Diagnóstica, Sao Paulo, Brazil) and the concentration of insulin was determined using a direct ELISA kit specific for rat and mouse analysis (Millipore, United States).
Monovascular rat liver perfusion was performed as previously described[34]. Briefly, the rat was anesthetized with urethane, 1.3 g/kg, i.p. (Sigma Chemical Co., United States), and hemoglobin-free, nonrecirculating liver perfusion was performed. Abdominal and thoracic cavities were opened and the portal vein (entry via) and the vena cava (exit via) cannulated. The perfusion fluid was Krebs/Henseleit-bicarbonate buffer, pH 7.4, containing 1 mg/mL BSA (Sigma Chemical Co., United States) saturated with an oxygen/carbon dioxide mixture (95/5%). Fluid was pumped in a constant flow (3-4 mL/min.g liver) through a temperature-regulated membrane oxygenator (37 °C) prior to entering the liver via the portal vein. The oxygen uptake in the outflowing perfusate was monitored continuously with a polarigraphic type of probe (Delta OHM HD2109.2, Italy) adequately positioned in a chamber at the exit of the perfusate. Liver viability was evaluated by bile production and oxygen consumption. The portal pressure was measured by using a vertically positioned, graduated fluid-filled column attached before the afferent cannula open to the atmospheric. After 20 min of stabilization previously determined (glucose release and portal pressure), 2 nmol AngII (Sigma Chemical Co., United States) or 40 nmol Epi (Sigma Chemical Co., United States) was injected in bolus into the portal vein cannula. Aliquots of perfusate were collected (0 and every 30 s until 5 min and 6, 8 and 10 min) for glucose determination.
Portal pressure was recorded during all experiments (0, 15, 30 and 45 s and 1-10 min). The portal pressure increase was determined over the basal pressure and the maximum increase measured. The portal hypertensive response (PHR; the area under the curve) was calculated from the graphic: Portal pressure increase vs time after agonist injection and expressed as cmH2O.min.
Metabolic effects were evaluated on the basis of oxygen consumption and glucose release by perfused liver. Oxygen consumption was calculated from input-output differences expressed as μmol O2 consumed/min.g liver. Glucose released was determined in perfusate aliquots using an enzymatic method (Glucose PAP kit, Labtest Diagnóstica, Sao Paulo, Brazil) and expressed as µmol glucose released/min.g liver. This parameter was also used to assure the liver viability. The amount of glucose released was calculated (area under the curve) from the graphic: Glucose increase vs time after agonist injection and expressed as μmol/min.g liver.
In order to avoid loss of the liver glycogen content during the 30 min of perfusion, a fragment of caudate lobe was removed after a rapid exsanguination at the beginning of the perfusion procedure. Quantification of the glycogen was based on the extraction of the polysaccharide with an alkaline solution (30% KOH) and its conversion into glucose during the reaction of the exergonic homogenized with a solution of sulfuric acid and anthrone[35]. The concentration of glycogen (expressed as mg/100 mg liver) was determined from a glucose standard curve. Furthermore, liver fragments were removed at the end of the experiment and processed by the company Histotech Teaching Blades (http://www.histotech.com.br/site/). The histological analysis of liver glycogen was performed using the periodic acid-Schiff (PAS) staining.
The results are expressed as mean ± SEM. Comparisons were performed by using Student’s t-test and a value of P < 0.05 was adopted as the level of significance. Analysis was performed using Graph Pad Prism 5.0 program.
Arterial blood pressure of SHR and rats submitted to drug hypertension (L-NAME) was evaluated before the perfusion experiments. The tail systolic blood pressure (mmHg) of L-NAME (169.1 ± 4.8; n = 12) and SHR groups (180.2 ± 5.9; n = 10) were higher (t-test, P < 0.001) when compared to WIS (126.4 ± 2.9; n = 9) and Wistar Kyoto (127.0 ± 2.0; n = 15), respectively. The glycemia and insulinemia of the rats used in the experiments are shown in Table 1; values of glycemia of normotensive animals were taken as the reference value. The glycemia of both the L-NAME and SHR groups was similar when compared to their respective control groups. The insulinemia of all groups were within normal range (0-118 pmol/L)[36] without difference between groups.
