Published online Jun 18, 2017. doi: 10.4254/wjh.v9.i17.757
Peer-review started: March 7, 2017
First decision: April 18, 2017
Revised: April 29, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: June 18, 2017
Processing time: 102 Days and 4 Hours
Beyond the metabolic functions, the liver recently has been defined as an organ of immune system (IS), which have central regulatory role for innate and adaptive immunity. The liver keeps a delicate balance between hepatic screening of pathogenic antigens and immune tolerance to self-antigens. Herbal treatments with immunological effects have potential to alter this hepatic immune balance towards either therapeutic side or diseases side by inducing liver injury via hepatotoxicity or initiation of autoimmune diseases. Most commonly known herbal treatments, which have therapeutic effect on liver and IS, have proven via in vitro, in vivo, and/or clinical studies were summarized in this review.
Core tip: Herbal treatment is the mother of modern medicine. The ancient habit of treating diseases with plants still goes on as either primary or complementary to conventional medical treatment. The other side of medallion is the fact that the liver is number one target organ for herbal toxicity. Furthermore, liver has been recently defined as an active organ of immune system, which have central regulatory role on innate and adaptive immune response. The delicate homeostasis between immediate and efficient defense against threats (immune surveilance of antigens) without triggering harmful immune response towards self-structures (periferal immune tolerance to self antigens) is controled by liver. Herbal formulas are not a single plant extract, but is an interacting mixture of ingredients that determines the final clinical outcome as therapeutic and hepatotoxic effect. This review aimed to drive attention on both potentials of herbals from the point of immunology, in order to initiate a motivation for feature studies defining the mechanisms of immunological interaction between herbals and liver.
- Citation: Balaban YH, Aka C, Koca-Caliskan U. Liver immunology and herbal treatment. World J Hepatol 2017; 9(17): 757-770
- URL: https://www.wjgnet.com/1948-5182/full/v9/i17/757.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i17.757
Herbal treatment is the mother of modern medicine. Because of cultural, economical and practical reasons, the ancient habit of treating diseases with plants still goes on as either primary or complementary to conventional medical treatment. The other side of medallion is the fact that the liver is number one target organ of herbal and dietary supplements (HDS) induced toxicity. The Food and Drug Administration (FDA) under the Dietary Supplement Health and Education Act (DSHEA) regulate herbal treatments since 1994[1]. The submissions of new herbal products to FDA require the dose and list of ingredients to be written on its bottle, however, documentation of safety and efficacy is not need to be reported. Furthermore, HDS can be obtained without prescription, medical advice or monitoring. Although the actual size of the problem is not well defined, HDS-induced hepatotoxicity accounts for 20% of cases of hepatotoxicity in the United States and the rates differ from 2.5% in India to 70% in Singapore[2].
The liver is prone to drug-induced liver injury (DILI) because of its functions on metabolizing chemicals and regulating immune response. DILI can develop either by dose related direct drug toxicity, or - much more commonly - as idiosyncratic reactions due to individual susceptibility to ingredients. The complex composition of HDS eases the both direct toxicity and idiosyncratic reactions during their metabolism in liver. Idiosyncratic DILI (IDILI) is in most instances characterized by a mild injury (ALT < 3 times upper normal limit) which normalized with continuous drug treatment. This phenomenon of clinical adaptation is a biochemical adaptive response of organelles such as endoplasmic reticulum and mitochondria metabolizing chemicals. It is hypothesized that defective clinical adaptation mechanisms result in severe IDILI with jaundice and liver failure, in < 0.1% population with susceptible human leucocyte antigen (HLA) type. Microbiota is the ecological community of commensal, symbiotic and pathogenic microorganisms that literally share our body space[3]. The microbiota of gut also determinates IDILI susceptibly by regulating hepatic immune-tolerance though lipopolysaccharides (LPS) induced T-cell response in liver. According to theory, haptens of metabolized chemicals covalently bind to proteins, and become antigenic peptides. While 70%-90% of population has immune tolerance, the rest develop adaptive immune response due to their susceptible type HLA and/or dysfunctional microbiota. If biochemical adaptation mechanisms cannot control this initiated mild injury, acute liver failure develops[4]. There are supporting evidences to this theory. The many top IDILI drugs are antibiotics changing the normal microbiota. The “immune check point therapy” for cancer treatments are FDA approved antibodies that aim to inhibit T cell immune-tolerant states, such as ipilimumab (anti-CTLA4), pembrolizumab (anti-PD-1) and nivolumab (anti-PD-1). The major side effect of these treatments is to make individuals to susceptible to haptens (so inducing IDILI) and auto-antigens (so inducing autoimmunity).
This review aimed to drive attention on both therapeutic mechanisms and hepatotoxic potentials of herbal treatments from the point of immunology. After defining basic immune system (IS), we summarized the role of liver as an immune regulatory organ and then the herbal treatments with therapeutic potential on liver.
IS has evolved to recognize and eliminate internal insults (i.e., cancer cells) or external invading pathogens (i.e., infections) by developing local or systemic response. IS composed of “classical lymphoid organs”; thymus, bone marrow, spleen, tonsils, lymph nodes and “peripheral immune organs”; skin, respiratory and gut mucosa-associated lymphoid tissues, adrenal glands. Additionally, the gut and liver are recently defined as active organs of IS. Although the primary functions of liver parenchymal cells are methabolical, they also carry out essential immune tasks. Beside all metabolic functions, liver has important role as an organ of IS.
IS begins to develop during intrauterine life. However, maturation of IS depends on antigenic stimulations from environment. The gut microbiota is initiated by maternal microorganisms gained during passage through birth channel and it dynamically change according to external conditions. The gut microbiota is necessary for proper “education” of IS. Although IS completes its maturation around teenage, lifelong antigenic stimulations from microbiota is needed for normal functioning of IS. Epidemiological observations and then, experimental data from germ-free animals leads to “hygiene hypothesis” and its modern extension called “microflora hypothesis”. According to these hypotheses, the higher levels of cleanliness and decreased exposure to microorganisms (driven by factors such as antibiotic use, xenobiotics, infection, or diet) during early childhood disrupt maturation if IS. In other words, the dysbiotic gut microbiota, which arisen during critical window of IS maturation, turns the differentiation of naïve immune gut dentritic cells (DCs) from generation of Treg (regulatory) cells by tolerogenic DCs into generation of effector T cells by immunogenic DCs. This shifts TH response from Th1 type (IFN-γ mediated) to Th2 type (IL-4 mediated). As a result, the risk for autoimmune and allergic diseases increases[5,6].
By definition IS has 2 parts; the innate IS and the adaptive IS. Although this division simplifies the understanding of immune processes, IS orchestrates whole immune cells during local or systemic responses.
The innate IS initiates first defense against insults, and is characterized by its ability to distinguish self from non-self. Its members are classic immune cells such as polymorphic nuclear leukocytes (neutrophils), monocytes, macrophages and DCs, natural killer (NK) cells, and innate lymphoid cells (ILCs), beside epithelial, endothelial and mesenchymal cells which are non-immune cells.
The inflammation during innate immune response is triggered by pattern-recognition receptors (PRRs). The 3 families of PRR, according to the structure they can recognize, are Toll-like receptors (TLRs), retinoic acid-inducible gene I-like receptors and nucleotide-binding oligomerization domain-like receptors. The cells expressing PRR can recognize conserved structures. For instance, miRNAs controls multiple immune processes such as regulating the innate immune responses of macrophages, dendritic cells and NK cells; involving in T-cell differentiation and function. Furthermore defective PRR function might lead to autoimmune or auto-inflammatory diseases, since nucleic acids (DNA and RNA) is commonly shared by the pathogen and host. Additionally, damage associated molecular patterns (DAMP), pathogen associated molecular patterns (PAMP), microbiome associated molecular patterns are the subtypes of PRR[6,7].
Mononuclear phagocyte system (MPS) composed by monocytes, macrophages, and DCs that have phenotypical and functional overlaping boundaries leading to uncertainty in differentiating them form each other. Antigen-presenting cells (APCs) express PRR and characterized by their ability to recognize, process and present antigens for activation of innate and adaptive immunity. The classical APCs include DC, monocytes and macrophages, although parenchymal cells can also act as APCs. MPS may be precursor of some APCs of liver, namely DCs and Kuppfer cells (KCs)[8].
ILCs are a recently identified family of heterogeneous variety of T cells and non-T cells, including NK cells, CD56+ Tcells, natural killer T cells (NKT), gamma/deltaT cells, mucosal- associated invariant T cells, lymphoid tissue-inducer cells and cells that produce IL-5, IL-13, IL-17 and IL-22. ILCs not only regulate innate and adaptive immune responses by promoting DC maturation into APCs, they have function in lymphoid tissue formation and the homeostasis of tissue stromal cells remodeling the tissues[9,10].