| Group | Glycemia (mg/dL) | n | Insulinemia (ng/mL) | n | Glycogen content (mg/100 mg liver) | n |
| WIS | 75.4 ± 4.2 | 9 | 2.1 ± 0.4 | 12 | 2.9 ± 0.2 | 10 |
| L-NAME | 80.7 ± 7.5 | 8 | 2.0 ± 0.4 | 12 | 2.3 ± 0.2 | 10 |
| WKY | 76.9 ± 4.0 | 9 | 3.8 ± 0.6 | 12 | 2.8 ± 0.2 | 10 |
| SHR | 86.2 ± 4.0 | 8 | 2.7 ± 0.4 | 13 | 2.8 ± 0.2 | 10 |
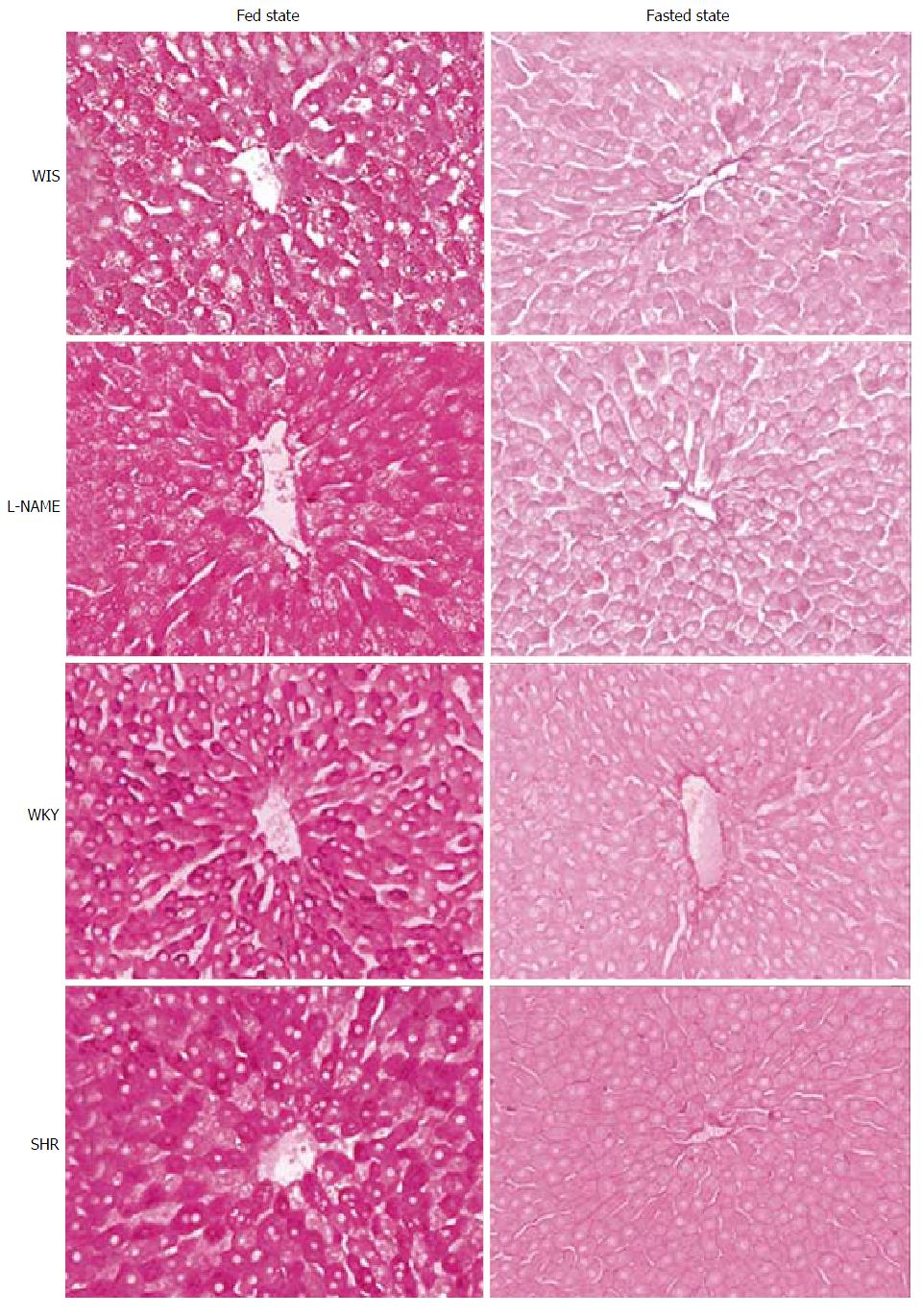
The perfusion experiments were performed in the morning when the animals, which have nocturnal habits, were in a well-fed state confirmed by hepatic glycogen content. No difference in liver glycogen content among groups (Table 1) was found. At the end of perfusion another fragment of the liver was removed for histological analysis for glycogen content (PAS staining) and compared to the perfused livers of animals left for 24 h of fasting. We observed that even after 30 min of perfusion, the hepatic glycogen of all groups was noticeably higher than in fasted animals (Figure 1).