The adaptive immunity evolutionarily developed later than innate immunity in high-class vertebrates. The adaptive immune response occurs as second phase of immune response, mediated mainly by lymphocytes, and characterized by the features of antigen-specific response and memory response. It is initiated by antigen presentation lymphocytes. The main lymphoid repertoire includes T-cells, B cells. B cells produce specific antibodies in response to a specific antigen. These antibodies are crucial for T cells activation against bacterial infections and development of active immunization after vaccination[7,11]. However, the major mediator of adaptive immune response is the T cells, which control both the establishment and regulation of adaptive immunity.
T cells are identified by CD3 and T cell receptors (TCRs) positivity, and have vital importance in the adaptive and innate immunity. Conventional T cells express alpha-beta type TCR. Gamma-delta T cells are located in skin, genitourinary tract mucosa and gut, as well as liver. The naive T cells produced in bone barrow migrates to thymus and differentiate into 2 main subtypes are Th (helper) and Ts (supressor). The differentiated T cells are exported to periphery, where they become effector T cells upon activation by APCs or B cells. T cell activation requires binding of TCR to major histocompatibility complex (MHC), as well as binding of co-stimulatory molecule present on T cells to its co-receptor on APCs (e.g., binding of CD28 to B7). Th cells express CD4, which recognizes antigens in the context of MHC class II, and are mainly regulatory cells. Ts are cytotoxic cells carrying CD8 receptors, which are activated by MHC class I molecules[11]. Recently, CD4+ cells have been divided into subsets according to their distinct cytokine production and function; Th1, Th2, T17, Treg, Tfh (follicular T helper). Some features of CD4+ cells are as shown on Table 1. Treg cells express CD4+CD25+ and are essential for maintaining immune homeostasis and self-tolerance. Treg cells either naturally produced from CD4+ thymocytes in the thymus or iTreg cells are induced at periphery from naive CD4+ T cells in response to the low-dose stimulation of TCR, TGF-beta and IL-2. Beside all these effector T cells, there are also memory T cells. Id3 is the key transcriptional regulator for controlling T-cell differentiation into either effector T cells or memory T cells by its action though mTORC signaling[7,9,12].
| Th1 | Th2 | T17 | Treg | Tfh | |
| Produced Cytokines | IFN-gama, TNF-alpha, IL-2 | IL-4, IL-5, IL-9, IL-10, IL-13 | IL-17A, IL-17F, IL-21, IL-22, IL-26 | TGF-beta, IL-10 | CXCR5, IL-21 |
| Immune response mediated aginst | Intracellular pathogens | Extracellular parasites, allergy, humoral response | Extracellular bacteria and fungi, autoimmunity | IgA secretion, self-tolerance | Differentiation of B cells |
| Master transcription factors for differentiation | T-bet | GATA-3 | RORct | Foxp3 | Bcl6 |
| Effected cells | Macrophages, cytotoxic cells activated | Eosinophils, mast cells activated | Neutrophils activated | B cells activated Th1, Th2, Th17 supressed | B cells activated |
DCs are professional APC, which can recognize foreign antigens by their PRR, initiate immune response and constitute a bridge between innate and adaptive immunity. They primary screen surrounding microenvironment by antigen sampling and direct IS towards pro- or anti-inflammatory response[6]. DCs are found throughout the body as immature DCs and subdivided as plasmacytoid (or lymphoid) DCs and myeloid DCs. Plasmacytoid DCs mediate anti-viral immunity by its capability of viral recognition and type 1 interferons secretion. The myeloid DCs constitute conventional MPS derived DCs in blood, interstitial DCs in tissues, Langerhan cells in skin and monocyte-derived DCs. mDC can internalise antigens by phagocytosis, pinocytosis or receptor-mediated endocytosis. After generation of peptide by proteolytic degradation within endocytic vesicles, it complexes with newly synthesized MHC class II molecule within endocytic compartment, and then is carried via the trans-Golgi network to the cell surface. The recognition and internalization of pathogens by DCs leads to maturation of them into professional APCs, which have altered adhesion molecule and chemokine receptor expression. After maturation DCs leave primary side of infection through lymphatic’s to carry the internalized pathogen to secondary lymphoid organ. The professional APCs can be either immunogenic DCs which express high levels of MHC and co-stimulatory molecules, and secrete IL-12, IL-18, IL-21 and IL-23 or tolerogenic DCs having low expression levels, express inhibitory receptors, such as programmed death ligand-1, and releasing suppressive cytokines, such as IL-10, IL-27 and TGF-beta. Immunogenic DCs stimulate naïve CD4+ T cells to differentiation into effector cell mediating adaptive immunity against specific pathogen. On the other hand, if the antigenic peptid is presented to naïve CD4+ T cell by tolerogenic DCs, immune tolerance develops either at thymus or periphery. The consequen result in thymus is either T cell apoptosis or T cell maturation into natural Treg cells. The mechanisms for peripheral immune tolerance are anergy of T cells and exhaustion of T cells. The anergy arises when T cells are inactivated due to lack of co-stimulation. The exhaustion of T cells is characterized by expression of inhibitory receptors, namely programmed death-1 (PD-1), cytotoxic T lymphocyte antigen-4 (CTLA4) and T cell immunoglobulin mucin-3. Interestingly in mice and humans, the lipid content of DCs in liver determines the maturation type of APCs, as lipid content decreases tolerant immune response is favoured. This phenomenon might be important for progression of simple steatosis into steatohepatitis. The relationship of autoimmune diseases with infection and environmental pollution and is very well known fact. It is though that the similarity between insulting antigen and self antigens of individuals with susceptible HLA haplotypes causes a shift during APCs maturation from tolerogenic DCs towards to immunogenic DCs, leading to differention of naive T cells into effector ratherden tolerogenic cells, and ending in loss of self-immune tolerance[1,9,11].
NK express CD56 in the absence of CD3, but NKT express both of them. NKT mediate anti-tumor effect by activating CD8+ T cells cytotoxicity or overriding the tolerogenic mechanisms through counter-regulation of Treg cells. NKT can identify glycolipid antigens and subtyped into two according to TCR expression profile. Type 1 or invariant NKT (iNKT) carry an invariant TCR alfa-chain pairing with a limited number of beta-chains, whereas type 2 NKT cells express a diverse array of TCRs that recognize CD1d which is MHC class I-like molecule. Innate T lymphocytes (ITLs) is composed of iNKT cells and gamma-delta T cells. ITLs regulate adaptive immune response through its key roles in initiation and polaration of APCs and other cells of IS. This feature of ITLs has made them target as immunomodulation for treatment of autoimmune diseases[1,9].
In order to keep homeostasis for survival, the immune response had to continuously adopt according to age, sex, dietary antigens, hormones (i.e., pregnancy and lactation), and external stress factors such as microbiota, environmental flora or exposed chemicals[13]. Therefore IS has a dynamic nature and has a wide “range of normal”. Beyond being a metabolic organ attached to gut, liver recently has been defined as central axis in IS controlling local and systemic immune reactions and tolerance. All types of liver cells have active immune function, including both parenchymal cells (hepatocytes, cholangiocytes) and non-parenchymal cells [liver sinusoidal endothelial cells (LSECs), hepatic satellite cells (HSCs) or ito cells, KCs, neutrophiles, mononuclear cells, lymphocytes (B cells, T cells, NK cells, NKT cells, ITL)]. Parenchymal cells occupy most of liver volume (78%-80%). Non-parenchymal cells and extracellular space represent the remaining 5%-6% and 14%-17%, respectively[11,14].
The unique anatomical and histological features of liver are important for its immune functions. The liver is located at the junction between systemic and portal circulation. It is supplied by approximately 1.5 L of blood every minute; 2/3 via the portal vein and 1/3 via the hepatic artery. The double blood supply carries a massive antigenic load from the gastrointestinal tract and systemic circulation to liver. The blood, coming from these two sources mixes within sinusoids, and then flows through hepatic lobule from peri-portal area towards central vein. The fenestrated structures of sinusoids enable intimate interaction of antigens and blood immune cells with hepatocytes, KCs and HSCs at space of Disse. The abundant cells of the innate and adaptive ISs are located in hepatic sinusoids, and have ability for pathogen sensing, phagocytosis, cytotoxicity, cytokine release and antigen presentation to T cells.
The antigen-rich blood passing through the liver sinusoids is “scanned” by IS, which is tightly regulated between activation and tolerance. The liver remains tolerant to harmless dietary antigens, products of commensal gut microbiota and auto-antigens, while responds to exogen toxins, a variety of blood-borne or gut originated viruses, bacteria and parasites, as well as to metastatic cells, which try to home to the liver. Therefore, immune roles of liver can be divided into 2 groups; immune surveillance and induction of peripheral immune tolerance[3,15]. Indeed, the hepatic IS plays predominantly tolerogenic role. This can clinically be observed in liver transplant patients, e.g., liver allograft from major MHC or even ABO mismatched donors can be transplanted; if combined transplantation is done with organs from the same donor, non-liver allografts are more likely to be accepted; “operational tolerance”, which describes a patient with clinically normal graft function without needing immunosuppression, developments in up to 50% of hepatic transplantations[16].