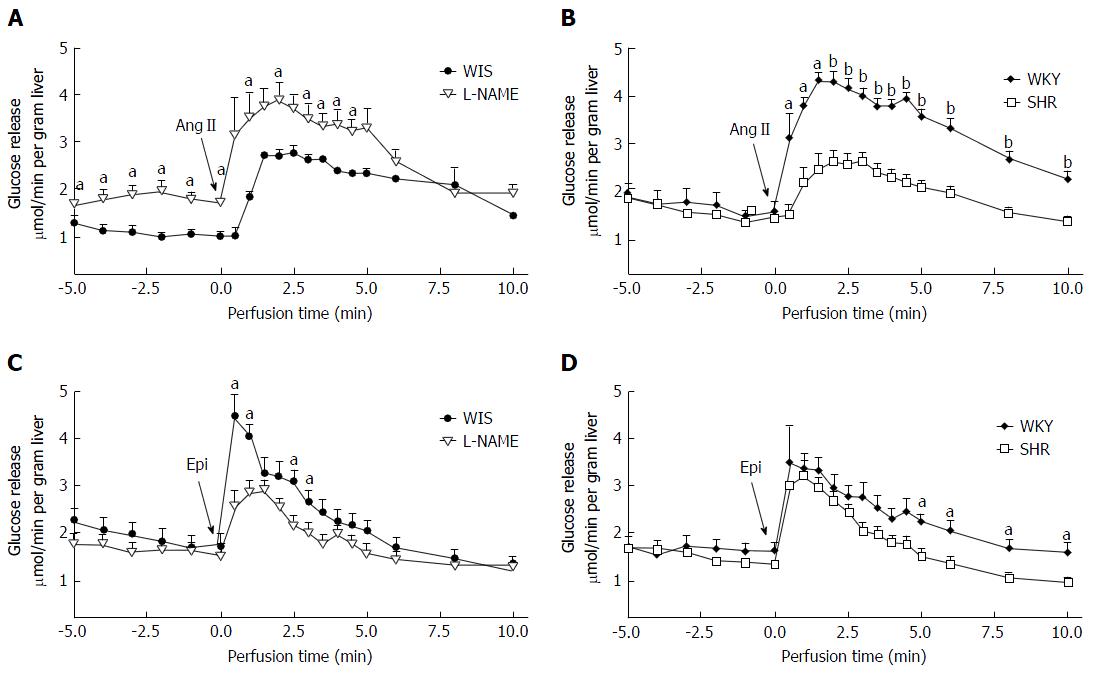
To ensure liver viability during the period of liver perfusion experiment (approximately 30 min), bile production and oxygen consumption were monitored. The bile was collected before and after injection of Epi or AngII. As the bile production before and after agonist injection were similar, the arithmetic average was used for statistical analysis. The bile production (mL/min.g liver) was similar among groups (WIS: 1.2 ± 0.1, n = 16; L-NAME: 1.2 ± 0.1, n = 15; WKY: 1.1 ± 0.1, n = 14; SHR: 1.1 ± 0.1, n = 13). The oxygen consumption was observed throughout the perfusion period ensuring the functioning of the organ. The basal oxygen consumption (μmol/min.g liver) of SHR (2.5 ± 0.1, n = 14) was lower (t-test, P = 0.0151) when compared to WKY (3.2 ± 0.2, n = 16). This parameter on L-NAME (3.1 ± 0.2, n = 15) was similar to WIS (3.2 ± 0.1; n = 17). After agonist injection, oxygen consumption was maintained but no standard response was observed: It remained the same in some experiments and increased in others. As the perfusion fluid did not contain glucose, its release was observed from the beginning of the experiment. Basal glucose release was similar in all groups (Figure 2A and B); after agonists injection its release continued throughout the entire experiment, ensuring hepatic viability.
Following AngII injection, the amount of glucose released (Figure 2A and B) from the L-NAME group was similar compared to the WIS group, whereas the amount released from SHR livers was lower than its WKY control group (Table 2).
The glucose release induced by epinephrine is shown in Figure 2C and D; the amount released (AUC) from the L-NAME group (4.2 ± 0.4) was lower when compared to its WIS control group (7.5 ± 0.9), whereas the SHR group was similar to the WKY group (Table 2).