The liver relies on its strong immunity for its immediate and efficiend defense against potentially toxic agents without triggering harmful immune response towards self-structures. Liver, primarily hepatocytes, synthesizes the major amount of proteins involved in local and systemic immune responses. These proteins are called acute phase reactants such as fibrinogen, proteinase inhibitors, complement proteins, PRRs [e.g., C reactive protein, lipopolysaccharide (LPS)-binding protein, peptidoglycan-recognition protein, soluble CD14], opsonizing proteins (e.g., mannose-binding lectin, serum amyloids), cytokines [e.g., IL-6, tumor necrosis factor-alpha (TNF-alpha) and transforming growth factor-beta (TGF-beta)], and hepcidin. The acute phase reactants function during innate immune response, mediate inflammation as well as tissue repair and regeneration. Their expression in hepatocytes is controlled by liver-enriched transcription factors (e.g., HNFs, C/EBPs), pro-inflammatory cytokines (e.g., IL-6, IL-22, IL-1β, TNF-α), and downstream signaling pathways (e.g., STAT3, NF-kappa B)[7,15].
Neutrophils are short-lived, circulating, phagocytic cells, which are recruited to site of infection by cytokines and chemokines, mainly IL-1 and IL-8. They are the first responders to infections and act by three main mechanisms; phagocytosis (requiring opsonization), generation of reactive oxygen species and degranulation (releasing enzymes and antimicrobial peptides), and formation of neutrophil extracellular traps (NETs). NETs are another mechanism of microbe killing. Nuclear DNA ligated with various microbicidal proteins released by activated neutrophils forms these webs. Under normal conditions the liver have few neutrophils, but they rapidly accumulate following necrosis. Neutrophils can rapidly shift their adhesive mechanisms in order to reqrude and form NETs in liver as a response to both endotoxin and bacteria[14].
Macrophages have been classified as classically activated macrophages (M1, secretes TNF-α, IL-1, IL-6, IL-8 and IL-12) or alternatively activated macrophages (M2, secretes IL-10 and TGF-β) based on their cytokine secretory patterns and proinflammatory vs immunoregulatory activity which however, are interchangeable functional states depending on the microenvironment the macrophages encounter[6,11]. KCs are fixed macrophages specialized at eliminating insoluble waste by phagocytosis and capable of processing and presenting antigens to T cells and participate in the regulation of the adaptive immune response. KCs reside on intravascular side of LSECs, and capture bacteria and able to bind component 3b under shear conditions while flowing through sinusoids. The role of KCs in microbial killing depends on the nature of the pathogen and on the recruited immune cells to the inflammation side. The characteristic feature of PRRs expression in liver is their constitutive expression and continuous low-level stimulation by endotoxins from gut. TLR4 is an PRRS expressed on all liver cells and it binds and clears endotoxins, and so initiates secretion of pro-inflammatory and anti-inflammatory cytokines[7].
Although, more than 80% of the CD3+ T cells are alpha-betaT-cells, the liver is also enrichmented by NKs and unconventional lymphocytes (NKT and gamma-delta T cells). The gamma-deltaT cells is 5 times higher in the liver (15%) then the periphery[4].
Hepatocytes, which constitutively express intercellular adhesion molecule-1, can directly interact with T cells through the fenestrations of LSECs. IFN-gamma primes hepatocytes to APCs by dose dependently enhancing HLA expression; from moderate HLA class I expression to enhanced HLA class II expression at low to high IFN-gamma levels. Hepatocyte primed naïve T cells either become effector T cells or undergoes apoptosis in the absence of co-stimulatory signals. On the other hand, cholangiocytes are relatively spared form antigenic stimulation from blood, but not from those one secreted into bile. Cholangiocytes can express TLRs, HLA class I at a low frequency and co-stimulatory molecules. Hepatotropic viruses (i.e., CMV) enhances HLA class I expression without inducing HLA class II. In pathological conditions such as that of PBC, cholangiocytes act as APC by overexpress HLA class II, as well as CD80 and CD86 co-stimulatory molecules. The limited experimental data supports that HSCs have capacity to act as APCs. The presentation of lipids to T-cells and NKT cells by HSCs can cause activation or tolerance in IS depending on co-stimulation[4,7].
Besides conferring strong local innate immunity, the liver regulates immune homeostasis as being a major site for induction of T cell mediated local and systemic adaptive immune response. Both resident and transiting T and B cells scattered throughout the parenchyma and the portal tracts become important effector cells of defensive adaptive immune in liver after activation by APCs. Both hepatic parenchymal and non-parenchymal cells can act as APCs depending on the stimulus and special cytokine milieu. The classical hepatic APCs are DCs and reticulo-endothelial system (including KCs and LSECs). However, hepatocytes and cholangiocytes become non-conventional APCs by expressing MHC II, if there is under pathological insult or persistent inflammation. Classical hepatic APCs constitutively express MHC class I-II, co-stimulatory receptors and molecules that promote antigen uptake (e.g., mannose and scavenger receptors). Under the physiological liver conditions, DCs are at immature developmental status and there is high production an anti-inflammatory cytokine (IL-10, TGF-b, TNF-a and prostaglandins) from reticulo-endothelial cells. This reduces capacity of APCs to activate effector T cells and lead to generation of anergic T cells and Treg cells. In other words, the tolerogenic nature of the liver by preferentially suppressing adaptive immunity is created by APCs, which kill or suppress effector CD4+ and CD8+ T cells and induce maturation of naïve T cells into Treg cells. Since diversion of portal flow results in the loss of immune-tolerance, it is hypothesized that physiological concentration of endotoxin is essential for maintain hepatic immune tolerance. LPS from gut microbiota drained to the liver by portal vein, modulates LSECs mediated CD4+ T cell activation by inducing secretion of IL-10 from LSECs and by down-regulating expression of MHC class II, CD80 and CD86 on LSEC. Another proposed mechanism for hepatic induction of peripheral immune-tolerance is clonal deletion/apoptosis of antigen-specific T cell at liver. HSCs may have a role in creation of tolerogenic microenvironment. HSCs have a capacity to serve as APCs, expand Treg cells, and promote T cell apoptosis (via B7-H1, PDL-1) or inhibit cytotoxic CD8+ T cells[4,9,15].
The local and systemic self-tolerance can be overridden and so autoimmune diseases can be initiated by several pathological immune mechanisms developed in liver. First of all, pathological antigen presentation might generate of auto reactive T cells and B cells due to defective clonal deletion (apoptosis of antigen-specific T cells). Similarly ILCs also switch on the autoimmunity by promoting antigen presentation with classical APCs, by releasing cytokines that polarize immune response towards effector T cells. ILCs may also be important mediators autoimmune liver injury by killing hepatocytes and/or bile duct epithelial cells. Pathological endotoxemia caused by dysbiotic microbiota may switch immune response from Th2 to Th1 predominance. Treg cells regulate both innate and adaptive immunity through regulation of CD4+ cells, KCs and LSECs. Therefore, defective function of Treg cells impairs hepatic immune tolerance leading to autoimmune hepatitis[4,7].
Hepatic IS is always active, regardless the overall response outcome. The nature of insult to liver and spectrum of activated cells determines the clinical picture. Healthy individuals have balanced immune surveillance of pathogens together with immune tolerance towards self-antigens. The over immune tolerance in liver leads to chronic infections with viruses or hepatic metastasis of cancer cells. In contrast the over activation of hepatic immune response causes fulminant hepatitis, allograft rejection or autoimmune diseases.
Hepatic immune hemoastasis is continuously rebalanced during clinical courses of cirrhosis. Patients with compensated cirrhosis have hyperactivated IS depending on underlying etiology of the liver diseases. Hepatic decompensation is associated with increased intestinal permeability. The episodic translocation of gut microbiota and their endotoxins into portal circulation triggers systemic and hepatic inflammation. PAMRs recognizing LPS, lipopeptides, glycopolymers, flagellin and bacterial DNA/RNA, activate innate and adaptive immunity. The released pro-inflammatory cytokines and chemokines cause hepatic injury and activation of DAMP. The viscous cycle between members of PRR, namely PAMP and DAMP exhausts IS and so, switches immune response from a predominantly ‘‘pro-inflammatory’’ to wards ‘‘immunodeficient’’ status. This very late stage of cirrhosis is clinically defined as acute- on-chronic liver failure (ACLF). The immune deficient state in ACLF patients is called cirrhosis associated immune deficiency[15].