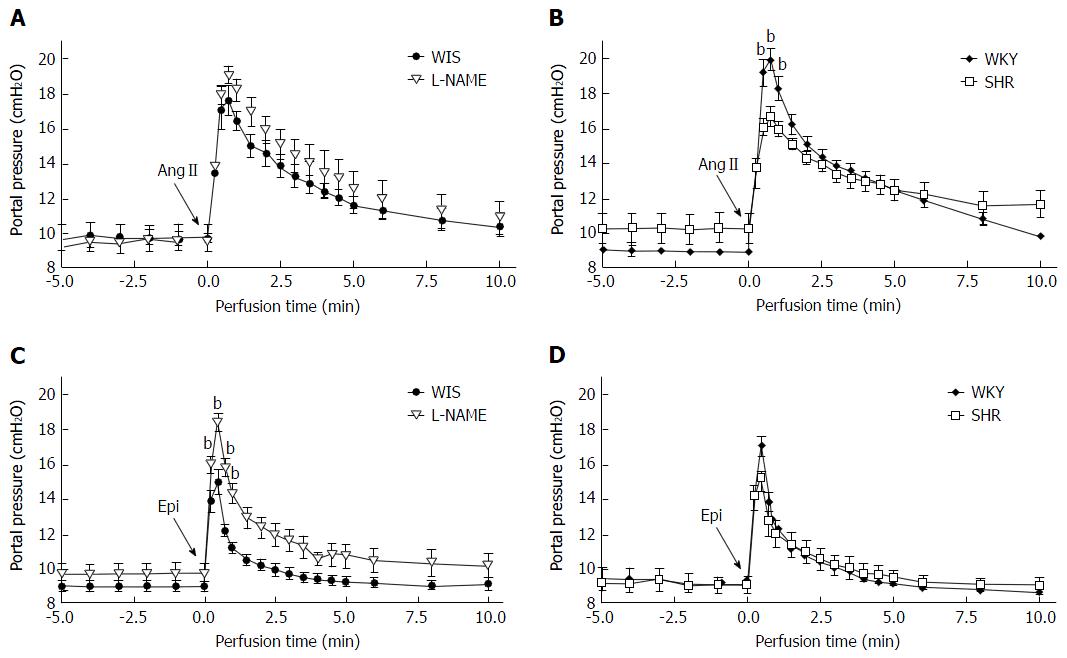
Basal portal pressure (before agonist injection) was similar in all groups. AngII (2 nmol) or Epi (40 nmol) was injected in portal vein and both agonists promoted portal vasoconstriction. Despite a 20-fold difference in agonists doses, the maximum portal pressure increase (cmH2O) induced by AngII and Epi was similar in among groups (AngII: WIS: 7.9 ± 1.2, n = 7; L-NAME: 7.6 ± 1.1, n = 7; WKY: 10.5 ± 0.3, n = 7; SHR: 6.5 ± 1.2, n = 10; Epi: WIS: 6.1 ± 0.7, n = 10; L-NAME: 8.9 ± 0.7, n = 8; WKY: 7.9 ± 0.7, n = 8; and SHR: 6.2 ± 0.5, n = 6).
The hepatic portal pressure increase after bolus injection of AngII was normalized after about 10 min of perfusion (Figure 3A and B). The curve prolife of portal pressure of L-NAME and SHR groups was similar to their control groups (WIS and WKY, respectively). The PHR induced by AngII in both L-NAME and SHR was similar when compared to their WIS and WKY control groups, respectively (Table 3). The effect of Epi in portal pressure was more transient than AngII. Following Epi injection, the portal pressure increase was normalized after about 5 min (Figure 1). The PHR induced by Epi in the L-NAME group was higher when compared to the WIS group. On the other hand, no difference in PHR of SHRs existed compared to the control WKY group (Table 3).
| Portal hypertensive response cmH2O.min | ||||
| Group | Angiotensin II | n | Epinephrine | n |
| WIS | 26.4 ± 3.2 | 7 | 8.2 ± 0.8 | 10 |
| L-NAME | 38.1 ± 4.8 | 7 | 18.5 ± 1.9b | 8 |
| WKY | 29.0 ± 1.1 | 7 | 10.0 ± 1.1 | 8 |
| SHR | 25.9 ± 3.7 | 10 | 10.5 ± 1.1 | 6 |
All key components of the RAAS are present in the normal liver and are up-regulated in response to chronic liver injury, with growing evidence that the intrahepatic RAAS plays important roles in both the pathophysiology of portal hypertension and liver fibrosis[18]. The use of ACE/AngII/AT1R axis inhibitors associated with ACE2/Ang (1-7)/Mas axis activation is a promising strategy-serving regimen to prevent and treat chronic liver diseases as well as acute liver injury[37]. Hepatic glucose metabolism can be modulated by NO directly inhibiting glycogen synthesis and gluconeogenesis, and indirectly inhibiting glycogen breakdown via the secretion of other intrahepatic mediators[38,39].