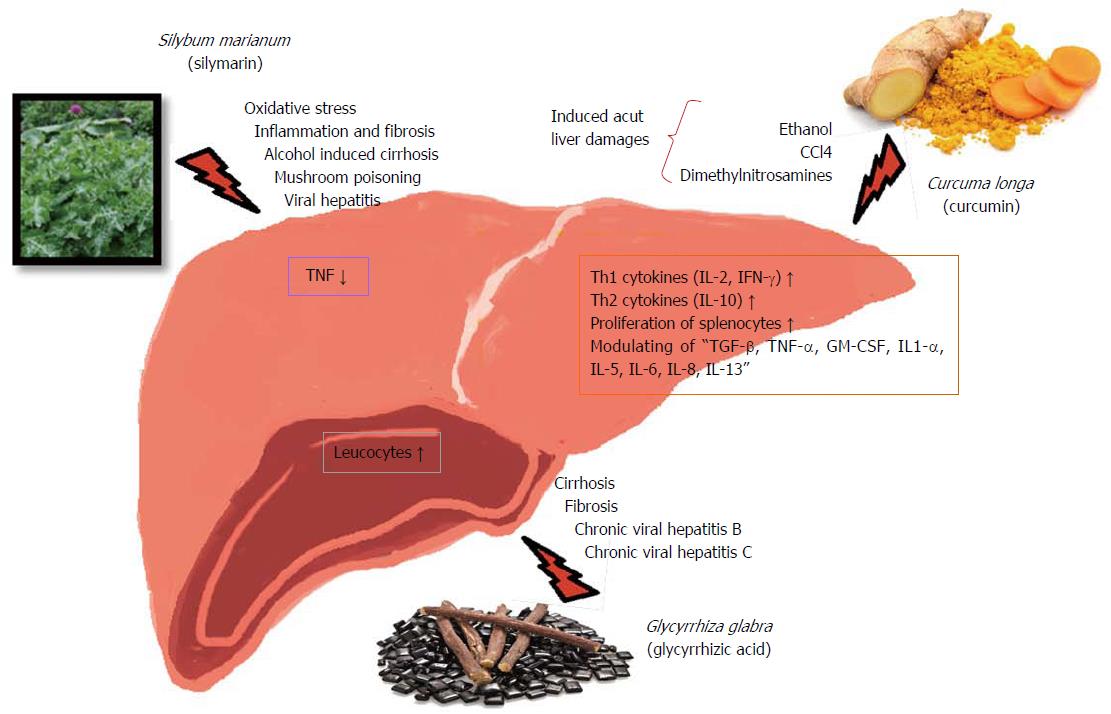
Herbal treatments are very often multifaceted blends of slightly processed medicinal plants, parts of the plants or products of the medicinal plants, which are traditionally accepted, comparatively low side-effects, and naturally compatible with the human body. Herbal remedies are applied for the treatment of a variety of indications and disorders, including hepatic as well as immunological problems (Table 2). Since scientific studies on herbal treatments have shown that they might effect cytokine and immunoglobulin secretion, cellular co-receptor expression, histamine release, lymphocyte proliferation, and cytotoxic activity, thus, herbal preparations might modify immune functions. In this study, literature was surveyed based on in vitro, in vivo and clinical studies on hepatoprotective, as well as immunostimulant and/or immunomodulator effective medicinal plants, which are lead by ethnopharmacological data. Table 2 was established, which covers common name, scientific name, effective part, known phytochemical content of the plant, ethnopharmacological/clinical effects, and medicinal preparation with their corresponding references. Due to complexity of the herbal treatments, the complete scientific data on mechanism of action is lacking, although clinical outcomes of herbal treatment are promising and leading the researchers to take on demanding scientific studies on the immune activity of herbal remedies (Table 3). The most applied medical plants in herbal treatments were selected and their mechanism of action on liver diseases and on immun system were searched to establish the Table 3. Flavonoid derivatives such as silybin, silymarin, obtained from milk-thistle [Silybum marianum (L.) Gaertn.] decreased alkaline phosphatase (completely) and gamma-glutamyl transpeptidase (partially) in CCl4 induced liver damage[17]. Moreover, it has been claimed that Silymarine containing preparations are the principal therapeutic of choice in liver diseases caused by oxidative stress. Many studies have proven that plant phyto compound Silymarin has medical applications to cure (alcoholic and non-alcoholic) fatty liver, cirrhosis, ischaemic injury, drug and chemically-induced hepatic toxicity, radiation toxicity, viral and toxic hepatitis by means of its anti-oxidative, anti-lipidperoxidative, anti-fibrotic, anti-inflammatory, liver regenerating and immunomodulating effects. Several studies have identified that continuous usage of Silymarin has significantly proved to increase the survival period of patients with alcohol-caused liver cirrhosis and primary liver cancer[18] (Figure 1). Scientific studies also have shown that, both silybin and silymarin normalized immunoregulatory failures by restoration of the cellular thiol status, T-cell activation (CD69), together with a substantial decrease in TNF[18,19]. Effects of the selected herbal medicines on immune and liver were summarized in Figure 1. Another study demonstrated that an edible plant Artichoke (Cynara scolymus L.) prevented CCl4 and oxidative stress-induced hepatotoxicity and it protected the liver[20]. Inulin, obtained from artichoke, stimulates components of the IS[21]. The extract of the rhizome Turmeric (Curcuma longa L.), which has hepatoprotective plant, amplified both Th1 (IL-2 and IFN gamma) and Th2 (IL-10) cytokines signifying its dual immune roles. Polysaccharide fraction of this rhizome showed potent immunostimulatory action in the direction of proliferation of splenocytes cell number and IL-10 secretion. Polysaccharides of the plant extract might be causative of these proliferative and cytokine release assets in murine splenocytes. In different studies have been shown that the cytokine productions (TGF-β, TNF-α, GM-CSF, IL-1α, IL-5, IL-6, IL-8, IL-10, IL-13, etc.) have been modulated by polysaccharide-enriched fractions[18,22-24]. Fennel (Foeniculum vulgare Mill.) and liquorice (Glycyrrhiza glabra L.) have also shown immunomodulatory and hepatoprotective effects[18,25-27].
| Common name | Scientıfic name | Effectıve part | Phytochemıcal content | Preparatıon on lıver dısorders1 | Ref. |
| Chaff-flower | Achyranthes aspera L. | Whole plant | Ecdysterone, achyranthine, betaine, pentatriaontane, 6-pentatriacontanone, hexatriacontane and tritriacontane | Natrossil natiris | [28-30] |
| Fennel | Foeniculum vulgare Mill. | Root | Coumarins (bergapten, ısopimpinellin, anthotoxin), flavonoids (quercetin, rutin) | Presselin dyspeptikum presselin, bupleurum compound phytomedicine, epagest lampugnani | [18,25,31] |
| Korean Ginseng, Chinese Red Ginseng | Panax ginseng Mey. | Root | Polysaccharides, saponins, ginsenoside | Tripid teguhsindo | [32-34] |
| Yarrow | Achillea sp. | Flower | Volatile oils, flavonoids, terpenoids, alkaloids, saponins, sesquiterpenlactones | Liv-52 drops, cheiranthol klein | [35-38] |
| Carqueja | Baccharis trimera (Less) DC | Epigeous part | Flavonoids, diterpenoids | Boldina Plata | [39-41] |
| Chicory | Cichorium intybus L. | Aerial part, root, leaf | Saccharides, methoxycoumarin cichorine, flavonoids, essential oils, anthocyanins | Natusor hepavesical soria natural, Liv-52 drops | [42-46] |
| Globe artichoke | Cynara cardunculus var. scolymus L. | Leaf | Sesquiterpenes lactones (cynaropicrin), flavonoids (cynaropicrin), phenolic acids (mainly caffeic acid derivatives) | Livstim mediherb, livton complex mediherb, lorbihepatic bioquimico, olocynan makros, rapacholin C herbapol wroclaw, farmasa, sylicynar herbapol poznan, alcafelol luper, bagohepat bago, armstrong, benevolus schwabe, boldina plata, cinarepa cristalfarma, colachofra EMS, cynarex roux-ocefa, herbapol wroclaw, cynarzym N altana, digestron loprofar, epagest lampugnani, figatil catarinense, salus, hecrosine B12 ortoquimica, hepatofalk falk, jurubileno ıbefar | [47,48] |
| Pale purple coneflower | Echinacea pallida (Nutt.) Nutt. Echinacea angustifolia (DC.) Hell. Echinacea purpurea (L.) Moench | Whole plant | Alkamides, polysaccharides, glycoproteins, cichoric acid (a derivate of caffeic acid) | Andrographis complex mediherb, kalbe, hepatin lapi, ımudator pyridam, herbal cleanse vitaplex | [49-52] |
| Faise daisy | Eclipta alba (syn E. prostrata L.) | Aerial part | Tannins, flavonoids, coumestans, saponins, alkaloids | Dipana promed | [53,54] |
| Chamomile | Matricaria chamomilla L. | Flower | Coumarin (herniarin and umbelliferone), phenylpropanoids (chlorogenic acid and caffeic acid), flavonoids (apigenin, apigenin-7-o-glucoside, luteolin, luteolin-7-o-glucoside, quercetin, rutin, naringenin), blue essential oils | Presselin dyspeptikum presselin, cholesol herbapol wroclaw, gotas digestivas bunker | [42,55-57] |
| Milk thisle | Silybum marianum (L.) Gaertn. | Seed | Polyphenolic flavonoids(silymarin, isosilyins, silibinins, silydianin silychristin) | Liverine cardinal, livermin korean ginseng, liverton sıffra, livosil-b centaur, livstim mediherb, livton complex mediherb, lomacholan lomapharm, phytohepar steigerwald, poikicholan lomapharm, prol procare, samarin berlin pharm, schwohepan S schworer, silegon teva, silibene merckle, silicur hexal, silimalon nikkho, silimarin benedetti, silimarit bionorica, silimax filofarm, silirex lampugnani, siliver farmasa, silliver abbott, silmar hennig, silvaysan sanum-kehlbeck, silybon micro, silygal ıvax, silyhexal hexal, sily-sabona sabona, mepha, sivylar ranbaxy, sylicaps herbapol lublin, sylicynar herbapol poznan, sylimarol herbapol pruszkow | [18,19,58,59] |
| Syliverin aflofarm, sylivit herbapol poznan, solas, vionin nf tempo scan pacific, alepa duopharm, apihepar madaus, vıa tris, aptivium liver support cynergen, ardeyhepan emonta, ardeypharm, bibol leloup hexa, bilisan duo repha | |||||
| Bioglan liver-vite bioglan, bupleurum complex mediherb, bupleurum compound phytomedicine, carsil sopharma, cefasliymarin cefak, cheiranthol klein, soho capsule/syrup, depatox progen, durasilymarin merck dura, eleparon sankyo, epagest lampugnani, flavobion zentiva, hegrimarin strathmann, strathmann, hepabene ratiopharm, merckle, ratiopharm, hepabesch strathmann, hepadigenor baliarda, hepaduran v otw, loges | |||||
| Capsule, hepamax dankos, hepa-merz sil merz, hepar-pasc pascoe, heparsyx n syxyl, heparviton bode, tempo scan pacific, hepatin lapi, kalbe, falk, darya-varia, hepatos, Yung shin, heplant spitzner, herbal liver formula faulding, worwag, legalon-madaus, laragon roemmers, ıfet, leveron vesco, limarin serum ınstitute, herbal cleanse vitaplex | |||||
| Dandelion | Taraxacum officinale G. | Root | Sesquiterpenes, saponins, phenolic compounds, flavonoids, sugars | Naturica DFM ıkapharmindo, livstim mediherb, livton complex mediherb, berberis complex blackmores, cholesol herbapol wroclaw, cinarepa cristalfarma, hepatofalk falk, herbal cleanse vitaplex | [60-63] |
| Radish | Raphanus sativus L. | Leaf, root | Flavanoids, terpenoids, alkaloids, saponins, sterols | Rapacholin AC herbapol wroclaw, rapacholin c herbapol wroclaw | [64-67] |
| Caper | Capparis spinosa L. | Root bark | Sugars (glucose, arabinose, mannose, galactose), lipid, volatile oils | Liv-52 drops | [68-71] |
| Kinkéliba | Combretum micranthum G. Don | Leaf | catechins, glycosylflavones, flavans, galloylated c-glycosylflavone derivatives, flavan-piperidine alkaloid | Tisane mediflor N°5 hepatique | [72-74] |
| Arjuna | Terminalia arjuna (Roxb.) Wight and Arn. | Bark | Arjunolic acid, tomentosic acid, arjunin, β-sitosterol, ellagic acid, leucodelphinidin, tannins | Liv-52 drops | [75-77] |
| Coffee senna | Cassia occidentalis L.(Senna occidentalis) | Leaf | Anthraquinones, saponins, sterols, triterpenes, quinines, tannins, flavonoids | Tisane mediflor N°5 hepatıque, Liv-52 drops | [78-80] |
| Liquorice, Licorice | Glycyrrhiza glabra L. | Root | Triterpene saponins, flavonoids, isoflavonoids and chalcones, glycyrrhizic acid | Tisane mediflor N°5 hepatıque, neo-minophagen C dexa, torii, curliv soho, soho capsule/syrup | [18,26,81] |
| Holy basil | Ocimum sanctum Linn. | Leaf | Volatile oils (eugenol, euginal, urosolic acid, carvacrol, linalool, limatrol, caryophyllene, methyl carvicol), anthocyans, alkaloids, flavonoids, tannins, carbohydrates,xylose, polysaccharides | Andrographis complex mediherb | [22,82-85] |
| Rosemary | Rosmarinus officinalis L. | Leaf | Diterpenoids, triterpenoids, phenolic acids, and flavonoids, carnosic acid, carnosol, rosmarinic acid | Tisane mediflor N°5 hepatıque, natusor hepavesical soria natural, cinarepa cristalfarma | [86-88] |
| Red sage, Danshen | Salvia miltiorrhiza Bunge. | Root | Tanshinones (tanshinone ı, tanshinoneıı, cryptotanshinone) miltirone and salvianolic acid a, b | Bupleurum complex mediherb | [18,89,90] |
| Common mallow | Malva sylvestris L. | Leaf | Amino acids/protein derivatives, flavonoids, mucilages, terpenoids, phenol derivatives, coumarin | Tisane mediflor N°5 hepatıque | [91-93] |
| Tinospora, Guduchi, Giloya | Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) | Root, stem | Flavonoids, alkaloids, sesquiterpenes, diterpenes arabinogalactan, syringine, cordiol, cordioside, cordifoliosides (a and b), berberine, tinosporine, giloin, giloinin | Dipana promed | [22,33,94-96] |
| Boldo, Boldu, Boldus | Peumus boldus Molina | Leaf | Alkaloids (isoquinoline-boldine, isoboldine, 6a,7-dehydroboldine, isocorydine, isocorydine-n-oxide, norisocorydine, laurolitsine, laurotetanine, n-methyllaurotetanine, reticuline, (-)-pronuciferine, sinoacutine), flavonoids, volatile oil, coumarin, resin, tannin) | Tisane mediflor N°5 hepatıque, natrossil natiris, natusor hepavesical soria natural, prinachol zurita, farmasa, alcafelol luper, berberis complex blackmores, boldina plata, boldopeptan neo quimica, colachofra ems, cynarzym N altana, eparema nycomed, figatil catarinense, gotas digestivas bunker, jurubileno ıbefar | [97-99] |
| Greater celandine | Chelidonium majus L. | Aerial parts | Isoquinoline alkaloids, such as sanguinarine, chelidonine, chelerythrine, berberine and coptisine, (-)-turkiyenine) | Livstim mediherb, livton complex mediherb, natusor hepavesical soria natural, schwohepan S schworer, berberis complex blackmores, chelicur hasco-lek, cynarzym N altana, hepatofalk falk, falk, darya-varia | [100-105] |
| Gale of the wind | Phyllanthus niruri L. | Whole plant | Flavonoids, alkaloids, terpenoids, lignans, polyphenols, tannins, coumarins and saponins | Natrossil natiris, meprofarm, dipana promed, gramuno graha, hepimun landson, ımudator pyridam | [106-108] |
| Kutki | Picrorhiza kurroa Royle ex Benth. | Rhizome, root | Iridoid glycoside (picrovil) | Dipana promed | [109,110] |
| Rhubarb | Rheum emodi | Rhizome | Anthraquinone (rhein, chrysophanol, aloe-emodin, emodin, physcion, and their glycosides) and stilbene (picetannol, resveratrol and their glycosides), flavonoids, glycosides, tannins, volatile oils, saponins | Natrossil natiris, boldopeptan neo quimica, eparema nycomed, SIT, hepatofalk falk | [111] |
| Magnolia-vine, Schisandra | Schisandra chinensis (Turcz.) Baill. | Fruit | Dibenzocyclooctadiene derivative lignans (or schisandra lignans), organic acids (citric, malic, fumaric and tartaric acid), sugars, vitamic C, vitamin E, phenolic acids, tannins, phytosterols, essential oil | Curliv soho, soho capsule/syrup, hepacell medikon, hepamax dankos | [31,112,113] |
| European black nightshade | Solanum nigrum L. | Whole plant | Glycoalkaloids, glycoproteins, polysaccharides, polyphenolic compounds (gallic acid, catechin, protocatechuic acid (pca), caffeic acid, epicatechin, rutin, naringenin) | Liv-52 drops, dipana promed | [114-117] |
| French tamaris | Tamarix gallica L. | Aerial parts | Tannin, tamarixin, tamauxetin, troupin, 4-methylcoumarin and 3,3’-di-o-methylellagic acid, tannic acid, 4-methylcoumarin and 3,3’-di-o-methylellagic acid | Liv-52 drops | [118,119] |
| Turmeric | Curcuma longa L. | Rhizome | Curcuminoid | Meprofarm, tripid teguhsindo, turmerik knop, aptivium liver support cynergen, chelicur hasco-lek, galena, ıvax, cinarepa cristalfarma, depatox progen, heparviton bode, tempo scan pacific, kalbe, hepatin lapi, falk | [22,31,120] |
| Ginger | Zingiber officinale Roscoe | Rhizome | Volatile oils, pungent phenol compounds [sesquiterpenoids,beta-sitosterol palmitate, isovanillin, glycol monopalmitate, hexacosanoic acid 2,3-dihydroxypropyl ester, maleimide-5-oxime, p-hydroxybenzaldehyde adenine, 6-gingerol, 6-shogaol, 1-(omega-ferulyloxyceratyl) glycerols] | Presselin dyspeptikum presselin, dipana promed, herbal cleanse vitaplex | [121-127] |
| Scientific name | Effect/mechanism on liver | Effect/mechanısm on immun system | Ref. |
| Curcuma longa L. | Acute liver damage by chemicals, e.g., ethanol, CCl4, Dimethylnitrosamines | Immunostimulant, immunomodulatory | [18,22-24] |
| The extract of the rhizome C. longa increased both Th1 (IL-2 and IFN gamma) and Th2 (IL-10) cytokines indicating its dual immune functions. NR-INF-02 significantly increased the IL-2 and IFN gamma levels in Con A stimulated splenic lymphocytes. The above results indicated that NR-INF-02 showed a specific immunity response by stimulating both Th1 and Th2 cells | |||
| Polysaccharide fraction of the rhizome showed potent immunostimulatory activity towards proliferation of splenocytes cell number and IL-10 secretion. Polysaccharides might be contributing to this proliferative and cytokine release property in murine splenocytes | |||
| Hot water extracts of the rhizome showed that the high polarity fraction exhibited stimulatory effects on PBMC. The cytokine productions (TGF-beta, TNF-alpha, GM-CSF, IL-1alpha, IL-5, IL-6, IL-8, IL-10, IL-13, etc.) have been modulated by a polysaccharide-enriched fraction.The proportion of CD14 positive stained PBMC was increased by the fraction | |||
| Anti-HIV-1 and HIV-2 | |||
| Foeniculum vulgare Mill. | Oxidative stress of the liver bacterial and viral infections anti-inflammatory, acute hepatotoxicity | Immunomodulatory | [18,25] |
| Antimicrobial, antifungal | |||
| Glycyrrhiza glabra L. | Cirrhosis fibrosis chronic viral hepatitis B and C | Immunomodulatory | [18,26,27] |
| Leukocyte count and phagocytic index (carbon clearance) was increased significantly with the treatment of water extract of G. glabra root. Zinc (45 mg/kg) in combination with ALE (0.75 g/kg) showed highly significant increase of leukocyte count and phagocytic index | |||