In the liver, both AngII and Epi cause vasoconstriction and glucose release. Although the liver is not considered the target organ in hypertension pathophysiology, it is an important metabolic regulator organ. To study hepatic effects of AngII and Epi, we used two different experimental models of hypertension: Pharmacological (systemic inhibition of NO synthase) and genetic (SHR). Chronic oral administration of L-NAME promotes a rapid deployment of hypertension in the first days of treatment that is largely mediated by the RAAS. The rats treated with ACE inhibitors, such as captopril and enalapril, or with AT1 receptor antagonists, such as losartan, restore blood pressure to near normal levels[40,41]. In our study, 10 d of L-NAME treatment were sufficient to induce a high level systolic blood pressure. On the other hand, the SHR strain is the most widely used phenotypic experimental model in hypertension research with specific potential in the study of polygenic hypertension, being associated with cardiac hypertrophy, heart failure, and renal dysfunction. Hepatic functions are also altered at the molecular level in this model of primary hypertension[42].
Treatment with L-NAME did not affect fasting glucose levels but reduced significantly insulin levels in blood and increased insulin sensitivity of rats[43]. Gouveia et al[44] described increased glycemia and insulinemia values for fasted or fed SHRs. We observed normal glycemia and insulinemia in both hypertension models in fed state, which contrasts with the studies that show changes in these metabolic parameters. The discrepancy may be due to the metabolic states of the animals in the studies.
Tarsitano et al[43] described how prolonged treatment (2-8 wk) with NO synthase inhibitor enhanced hepatic glycogen levels. In our study, as the treatment with L-NAME was only for 10 d, the amount of liver glycogen was similar to the WIS group. This short period of treatment might not have been enough to observe possible changes in the glycogen content. Chronic or acute administration of an inhibitor of NO synthesis (L-NAME or L-NNMA) was shown to alter systemic RAAS, decreasing plasma level AngII as well as renin activity[45]. Nevertheless, hepatic glucose release profile induced by AngII in chronically treated L-NAME animals was similar to the control, which suggests that NO is not involved in the glucose release after induction.
Interestingly, in the L-NAME group, the glucose release induced by Epi was lower than in the control group, suggesting that this effect may be related to the inhibition of NO synthesis. In cultured rat hepatocytes, Hodis et al[46] observed that glycogenolysis occurs via α-adrenergic stimulation and signaling cascade that involves the production of NO. Similarly, our results suggest that the chronic inhibition of NO synthase might inhibit hepatic glycogenolysis, which in turn decreases the release of glucose in the perfusate during the experiment. Therefore, the differences in glucose release following the L-NAME treatment evidenced that the increase in hepatic glycogenolysis was probably mediated by NO when activated by Epi but not by AngII.
In the SHR group, it was described that muscle glycogen content was lower, but livers presented similar levels of glycogen in the fed and fasted states[44]. Likewise, we found similar amounts of liver glycogen in the SHR and WKY groups. Despite this similarity, after AngII in bolus injection, glucose released was lower in the SHR group compared to the control group. This result suggests that glucose release is not necessarily related to glycogen content, but may be due to a possible difference in glycogen phosphorylase activation by increased [Ca2+]i induced by AngII[47]. On the contrary, in this hypertension model, glucose release induced by Epi was similar when compared to the control.
Both AngII and Epi are potent physiological vasoconstrictors. We observed that although these agonists led to similar maximum increases of the portal pressure, AngII promoted a higher PHR, even using doses 20-fold lower. These response differences may be related to the prolonged responses induced by AngII in the liver or with the amount of AngII receptor vs Epi receptor. An enhanced AngII-mediated vasoconstriction was observed in healthy elderly individuals and this apparent increase is due, at least in part, to the potentiation of α-adrenergic vasoconstriction. These findings suggest that cross-talk between RAAS and adrenergic systems may be an important regulator of resting vascular tone and muscle blood flow with advancing age[48]. Cross-talk between the α1-adrenergic receptor (α1R) and AT1R potentially exists on two levels: Receptor heterodimerization between α1R and AT1R and second messenger level[49].
No difference in the PHR of AngII in the pharmacologic hypertensive model was found, which suggests no changes in the expression of hepatic AT1 receptor. Our result contrasts with AT1R up-regulation described in the L-NAME model in other tissues such as the aorta[50], adrenals[51] and heart[52].
On the other hand, in L-NAME-treated animals, Epi induced increased PHR. It was shown that in rats, chronic inhibition of NO synthase produces endothelial dysfunction, increased vascular response to adrenergic stimulation, and perivascular inflammation[53]. NO is also involved in regulation of sympathetic nerve activity in human skin and muscle cells[54]. Therefore, this increased hypertensive effect in the liver of L-NAME-treated rats may be related to increased sympathetic vascular activity. The disparity between the effects of portal vasoconstriction (higher) and glucose released (lower) in the L-NAME group is a further indication that these effects might be dissociated in two components: One with direct action in the hepatocyte and the other as a presinusoidal response.
We also observed similar vasoconstrictor effect of AngII in the SHR group. Although in this strain, higher levels of AT1R gene expression was described in brain regions involved in arterial blood pressure control[55]. Despite widely described sympathetic hyperactivity in this model[56-58], in this work, PHR to Epi on SHRs was similar to the control group.
In conclusion, AngII and Epi responses are altered in different ways in these two models of hypertension. Our results suggest that inhibition of NO production seems to be involved in the hepatic hemodynamic and metabolic effects induced by Epi but not by AngII. Furthermore, diminished glucose release induced by AngII in SHR is not related to glycogen content, but to the glycogen phosphorylase activation by AngII, that is under investigation.
In a normal liver, angiotensin (Ang)I is rapidly converted in AngII by hepatic angiotensin converting enzyme, and AngII promotes hypertensive response mediated by the AT1 receptor. Besides this hemodynamic effect, AngII induces metabolic effects (glucose release and O2 consumption). Epinephrine promotes hepatic metabolic (glucose and lactate release and O2 consumption increase) as well as hemodynamic (vasoconstriction) effects. It has also been described as a correlation between the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system; the latter is activated by AngII and plays a fundamental role in the homeostasis of blood pressure control. In hypertension, sympathetic hyperactivity is described but little is known about this hyperactivity in the liver. The hepatic response to AngII and Epinephrine in hypertension has not been studied yet. Therefore, the relevance of this study is to understand the hepatic effects of these hormones in two different hypertensive models.
The RAAS and the catecholaminergic system are present in the normal liver. The interaction of RAAS with the catecholaminergic sympathetic nervous system in the liver of hypertensive animals might bring to light relevant aspects of the relationship among metabolic disorders such as hypertension, type II diabetes, obesity, and hypertriglyceridemia.
No descript of hemodynamic and metabolic effects of the two hormones AngIIand Epi exists in the literature on RAAS and the catecholaminergic system in the livers of hypertensive rats. This is the first study evaluating hemodynamic and metabolic effects of the two hormones AngII and Epi. Inhibition of NO production in the L-NAME model increased hepatic hemodynamic and metabolic effects induced by Epi but not by AngII. Furthermore, diminished glucose release induced by AngII in SHRs is not related to glycogen content. Therefore, the hepatic effect of AngII or Epi is different depending on the pathophysiology of systemic arterial hypertension
Although not target organs in hypertension, RAAS and sympathetic nervous system are overexpressed, elucidating the hepatic role of these systems, which can bring knowledge about metabolic-related comorbidities and therapeutics.
The portal hypertensive response represents the area under the curve and was calculated from the graphic: Portal pressure increase (cmH2O) vs time after agonist injection (min) and expressed as cmH2O.min. It considers not only the perfusion pressure increase but the effect of the agonist over time.
In this paper, authors give some new information about the effects of Epi and AngII on glucose release, finding that inhibition of NO production seems to be involved in the hepatic hemodynamic and metabolic effects induced by Epi but not by AngII.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tang JM S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Gonçalves PB, Ferreira R, Gasperin B, Oliveira JF. Role of angiotensin in ovarian follicular development and ovulation in mammals: a review of recent advances. Reproduction. 2012;143:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Zhang Z, Liu C, Gan Z, Wang X, Yi Q, Liu Y, Wang Y, Lu B, Du H, Shao J. Improved Glucose-Stimulated Insulin Secretion by Selective Intraislet Inhibition of Angiotensin II Type 1 Receptor Expression in Isolated Islets of db/db Mice. Int J Endocrinol. 2013;2013:319586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1245] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 4. | Chen YH, Huang CH, Lu HI, Chen CH, Huang WT, Hsieh MJ, Rau KM, Chang AY, Lin WC, Li SH. Prognostic impact of renin-angiotensin system blockade in esophageal squamous cell carcinoma. J Renin Angiotensin Aldosterone Syst. 2015;16:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One. 2013;8:e63847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Moniwa N, Varagic J, Ahmad S, VonCannon JL, Simington SW, Wang H, Groban L, Brosnihan KB, Nagata S, Kato J. Hemodynamic and hormonal changes to dual renin-angiotensin system inhibition in experimental hypertension. Hypertension. 