| Silybum marianum (L.) Gaertn. | Oxidative stress inflammation and fibrosis alcohol-induced cirrhosis mushroom poisoning viral hepatitis | Immunomodulatory | [18,19] |
| Flavonoids from S. marianum normalize immunoregulatory defects via restoration of the cellular thiol status. T-cell activation (CD69), along with a significant decrease in TNF |
Herbal drugs are composed of complex mixtures of phytochemicals, unlike conventional and plant originated single compound drugs, which are composed of known chemical constituents and are precisely quantified. For that reasons studying the clinical effects of individual chemical constituents separately will not be accurate, due to various reasons, such as the synergistic or inhibiting effects of phytochemicals on each other and neutralization of harmful chemicals in the mixture by other compounds, which provides a flawless combination for therapeutic purposes. Aspects such as, absorption, distribution intrinsic concentration and metabolism of the drug should be known precisely to determine the dosage, safety margin and length of treatment. Moreover, future research should include characterization of multifactorial mechanisms of action, elucidation of adverse effects and well-designed clinical trials in pediatrics and geriatrics as well.
Scientists as well as immunologists, who study herbal treatments in hepatic diseases most be ready to face challenges and opportunities. In vivo and clinical molecular researches on immunomodulatory, immunoenhancing, immunostimulant effects of herbal treatments will offer novel perceptions into IS and immunotherapy. Not only single plant extract, but the interactions of the ingredients in a given herbal treatment formula determines the final clinical picture by finely tuning the balance between therapeutic effect and hepatotoxicity. Feature studies must pricely define the interaction between the liver as an organ regulating local and systemic immune response and complex action mechanisms of herbal treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chan SPL, He ST S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 2. | Liu J, Liu S, Cao X. Highlights of the advances in basic immunology in 2011. Cell Mol Immunol. 2012;9:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Navarro VJ, Khan I, Björnsson E, Seeff LB, Serrano J, Hoofnagle JH. Liver injury from herbal and dietary supplements. Hepatology. 2017;65:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 4. | Dara L, Liu ZX, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int. 2016;36:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Elmore SA. Enhanced histopathology of the immune system: a review and update. Toxicol Pathol. 2012;40:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Marques PE, Oliveira AG, Chang L, Paula-Neto HA, Menezes GB. Understanding liver immunology using intravital microscopy. J Hepatol. 2015;63:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Strauss O, Dunbar PR, Bartlett A, Phillips A. The immunophenotype of antigen presenting cells of the mononuclear phagocyte system in normal human liver--a systematic review. J Hepatol. 2015;62:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Invernizzi P. Liver auto-immunology: the paradox of autoimmunity in a tolerogenic organ. J Autoimmun. 2013;46:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Levine J. The impact of immune dysregulation on the development of autoimmune gastrointestinal and liver disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:322-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 11. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 847] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 12. | Food and Drug Administration. Dietary Supplements. 21 January 2016. Accessed: February 15 2017; Available from: http://www.fda.gov/Food/DietarySupplements/default.htm. |
| 13. | Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 2015;4:143-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1116] [Article Influence: 124.0] [Reference Citation Analysis (1)] |
| 15. | Lederberg J, Mc Cray AT. ‘Ome Sweet ‘Omics-a genealogical treasury of words. Scientist. 2001;15:8. |
| 16. | Emond JC, Griesemer AD. Tolerance in clinical liver transplantation: The long road ahead. Hepatology. 2017;65:411-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Muriel P, Mourelle M. Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J Appl Toxicol. 1990;10:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Rajaratnam M, Prystupa A, Lachowska-Kotowska P, Zaluska W, Filip R. Herbal medicine for treatment and prevention of liver diseases. JPCCR. 2014;8:55-60. [DOI] [Full Text] |
| 19. | Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr Cancer Ther. 2007;6:146-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Mehmetçik G, Ozdemirler G, Koçak-Toker N, Cevikbaş U, Uysal M. Effect of pretreatment with artichoke extract on carbon tetrachloride-induced liver injury and oxidative stress. Exp Toxicol Pathol. 2008;60:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | López-Molina D, Navarro-Martínez MD, Rojas Melgarejo F, Hiner AN, Chazarra S, Rodríguez-López JN. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry. 2005;66:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Adhvaryu MR, Reddy N, Parabia MH. Effects of four Indian medicinal herbs on Isoniazid-, Rifampicin- and Pyrazinamide-induced hepatic injury and immunosuppression in guinea pigs. World J Gastroenterol. 2007;13:3199-3205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacognosy Res. 2013;5:71-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Yue GG, Chan BC, Hon PM, Kennelly EJ, Yeung SK, Cassileth BR, Fung KP, Leung PC, Lau CB. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int J Biol Macromol. 2010;47:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | He W, Huang B. A review of chemistry and bioactivities of a medicinal spice: Foeniculum vulgare. J Med Plant Res. 2011;5:3595-3600. |
| 26. | Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 781] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 27. | Mazumder PM, Pattnayak S, Parvani H, Sasmal D, Rathinavelusamy P. Evaluation of immunomodulatory activity of Glycyrhiza glabra l roots in combination with zing. Asian Pac J Trop Biomed. 2012;2:15-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Bafna AR, Mishra S. Effect of methanol extract of Achyranthes aspera linn. on rifampicin-induced hepatotoxicity in rats. Ars Pharmaceutica. 2004;45:343-351. |
| 29. | Chakrabarti R, Vasudeva RY. Achyranthes aspera stimulates the immunity and enhances the antigen clearance in Catla catla. Int Immunopharmacol. 2006;6:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Srivastav S, Singh P, Mishra G, Jha KK, Khosa RL. Achyranthes aspera-An important medicinal plant: A review. J Nat Prod Plant Resour. 2011;1:1-14. |
| 31. | Duke JA. Handbook of phytochemical constituent grass, herbs and other economic plants. CRC press. Boca Raton, FL. 1992;54-59, 197-199, 224-229, 257-259, 438-441. |
| 32. | Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Tang W, Eisenbrand G. Panax ginseng CA Mey. In Chinese drugs of plant origin Springer Berlin Heidelberg. 1992;711-737. |
| 35. | Nemeth E, Bernath J. Biological activities of yarrow species (Achillea spp.). Curr Pharm Des. 2008;14:3151-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Saeidnia S, Gohari A, Mokhber-Dezfuli N, Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 2011;19:173-186. [PubMed] |
| 37. | Sharififar F, Pournourmohammadi S, Arabnejad M. Immunomodulatory activity of aqueous extract of Achillea wilhelmsii C. Koch in mice. Indian J Exp Biol. 2009;47:668-671. [PubMed] |