2013;61:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Rajamohan SB, Raghuraman G, Prabhakar NR, Kumar GK. NADPH oxidase-derived H(2)O(2) contributes to angiotensin II-induced aldosterone synthesis in human and rat adrenal cortical cells. Antioxid Redox Signal. 2012;17:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med. 2005;146:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Gioli-Pereira L, Nascimento EA, Santos EL, Bracht A, Juliano MA, Pesquero JB, Borges DR, Kouyoumdjian M. Fate of bradykinin on the rat liver when administered by the venous or arterial route. J Gastroenterol Hepatol. 2005;20:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Borges DR, Limãos EA, Prado JL, Camargo AC. Catabolism of vasoactive polypeptides by perfused rat liver. Naunyn Schmiedebergs Arch Pharmacol. 1976;295:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Nascimento EA, Gioli-Pereira L, Carvalho LT, Santos EL, Pesquero JB, Kouyoumdjian M, Borges DR. Hemodynamic and metabolic effects of angiotensin II on the liver. Peptides. 2005;26:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Carvalho LT, Nascimento EA, Teixeira FO, Nagaoka MR, Borges DR, Kouyoumdjian M. Hepatic conversion of angiotensin I and the portal hypertensive response to angiotensin II in normal and regenerating liver. J Gastroenterol Hepatol. 2007;22:1543-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Reisenleiter F, Katz N, Gardemann A. Control of hepatic carbohydrate metabolism and haemodynamics in perfused rat liver by arterial and portal angiotensin II. Eur J Gastroenterol Hepatol. 1996;8:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Huang ML, Li X, Meng Y, Xiao B, Ma Q, Ying SS, Wu PS, Zhang ZS. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol. 2010;37:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Grace JA, Herath CB, Mak KY, Burrell LM, Angus PW. Update on new aspects of the renin-angiotensin system in liver disease: clinical implications and new therapeutic options. Clin Sci (Lond). 2012;123:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Gardemann A, Püschel GP, Jungermann K. Nervous control of liver metabolism and hemodynamics. Eur J Biochem. 1992;207:399-411. [PubMed] |
| 20. | Fabbri E, Buzzi M, Biondi C, Capuzzo A. Alpha-adrenoceptor-mediated glucose release from perifused catfish hepatocytes. Life Sci. 1999;65:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Vardanega-Peicher M, Lopes G, Lima FB, Curi R, Nakano LC, Bazotte RB. Time sequence of changes in the responsiveness of glycogen breakdown to adrenergic agonists in perfused liver of rats with insulin-induced hypoglycemia. Braz J Med Biol Res. 2000;33:805-813. [PubMed] |
| 22. | Manzl C, Schubert M, Schwarzbaum PJ, Krumschnabel G. Effects of chemical anoxia on adrenergic responses of goldfish hepatocytes and the contribution of alpha- and beta-adrenoceptors. J Exp Zool. 2002;292:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 23. | Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 436] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 25. | Beevers G, Lip GY, O’Brien E. ABC of hypertension: The pathophysiology of hypertension. BMJ. 2001;322:912-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Biancardi VC, Bergamaschi CT, Lopes OU, Campos RR. Sympathetic activation in rats with L-NAME-induced hypertension. Braz J Med Biol Res. 2007;40:401-408. [PubMed] |
| 27. | Pollock DM, Polakowski JS, Divish BJ, Opgenorth TJ. Angiotensin blockade reverses hypertension during long-term nitric oxide synthase inhibition. Hypertension. 1993;21:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Dornas WC, Silva ME. Animal models for the study of arterial hypertension. J Biosci. 2011;36:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Kuriyama S, Kawashima K, Sokabe H. Plasma renin activity determined by two different methods in spontaneously hypertensive rats. Jpn Heart J. 1982;23:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Nakamura Y, Ono H, Zhou X, Frohlich ED. Angiotensin type 1 receptor antagonism and ACE inhibition produce similar renoprotection in N(omega)-nitro-L& gt; -arginine methyl ester/spontaneously hypertensive rats. Hypertension. 2001;37:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Teng J, Fukuda N, Suzuki R, Takagi H, Ikeda Y, Tahira Y, Kanmatsuse K. Inhibitory effect of a novel angiotensin II type 1 receptor antagonist RNH-6270 on growth of vascular smooth muscle cells from spontaneously hypertensive rats: different anti-proliferative effect to angiotensin-converting enzyme inhibitor. J Cardiovasc Pharmacol. 2002;39:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Pu Q, Larouche I, Schiffrin EL. Effect of dual angiotensin converting enzyme/neutral endopeptidase inhibition, angiotensin converting enzyme inhibition, or AT1 antagonism on coronary microvasculature in spontaneously hypertensive rats. Am J Hypertens. 2003;16:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | CIOMS International Guiding Principles for Biomedical Research Involving Animals. Altern Lab Anim. 1985;12:ii. [PubMed] |