| 38. | Yaeesh S, Jamal Q, Khan AU, Gilani AH. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother Res. 2006;20:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Paul EL, Lunardelli A, Caberlon E, de Oliveira CB, Santos RC, Biolchi V, Bastos CM, Moreira KB, Nunes FB, Gosmann G. Anti-inflammatory and immunomodulatory effects of Baccharis trimera aqueous extract on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation. 2009;32:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Soicke H, Leng-Peschlow E. Characterisation of flavonoids from Baccharis trimera and their antihepatotoxic properties. Planta Med. 1987;53:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Torres LM, Gamberini MT, Roque NF, Lima-Landman MT, Souccar C, Lapa AJ. Diterpene from Baccharis trimera with a relaxant effect on rat vascular smooth muscle. Phytochemistry. 2000;55:617-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Amirghofran Z, Azadbakht M, Karimi MH. Evaluation of the immunomodulatory effects of five herbal plants. J Ethnopharmacol. 2000;72:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Gadgoli C, Mishra SH. Antihepatotoxic activity of Cichorium intybus. J Ethnopharmacol. 1997;58:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Gilani AH, Janbaz KH. Evaluation of the liver protective potential of Cichorium intybus seed extract on Acetaminophen and CCl(4)-induced damage. Phytomedicine. 1994;1:193-197. [PubMed] [DOI] [Full Text] |
| 45. | Heibatollah S, Reza NM, Izadpanah G, Sohailla S. Hepatoprotective effect of Cichorium intybus on CCl4-induced liver damage in rats. Afr J Biochem Res. 2008;2:141-144. |
| 46. | Street RA, Sidana J, Prinsloo G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid Based Complement Alternat Med. 2013;2013:579319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Braun L, Cohen M. Herbs & Natural Supplements: An Evidence-based guide (3rd ed.). Chatswood, NWS: Churchill Livingstone/Elsevier 2010; 207, 532. |
| 48. | Menin B, Moglia A, Comino C, Hakkert JC, Lanteri S, Beekwilder J. In vitro callus-induction in globe artichoke (Cynara cardunculus L. var. scolymus) as a system for the production of caffeoylquinic acids. J Hortic Sci Biotech. 2013;88:537-542. [DOI] [Full Text] |
| 49. | Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt.,Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 50. | Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2:247-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Binns SE, Livesey JF, Arnason JT, Baum BR. Phytochemical variation in echinacea from roots and flowerheads of wild and cultivated populations. J Agric Food Chem. 2002;50:3673-3687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Rusu MA, Tamas M, Puica C, Roman I, Sabadas M. The hepatoprotective action of ten herbal extracts in CCl4 intoxicated liver. Phytother Res. 2005;19:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Dalal S, Kataria SK, Sastry KV, Rana SVS. Phytochemical screening of methanolic extract and antibacterial activity of active principles of hepatoprotective herb, Eclipta alba. Ethnobot Leaflets. 2010;2010:3. |
| 54. | Mithun NM, Shashidhara S, Vivek Kumar R. Eclipta alba (L.) A review on its phytochemical and pharmacological profile. Pharmacologyonline. 2011;1:345-357. |
| 55. | Salamon I. Chamomile a medicinal plant. J Herbs Spices Med Plants. 1992;10:1-4. |
| 56. | Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 57. | Tavakol HS, Farzad K, Fariba M, Abdolkarim C, Hassan G, Seyed-Mostafa HZ, Akram R. Hepatoprotective effect of Matricaria chamomilla.L in paraquat induced rat liver injury. Drug Res (Stuttg). 2015;65:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 460] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 59. | Madani H, Talebolhosseini M, Asgary S, Nader GH. Hepatoprotective activity of Silybum marianum and Cichorium intybus against thioacetamide in rat. Pak J Nutr. 2008;7:1726. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Martinez M, Poirrier P, Chamy R, Prüfer D, Schulze-Gronover C, Jorquera L, Ruiz G. Taraxacum officinale and related species-An ethnopharmacological review and its potential as a commercial medicinal plant. J Ethnopharmacol. 2015;169:244-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Schütz K, Carle R, Schieber A. Taraxacum--a review on its phytochemical and pharmacological profile. J Ethnopharmacol. 2006;107:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 62. | Yoon TJ. Effect of water extracts from root of Taraxacum officinale on innate and adaptive immune responses in mice. Korean J Food Nutr. 2008;21:275-282. |
| 63. | You Y, Yoo S, Yoon HG, Park J, Lee YH, Kim S, Oh KT, Lee J, Cho HY, Jun W. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol. 2010;48:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 64. | Baek SH, Park M, Suh JH, Choi HS. Protective effects of an extract of young radish (Raphanus sativus L) cultivated with sulfur (sulfur-radish extract) and of sulforaphane on carbon tetrachloride-induced hepatotoxicity. Biosci Biotechnol Biochem. 2008;72:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Chaturvedi P, George S, Machacha CN. Protective role of Raphanus sativus root extract on paracetamol-induced hepatotoxicity in albino rats. Int J Vitam Nutr Res. 2007;77:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Popovic M, Lukie V, Jakovljevie V, Mikov M. The effect of the radish juice on liver function. Fitoterapia. 1993;64:229-231. |
| 67. | Syed SN, Rizvi W, Kumar A, Khan AA, Moin S, Ahsan A. In Vitro Antioxidant and In Vivo Hepatoprotective Activity of Leave Extract of Raphanus sativus in Rats Using CCL 4 Model. Afr J Tradit Complement Altern Med. 2014;11:102-106. |
| 68. | Aghel N, Rashidi I, Mombeini A. Hepatoprotective activity of Capparis spinosa root bark against CCl4 induced hepatic damage in mice. Iran J Pharm Res. 2010;285-290. |
| 69. | Arena A, Bisignano G, Pavone B, Tomaino A, Bonina FP, Saija A, Cristani M, D’Arrigo M, Trombetta D. Antiviral and immunomodulatory effect of a lyophilized extract of Capparis spinosa L. buds. Phytother Res. 2008;22:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Sher H, Alyemeni MN. Ethnobotanical and pharmaceutical evaluation of Capparis spinosa L, validity of local folk and Unani system of medicine. J Med Plant Res. 2010;4:1751-1756. [DOI] [Full Text] |
| 71. | Tlili N, Elfalleh W, Saadaoui E, Khaldi A, Triki S, Nasri N. The caper (Capparis L.): ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia. 2011;82:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 72. | Bitsindou M, Lejoly J. Plants used in hepatoprotective remedies in traditional African medicine. In WOCMAP I-Medicinal and Aromatic Plants Conference: part 2 of 4. 1992;332:73-80. |
| 73. | Dicarlo FJ, Haynes LJ, Sliver NJ, Phillips GE. Reticuloendothelial system stimulants of botanical origin. J Reticuloendothel Soc. 1964;1:224-232. [PubMed] |
| 74. | Welch CR. Chemistry and pharmacology of Kinkéliba (Combretum micranthum), a West African medicinal plant. Doctoral dissertation, Rutgers University-Graduate School-New Brunswick. 2010;. |
| 75. | Halder S, Bharal N, Mediratta PK, Kaur I, Sharma KK. Anti-inflammatory, immunomodulatory and antinociceptive activity of Terminalia arjuna Roxb bark powder in mice and rats. Indian J Exp Biol. 2009;47:577-583. [PubMed] |
| 76. | Manna P, Sinha M, Sil PC. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complement Altern Med. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | Paarakh PM. Terminalia arjuna (Roxb.) Wt. and arn: A review. Int J Pharmacol. 2010;6:515-534. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 78. | Abongwa M, Rageh GA, Arowolo O, Dawurung C, Oladipo O, Atiku A. Efficacy of Senna occidentalis in the amelioration of tetracycline induced hepatoand nephro-toxicities in rabbits. Toxicol Lett. 2010;196S:S207: P206-009. |
| 79. | Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Yadav JP, Arya V, Yadav S, Panghal M, Kumar S, Dhankhar S. Cassia occidentalis L.: a review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia. 2010;81:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 81. | Kaur R, Kaur H, Dhindsa AS. Glycyrrhiza glabra: a phytopharmacological review. IJPSR. 2013;4:2470. [DOI] [Full Text] |
| 82. | Jeba CR, Vaidyanathan R, Rameshkumar G. Immunomodulatory activity of aqueous extract of Ocimum sanctum in rat. Int J Pharm Biomed Res. 2011;2:33-38. |
| 83. | Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 84. | Kumar A, Rahal A, Chakraborty S, Tiwari R, Latheef SK, Dhama K. Ocimum sanctum (Tulsi): a miracle herb and boon to medical science-A Review. Int J Agron Plant Prod. 2013;4:589. |
| 85. | Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol. 2005;49:125-131. [PubMed] |
| 86. | al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol. 1999;37:124-130. [PubMed] |
| 87. | Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, Stojanovic I, Gerothanassis IP, Tzakos AG. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 88. | Sakr SA, Lamfon HA. Protective effect of rosemary (Rosmarinus officinalis) leaves extract on carbon tetrachloride-induced nephrotoxicity in albino rats. Life Sci J. 2012;9:779-785. |
| 89. | Lee TY, Mai LM, Wang GJ, Chiu JH, Lin YL, Lin HC. Protective mechanism of salvia miltiorrhiza on carbon tetrachloride-induced acute hepatotoxicity in rats. J Pharmacol Sci. 2003;91:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Wang BQ. Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J Med Plant Res. 2010;4:2813-2820. |
| 91. | Gasparetto JC, Martins CA, Hayashi SS, Otuky MF, Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L.: a millennial herbal medicine. J Pharm Pharmacol. 2012;64:172-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 92. | El Ghaoui WB, Ghanem EB, Chedid LA, Abdelnoor AM. The effects of Alcea rosea L., Malva sylvestris L. and Salvia libanotica L. water extracts on the production of anti-egg albumin antibodies, interleukin-4, gamma interferon and interleukin-12 in BALB/c mice. Phytother Res. 2008;22:1599-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Hussain L, Ikram J, Rehman K, Tariq M, Ibrahim M, Akash MSH. Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turk J Biol. 2014;38:396-402. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Kavitha BT, Shruthi SD, Rai SP, Ramachandra YL. Phytochemical analysis and hepatoprotective properties of Tinospora cordifolia against carbon tetrachloride-induced hepatic damage in rats. J Basic Clin Pharm. 2011;2:139-142. [PubMed] |
| 96. | Sinha K, Mishra NP, Singh J, Khanuja SPS. Tinospora cordifolia (Guduchi), a reservoir plant for therapeutic applications: A Review. Indian J Tradit Know. 2004;3:257-270. |
| 97. | EMEA - European Medical Agency-Committee on Herbal Medicinal Products, List of References Supporting the Assessment Report on: Peumus boldus, London: European Medicines Agency Evaluation of Medicines for Human Use, 2009. . |
| 98. | González-Cabello R, Speisky H, Bannach R, Valenzuela A, Fehér J, Gergely P. Effects of boldine on cellular immune functions in vitro. J Investig Allergol Clin Immunol. 1993;4:139-145. [PubMed] |
| 99. | Lanhers MC, Joyeux M, Soulimani R, Fleurentin J, Sayag M, Mortier F, Younos C, Pelt JM. Hepatoprotective and anti-inflammatory effects of a traditional medicinal plant of Chile, Peumus boldus. Planta Med. 1991;57:110-115. [PubMed] [DOI] [Full Text] |
| 100. | Gilca M, Gaman L, Panait E, Stoian I, Atanasiu V. Chelidonium majus--an integrative review: traditional knowledge versus modern findings. Forsch Komplementmed. 2010;17:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Kadan G, Gözler T, Shamma M. (-)-Turkiyenine, a new alkaloid from Chelidonium majus. J Nat Prod. 1990;53:531-532. [DOI] [Full Text] |
| 102. | Küpeli E, Koşar M, Yeşilada E, Hüsnü K, Başer C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72:645-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 103. | Mehta VB, Sathaye SS, Amin PD. Hepatoprotective potental of Chelidonium majus and Myrica cerifera in drug induced hepatotoxicity. Drug Manufact. 2006;43:12. |
| 104. | Mitra S, Gole M, Samajdar K, Sur RK, Chakraborty BN. Antihepatotoxic activity of Chelidonium majus. Int J Pharmacogn. 1992;30:125-128. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Song JY, Yang HO, Pyo SN, Jung IS, Yi SY, Yun YS. Immunomodulatory activity of protein-bound polysaccharide extracted from Chelidonium majus. Arch Pharm Res. 2002;25:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Amin ZA, Abdulla MA, Ali HM, Alshawsh MA, Qadir SW. Assessment of in vitro antioxidant, antibacterial and immune activation potentials of aqueous and ethanol extracts of Phyllanthus niruri. J Sci Food Agric. 2012;92:1874-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 107. | Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol. 2006;58:1559-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 108. | Harish R, Shivanandappa T. Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem. 2006;95:180-185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 109. | Sharma ML, Rao CS, Duda PL. Immunostimulatory activity of Picrorhiza kurroa leaf extract. J Ethnopharmacol. 1994;41:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 110. | Verma PC, Basu V, Gupta V, Saxena G, Rahman LU. Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa royle ex benth. (kutki). Curr Pharm Biotechnol. 2009;10:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 111. | Rehman H, Begum W, AnjumF , Tabasum H. Rheum emodi (Rhubarb): A fascinating herb. J Pharmacogn Phytochem. 2014;3:89-94. |
| 112. | Chan SW. Panax ginseng, Rhodiola rosea and Schisandra chinensis. Int J Food Sci Nutr. 2012;63 Suppl 1:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Ekiert R, Szopa A, Ekiert H, Krzek J, Dzik E. Analysis of lignans in Schisandra chinensis fruits, leaves, biomasses from in vitro cultures and food supplements. J Funct Foods. 2013;5:1576–1581. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Atanu FO, Ebiloma UG, Ajayi EI. A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol Mol Biol Rev. 2011;6:1-8. |
| 115. | Jain R, Sharma A, Gupta S, Sarethy IP, Gabrani R. Solanum nigrum: current perspectives on therapeutic properties. Altern Med Rev. 2011;16:78-85. [PubMed] |
| 116. | Li J, Li QW, Gao DW, Han ZS, Lu WZ. Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phytother Res. 2009;23:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)-induced oxidative damage in rats. Chem Biol Interact. 2008;171:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 118. | Huseini HF, Alavian SM, Heshmat R, Heydari MR, Abolmaali K. The efficacy of Liv-52 on liver cirrhotic patients: a randomized, double-blind, placebo-controlled first approach. Phytomedicine. 2005;12:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 119. | Sehrawat A, Sultana S. Tamarix gallica ameliorates thioacetamide-induced hepatic oxidative stress and hyperproliferative response in Wistar rats. J Enzyme Inhib Med Chem. 2006;21:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Araújo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 385] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 121. | Abdel-Azeem AS, Hegazy AM, Ibrahim KS, Farrag AR, El-Sayed EM. Hepatoprotective, antioxidant, and ameliorative effects of ginger (Zingiber officinale Roscoe) and vitamin E in acetaminophen treated rats. J Diet Suppl. 2013;10:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Abdullah N, Saat NZM, Hasan HA, Budin SB, Kamaralzaman S. Protective effect of the ethanol extract of Zingiber officinale Roscoe on paracetamol induced hepatotoxicity in rats. Jurnal Sains Kesihatan Malaysia. 2004;2:85-95. |
| 123. | Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 736] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 124. | Bao L, Deng A, Li Z, Du G, Qin H. [Chemical constituents of rhizomes of Zingiber officinale]. Zhongguo Zhongyao Zazhi. 2010;35:598-601. [PubMed] |
| 125. | Carrasco FR, Schmidt G, Romero AL, Sartoretto JL, Caparroz-Assef SM, Bersani-Amado CA, Cuman RK. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: evidence for humor- and cell-mediated responses. J Pharm Pharmacol. 2009;61:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 126. | Ezeonu CS, Egbuna PAC, Ezeanyika LUS, Nkwonta CG, Idoko ND. Antihepatotoxicity studies of crude extract of Zingiber officinale on CCl 4 inducedtoxicity and comparison of the extract’s fraction D hepatoprotective capacity. Res J Med Sci. 2011;5:102-107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Kumar G, Karthik L, Rao KB. A review on pharmacological and phytochemical properties of Zingiber officinale Roscoe (Zingiberaceae). J Pharm Res. 2011;4:2963-2966. |