| 34. | Borges DR, Kouyoumdjian M. The recognition site for hepatic clearance of plasma kallikrein is on its heavy chain and is latent on prokallikrein. J Hepatol. 1992;16:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Seifter S, Dayton S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950;25:191-200. [PubMed] |
| 36. | Tietz NW, Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics. St. Louis, Mo. : Elsevier Saunders 2006; . |
| 37. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Casada M, Dìaz-Guerra MJ, Boscà L, Martìn-Sanz P. Characterization of nitric oxide dependent changes in carbohydrate hepatic metabolism during septic shock. Life Sci. 1996;58:561-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Sprangers F, Sauerwein HP, Romijn JA, van Woerkom GM, Meijer AJ. Nitric oxide inhibits glycogen synthesis in isolated rat hepatocytes. Biochem J. 1998;330:1045-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 513] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 41. | Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension. 1998;32:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Svoboda DS, Kawaja MD. Changes in hepatic protein expression in spontaneously hypertensive rats suggest early stages of non-alcoholic fatty liver disease. J Proteomics. 2012;75:1752-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Tarsitano CA, Paffaro VA, Pauli JR, da Silva GH, Saad MJ, Salgado I, da Cruz-Höfling MA, Hyslop S. Hepatic morphological alterations, glycogen content and cytochrome P450 activities in rats treated chronically with N(omega)-nitro-L-arginine methyl ester (L-NAME). Cell Tissue Res. 2007;329:45-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Gouveia LM, Kettelhut IC, Foss MC. Abnormalities of glucose metabolism in spontaneously hypertensive rats. Braz J Med Biol Res. 2000;33:1357-1362. [PubMed] |
| 45. | Garcia GE, Brown MR, Wead LM, Braun S, Gabbai FB. Effect of reduction of nitric oxide on plasma and kidney tissue angiotensin II levels. Am J Hypertens. 1997;10:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Hodis J, Kutinová-Canová N, Potmesil P, Kameníková L, Kmonícková E, Zídek Z, Farghali H. The role of adrenergic agonists on glycogenolysis in rat hepatocyte cultures and possible involvement of NO. Physiol Res. 2007;56:419-425. [PubMed] |
| 47. | Keppens S, De Wulf H, Clauser P, Jard S, Morgat JL. The liver angiotensin receptor involved in the activation of glycogen phosphorylase. Biochem J. 1982;208:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Barrett-O'Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE. Angiotensin II potentiates α-adrenergic vasoconstriction in the elderly. Clin Sci (Lond). 2013;124:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Batenburg WW, van Esch JH, Garrelds IM, Jorde U, Lamers JM, Dekkers DH, Walther T, Kellett E, Milligan G, van Kats JP. Carvedilol-induced antagonism of angiotensin II: a matter of alpha1-adrenoceptor blockade. J Hypertens. 2006;24:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Lee J, Kim S, Oh Y, Ryu SY, Kim SW. Upregulation of vascular renin-angiotensin and endothelin systems in rats inhibited of nitric oxide synthesis. Pharmacol Res. 2002;46:383-387. [PubMed] |
| 51. | Usui M, Ichiki T, Katoh M, Egashira K, Takeshita A. Regulation of angiotensin II receptor expression by nitric oxide in rat adrenal gland. Hypertension. 1998;32:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Katoh M, Egashira K, Usui M, Ichiki T, Tomita H, Shimokawa H, Rakugi H, Takeshita A. Cardiac angiotensin II receptors are upregulated by long-term inhibition of nitric oxide synthesis in rats. Circ Res. 1998;83:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Hsieh NK, Wang JY, Liu JC, Wang SD, Chen HI. Nitric oxide inhibition accelerates hypertension and induces perivascular inflammation in rats. Clin Exp Pharmacol Physiol. 2004;31:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol. 2009;587:4977-4986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin Exp Pharmacol Physiol. 2006;33:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Berg T, Jensen J. Simultaneous parasympathetic and sympathetic activation reveals altered autonomic control of heart rate, vascular tension, and epinephrine release in anesthetized hypertensive rats. Front Neurol. 2011;2:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Herring N, Lee CW, Sunderland N, Wright K, Paterson DJ. Pravastatin normalises peripheral cardiac sympathetic hyperactivity in the spontaneously hypertensive rat. J Mol Cell Cardiol. 2011;50:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Scridon A, Gallet C, Arisha MM, Oréa V, Chapuis B, Li N, Tabib A, Christé G, Barrès C, Julien C. Unprovoked atrial tachyarrhythmias in aging spontaneously hypertensive rats: the role of the autonomic nervous system. Am J Physiol Heart Circ Physiol. 2012;303:H386-H392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |