Published online Jun 8, 2017. doi: 10.4254/wjh.v9.i16.733
Peer-review started: February 12, 2017
First decision: March 10, 2017
Revised: March 22, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: June 8, 2017
Processing time: 129 Days and 2.3 Hours
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a recently introduced technique aimed to perform two-stage hepatectomy in patients with a variety of primary or secondary neoplastic lesions. ALPSS is based on a preliminary liver resection associated with ligation of the portal branch directed to the diseased hemiliver (DH), followed by hepatectomy after an interval of time in which the future liver remnant (FLR) hypertrophied adequately (partly because of preserved arterialization of the DH). Multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) play a pivotal role in patients’ selection and FLR assessment before and after the procedure, as well as in monitoring early and late complications, as we aim to review in this paper. Moreover, we illustrate main abdominal MDCT and MRI findings related to ALPPS.
Core tip: Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a variant of two-stage hepatectomy aimed to obtain rapid hypertrophy of the future liver remnant. Given its recent introduction, there are still controversies on indications and safety issues. Cross-sectional imaging by means of multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) play a key role in the multidisciplinary process of patients’ selection and postoperative management. This review aims to emphasize such a role and illustrate main abdominal ALPPS-related findings on MDCT or MRI.
- Citation: Zerial M, Lorenzin D, Risaliti A, Zuiani C, Girometti R. Abdominal cross-sectional imaging of the associating liver partition and portal vein ligation for staged hepatectomy procedure. World J Hepatol 2017; 9(16): 733-745
- URL: https://www.wjgnet.com/1948-5182/full/v9/i16/733.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i16.733
Resection is the only treatment proven to achieve long-term survival in patients with primary hepatic malignancies or selected liver metastases[1,2]. Over the last years, advances in surgical techniques, systemic chemotherapy and intensive care improved the outcome of liver resection, leading to wider criteria for operability compared to the past[3]. However, adequate future liver remnant (FLR) (i.e., the liver remnant planned to be left in situ) is still a critical factor in selecting patients when extended hepatectomy is required, given the need to minimize the risk of postoperative liver failure[4,5]. FLR should be at least 25%-30% of the liver volume in patients with normal preoperative liver function, 30% in chronic liver disease, and 40% in the setting of chemotherapy-related injury or cirrhosis[6,7]. Borderline FLR volumes pose the dilemma of whether attempting radical surgery vs performing palliative treatments[7].
In the 2000s, two-stage hepatectomy after preoperative percutaneous portal vein embolization (PVE) or portal vein ligation (PVL) has been proposed as a strategy to resect primarily inoperable tumors after having increased the FLR[8,9]. This approach combines the technical advantages of two-stage hepatectomy (i.e., wedge resections of lesions in the FLR in the case of bilobar tumors) with the compensatory hypertrophy of the FLR induced by PVE or PVL performed at the time of first surgery[10]. The mechanism with which PVE and PVL lead to hypertrophic FLR is complex, involving both the diversion of portal blood flow and release of growth factors[7]. Since hypertrophy usually takes at least 4 wk to be completed, this technique shows high failure rate because of insufficient FLR growth and/or tumor progression during the interval of time between the two stages[11,12].
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is a two-stage hepatectomy procedure introduced in September 2007 by Schnitzbauer et al[13] to obtained more rapid and larger increase of the FLR volume compared to conventional staged hepatectomy (40%-80% within 6-9 d vs 8%-27% within up to 60 d, respectively)[4,6,7,13-15]. The key technical point in ALPPS is the preservation of hepatic artery blood flow to the diseased hemiliver (DH) at the time of first surgical stage. Preserved arterialization leads the DH to act as a vital auxiliary liver and assist the growth of FLR through metabolic and synthetic functions[16,17]. ALPPS achieves a high rate of tumor complete resection (83%)[18], given the successful rate of adequate FLR growth (78%-91%)[19]. Additionally, the reduced interval of time between surgical steps translates into lower tumor progression rate, less adhesions during second surgery, faster patients recovery and prompter starting of adjuvant chemotherapy[4,15,20,21].
ALPPS is becoming increasingly popular in patients candidate to extended hepatectomy. To our knowledge, though imaging plays a key-role in planning the procedure and monitoring the results of both surgical stages, radiological findings related to ALPSS have been poorly reported. In this review, we aimed to summarize the current role for multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) in the procedure, which enable detailed view of pre- and postoperative anatomy, as well as prompt and reliable identification of complications. We also illustrated main cross-sectional imaging findings related to ALPPS, with special emphasis on normal aspects.
There is controversy on which lesions should be treated with ALPPS[6], given initially reported high mortality rates (up to 22% in some series)[22]. It should be kept in mind that ALPPS is an “extrema ratio” procedure to be proposed after careful, multidisciplinary patient selection[6,23,24]. Morbidity and mortality amount up to 14% and 6.6% in experienced centers applying strict selection criteria[10,25-27]. Best results have been obtained in patients with bilobar metastases from colorectal cancer with predictable radical resection, absence of extrahepatic disease and partial or complete response to chemotherapy[2]. Other treatable lesions include hepatocellular carcinoma, cholangiocarcinoma (intrahepatic or hilar), gallbladder carcinoma, and metastases from breast cancer or neuroendocrine tumors[7,25,26]. However, higher postoperative mortality was reported for non-colorectal liver metastases[7]. ALPPS can be also offered as first-line treatment or salvage-therapy after failed PVE[20,25,28-32].
Contraindications to ALPPS include unresectable lesions in the FLR, unresectable extrahepatic metastases, infiltration of the retrohepatic avascular space, severe portal hypertension, high anesthesiology risk, medical contraindications to major hepatectomy, impossibility to achieve negative margins, and unresectable primary tumor in extrahepatic locations[26]. ALPPS is not reccomended in patients with advanced liver cirrhosis, because liver regeneration in the context of chronic liver disease is less predictable[7,33]. On the other hand, some Authors attempted ALPPS in selected cirrhotic patients[34].
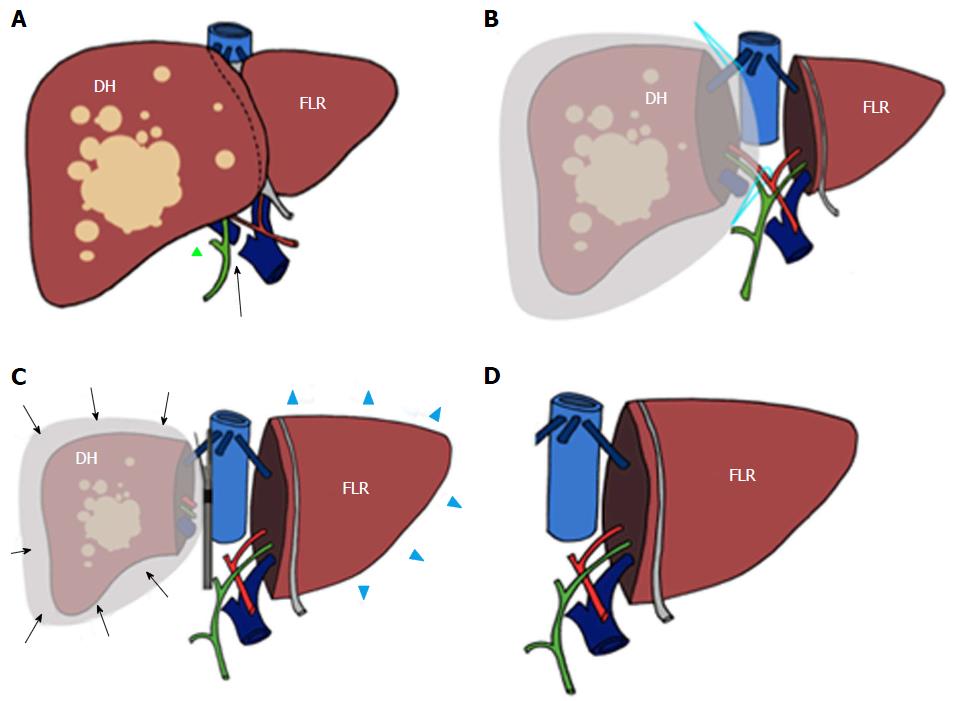
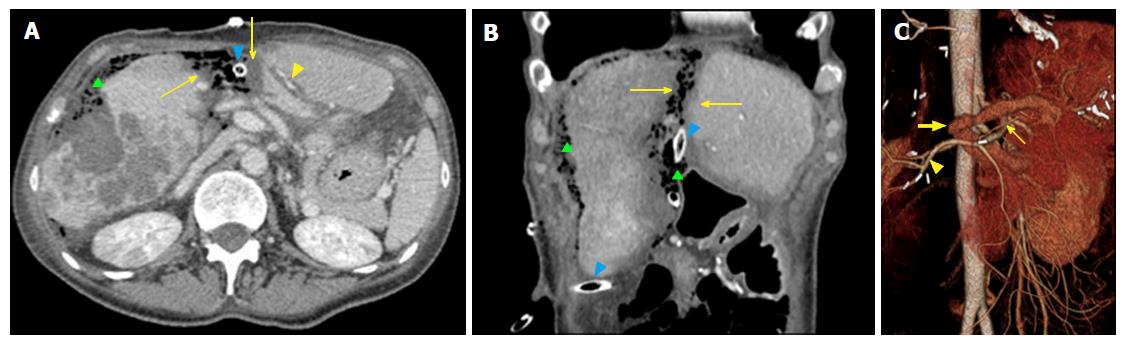
Elective indication to ALPPS is right trisectionectomy[7], in which FLR and DH consist of Couinaud segments 2-3 vs 4-8 (Figures 1 and 2), respectively. Other technical approaches include right hepatectomy (leaving a segments 2-4 FLR), left hepatectomy (leaving segments 5-8 FLR), central hepatectomy (segments 4, 5 and 8 FLR) or monosegmental ALPPS[35-37].
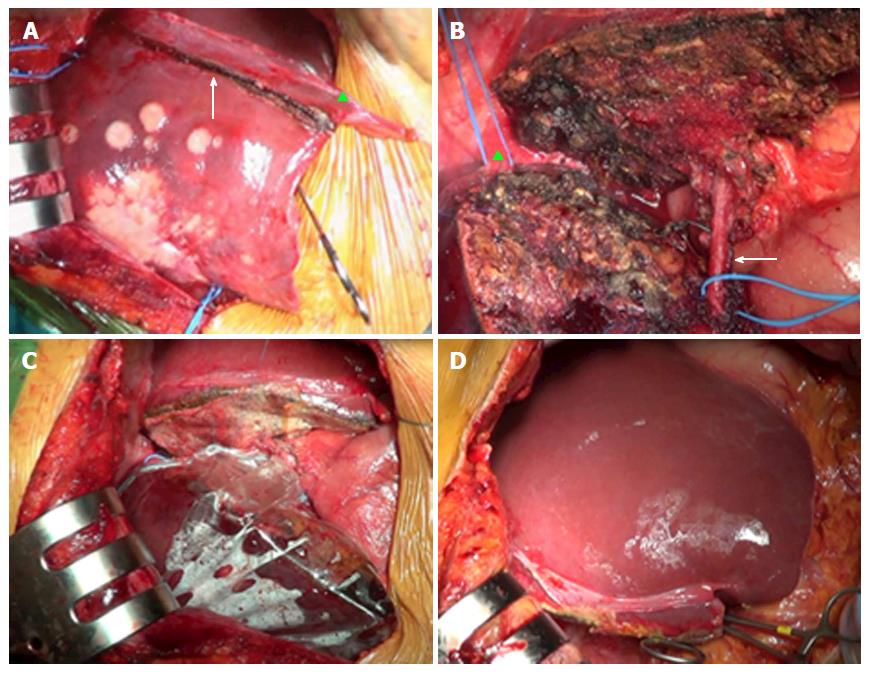
ALPSS includes two consecutive surgical stages (stage 1 and stage 2). During stage 1, the portal branch directed to the DH side is sectioned and sutured in order to divert the portal flow to the FLR. Hepatectomy is subsequently performed to separate the FLR from DH completely (complete ALPPS) or partially (partial ALPPS)[32,38]. If affected by metastases, the FLR is cleaned up by wedge resections and/or intraoperative radiofrequency ablation[17,26]. At the end of the procedure, DH is left in situ, often after having enveloped it into a hermetic bag made of plastic or a biodegradable type-I acellular collagen membrane[39]. The rationale for using the bag is to avoid adhesions and obtain an easier removal of DH on surgical stage 2, as well as better drainage or identification of collections (Figures 1 and 2)[7]. The purpose of stage 1 is to induce hypertrophy of the FLR (in which arterial and portal vascular supply is preserved) and atrophy of the DH (in which arterial supply alone is preserved). Cholecystectomy is also performed[40]. In the case of perihilar cholangiocarcinoma, biliary continuity is obtained by performing Roux-en-Y bilioenteric anastomosis[26]. After stage 1 completion, two drains are placed along the transection line and within the plastic bag, respectively.
Stage 2 is scheduled 7-14 d from stage 1[2]. Hepatectomy is completed by removing atrophic DH after transecting the serving hepatic artery, hepatic duct and hepatic veins (e.g., right hepatic and middle hepatic veins in the case of right trisectionectomy, or right hepatic vein only in the case of right hepatectomy).
First-line imaging after both surgical stages 1 and 2 is represented by ultrasonography (US) with Color-Doppler examination. In our experience, US permits a “quick-and-dirty” evaluation at patient’s bedside to screen for gross complications (e.g., collections) and assess the patency of FLR portal vein, hepatic artery branches and hepatic vein. However, early postoperative US is limited by lack of patients’ collaboration and reduced acoustic windows because of bowel gas and surgical dressing material[41]. Furthermore, US lacks panoramicity, i.e., the capability to represent a section or a 3D reconstruction of the entire liver within a single image. Consequently, though this technique is useful in initial diagnosis of liver abnormalities, it has no direct role in selecting patients for ALPPS (e.g., by assessing the number of lesions in the FLR or estimating its volume). Thus, cross-sectional imaging with MDCT and/or MRI is mandatory in the preoperative patients’ selection, in evaluating postoperative increase in FLR volume and in assessing complications.
Because of wide panoramicity, fast acquisition time and lesser costs, MDCT should be regarded as the cross-sectional modality of choice to image patients before and after ALPPS. Our institutional protocol is summarized in Table 1. Fast acquisition makes MDCT feasible in less collaborating patients, with the possibility to extend the examination to the thorax and/or the lower abdomen if needed. Moreover, the multiphasic MDCT protocol has the advantage of providing all-in-one evaluation of liver neoplasms (in terms of both tumor burden and characterization), extrahepatic disease or complications, and the status of arterial, portal and venous structures for the purpose of preoperative planning and complications assessment. Multiplanar reformations and 3D reconstruction are of help in interpreting images and communicating imaging results to referring clinicians.
| Scan phase (timing from contrast injection) | Scal lenght | Scanning parameters | Rationale in the preoperative phase | Rationale in the postoperative phases |
| Unenhanced | Upper abdomen | KVp 120 mA modulated between 200-450 Tube rotation 0.6 s Pitch 0.984 Noise index 16.10 Collimation 1.25 mm (0.625 for the angiographic phase) Image reconstruction thickness 1.25 mm | Identifying potential confounders in image interpretation (e.g., lesion’s or vascular calcifications). Measuring baseline attenuation of target lesions (e.g., fat-containing HCC) or in diffuse liver disease (e.g., steatosis) | Identifying potential confounders in image interpretation (e.g., surgical clips). Measuring the attenuation of intra-abdominal collections (biloma vs hematoma) |
| This phase is not required if recent prior imaging is available. | This phase in not mandatory in repeated follow-up examinations | |||
| Angiographic phase (20) | Upper abdomen | Assessing the patency and anatomic variants of the hepatic artery and its branches, both on source images and MIP reconstructions | Assessing the sources of suspicious active postoperative bleeding | |
| Delayed arterial (35-40 s) | Upper abdomen | Assessing hypervascular focal liver lesions (malignant and benign ones) | Assessing the patency of the hepatic artery and its branches. Identifying the recurrence of hypervascular tumors in the delayed post-operative period | |
| Venous (70 s) | Whole abdomen | Assessing lesions’ enhancement pattern for the purpose of identification/characterization. Assessing the patency and anatomic variants of the portal trunk and intrahepatic branches, both on source images and MIP reconstructions. Identifying additional abdominal findings potentially contraindicating ALPSS. Assessing for signs of chronic liver disease (including splenomegaly, venous collaterals and ascites) | Assessing the portal status (absence of flow in the ligated portal branch and patency of the FLR branch). Assessing successful tumor cleaning up in the FLR before surgical stage 2. Ruling out thrombosis of the portal braches, hepatic veins and inferior vena cava. Identifying tumor relapse | |
| Delayed (3-5 min) | Upper or whole abdomen, depending on findings on previous scans | Assessing lesions’ enhancement pattern for the purpose of identification/characterization. Identifying additional findings potentially contraindicating ALPSS (e.g., peritoneal carcinosis). This phase is not mandatory | Assessing venous bleeding. This phase in not mandatory |
Given limited availability and longer acquisition times, MRI should be reserved to inconclusive MDCT cases, especially in the preoperative phase, i.e., when there is less risk of image quality degradation because of reduced patients’ collaboration. Similarly to other liver applications[41-44], MRI should be performed with 1.5 Tesla or 3.0 Tesla magnets, equipped with highly performing gradients and multi-element surface coils (preferably 8-16 elements) implementing parallel imaging. Our MRI protocol is illustrated in Table 2.
| Sequence | Weightening | Acquisition plane | Technical clues | Rationale in the preoperative phase | Rationale in the postoperative phase |
| Half fourier acquisition single-shot turbo spin echo/single shot fast spin echo | T2 | Coronal, transverse | - | Ruling out signs of chronic liver disease, including splenomegaly and/or ascites. Detection of parenchymal low signal intensity in iron accumulation | Detection of perihepatic/abdominal collection and/or ascites |
| GE in-phase/out of-phase | T1 | Transverse | Dual echo, breath hold sequence with slice thickness 6 mm | Characterization of fat-containing lesions. Detection of signal intensity patterns of liver steatosis or hemochromatosis | Evaluation of the postoperative status of liver parenchyma. Characterization of tumor recurrence |
| MRCP | T2 | Radial coronal acquisition (2D) or oblique coronal (3D) | 2D and/or 3D technique | Evaluation of anatomic variants complicating or contraindicating surgery. Assessing the Bismuth category of hilar cholangiocarcinoma | Assessment of biliary strictures (site, extent) and biliary dilation upstream |
| Dynamic study with fat saturated 3D GE | T1 | Transverse | Thin slice thickness (3 mm). Baseline acquisition followed by early arterial, late arterial, venous and delayed phases | Detection and characterization of liver lesions | Detection and characterization of parenchymal abnormalities, including tumor recurrence |
| Single-shot echoplanar imaging | Diffusion | Transverse | b values 50 and 400 and 800 s/mm2 (1.5T) or 50 and 800 and 1200 s/mm2 (3.0T). Nominal acquisition time about 3 min (1.5T) and 4 min (3T) | Detection and characterization of smaller lesions (< 1 cm in size) | Detection of parenchymal/periportal edema. Detection and characterization of smaller lesions (< 1 cm in size) |
| Fat saturated Turbo spin echo | T2 | Transverse | Respiratory triggered, with slice thickness 6 mm. Nominal acquisition time 1.50 min | Detection and characterization of liver lesions. | Detection of parenchymal/periportal edema. Detection and characterization of liver lesions. Assessment of collections |
| GE in-phase/out of-phase | T1 | Transverse | Same sequence as (2), acquired in the hepatobiliary phase (15-20 min after contrast injection) | Detection and characterization of liver lesions | Detection and characterization of liver abnormalities |
| Fat saturated 3D GE | T1 | Transverse | Same sequence as (4), with modified flip angle (35°) to increase lesion-to-parenchyma conspicuity. Acquired in the hepatobiliary phase | ||
| Contrast-enhanced | T1 | Oblique coronal | Thin-slice (1 mm) fat saturated 3D fast low angle shot (FLASH) sequence acquired | Functional evaluation of biliary obstruction (if present) | Detection of active bile leakage. Functional assessment of bile duct strictures and patency of bilioenteric anastomosis |
| MRCP |
Hepatobiliary contrast agents such as gadoxetic acid and/or gadobenate dimeglumine improve the detection and characterization of focal liver lesions by representing the vascularity and the presence/absence of hepatocellular contrast uptake at one time[45,46]. When liver metastases are the cause for ALPPS, preoperative MRI with diffusion-weighted imaging and hepatobiliary contrast agents should be regarded as the method of choice for detailed identification of small lesions potentially affecting ALPPS feasibility or FLR cleaning up[47]. Furthermore, hepatobiliary contrast agents are of help in assessing tumor relapse after surgery.
Magnetic resonance cholangiopancreatography (MRCP) should be used preoperatively to evaluate whether biliary tree anatomic variants are at risk of increasing surgical difficulty, or to assign the Bismuth category of cholangiocarcinoma extension[48]. In the postoperative phases, this technique can be of help in assessing the content of fluid collections (fluid vs hemorrhagic) or early and late biliary complications. In particular, 3D T1-weighted MRCP acquired in the delayed phase after gadoxetic acid administration is useful in confirming clinical suspicion of biliary leakage (e.g., persisting postoperative fluid collections associated with clinical sign of biliary sepsis) by showing active contrast extravasation[44]. The presence of endobag after surgical stage 1 can avoid gadoxetic acid-based MRCP, since bile leakage can be actively monitored through the internal surgical drainage.
Preoperative findings are essential to understand whether ALPPS is feasible or not based on tumor burden, liver status and presence of ancillary findings with potential surgical significance. There are five main goals of cross-sectional imaging in this setting.
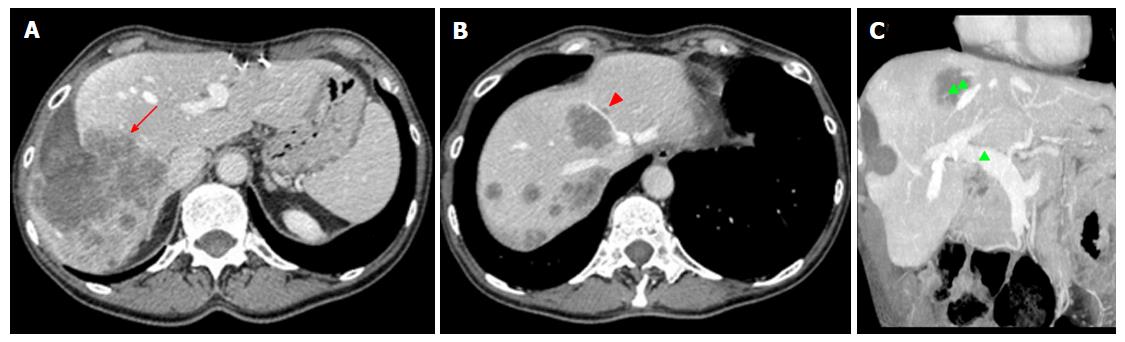
The first task for imaging is accurate detection and characterization of liver lesions. Radiologists should carefully report the number, size, and location of individual lesions, as well as their relationship with surgically relevant anatomic structures, including the hepatic artery, main portal branches, hepatic veins, and first- to second-order biliary branches (Figure 3). This will help the surgeon to establish lesions resectability and the risk for intraoperative complications (e.g., lesions close to the retrohepatic course of inferior vena cava, a region at higher risk of intraoperative bleeding). Second, imaging aims to evaluate the status of liver parenchyma, looking for signs of cirrhosis, cholestasis, steatosis or any other pathologic change attributable to the effects of lesions, diffuse liver disease or chemotherapy. Liver status may influence operability, regardless of the FLR volume (see below). Third, it is crucial to identify vascular and biliary anatomy variants of potential surgical significance (e.g., aberrant and/or accessory branches)[49]. Fourth, any extrahepatic finding potentially affecting the feasibility of ALPPS should be evaluated, including large, inoperable primary cancer on other sites, as well as portal hypertension (including splenomegaly and venous collaterals).
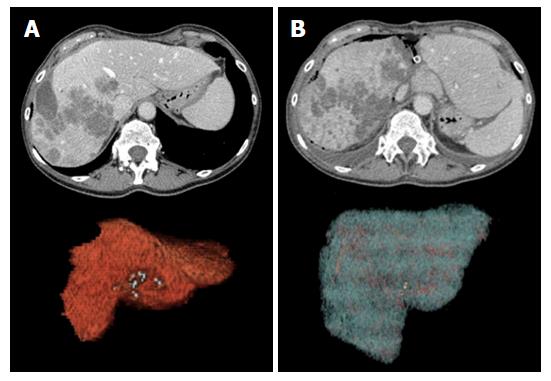
The final key step in preoperative imaging is liver volumetry (LV) of the FLR and the whole liver. FLR volume should be calculated by excluding major vessels and FLLs, in order to obtain a reasonable estimate of final viable liver tissue supporting liver function. FLR should be no lower than 25%-30% of preoperative liver volume in patients with normal liver function, and no lower than 40% in patients with underlying chronic liver disease or liver dysfunction (including the effects of chemotherapy)[7,23,50-52]. Many dedicated liver volumetry software are currently available, most times implemented in the picture archive and communication systems used for routine image analysis. In our Institution, abdominal radiologists perform LV together with liver surgeons, with the objective of reliable volumes definition according to the intended lines of resection.
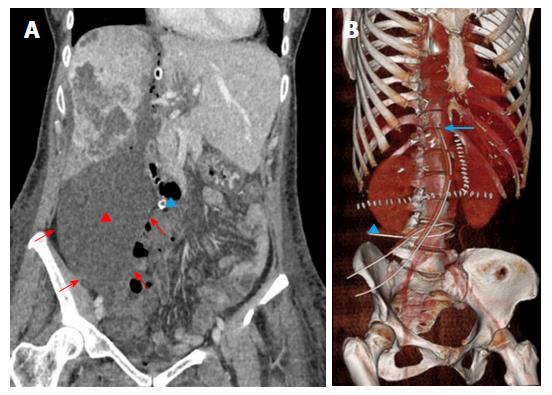
Goals: In uncomplicated patients, post-stage 1 imaging is performed at the time adequate FLR hypertrophy is expectedly achieved (about 6-9 d from surgery)[7]. Cross-sectional imaging is mandatory to calculate the increase in volume of the FLR using LV (Figure 4), to confirm tumor-free status of the FLR, and to verify the expected changes in the DH (atrophy and persistent portal devascularization).
In the case of US and/or clinical suspicion, MDCT or MRI must be anticipated to guarantee early assessment and intervention. Cross-sectional imaging is also of help in ruling-out surgical complications or insufficient FLR volume as a cause for postoperative liver failure.
Normal findings: Normal hypertrophic FLR is represented in Figure 4. Enlargement can be easily appreciated on transverse and reformatted 2D images, though precise estimation should be always performed on 3D reconstructions obtained with LV. The magnitude of expected FLR increase ranges between 61% and 93% compared to the baseline volume[50]. In our center, a minimum increase of 40% is needed for completing the procedure. It is of paramount importance to distinguish between true parenchymal hypertrophy and liver enlargement from postoperative liver edema or congestion. Measurement of Hounsfield units (HU) on MDCT can be of help in the distinction, since edematous parenchyma shows significantly lower attenuation compared to unaffected liver[20]. In rare cases in which doubts persist, MRI can be of help in differential diagnosis by showing parenchymal and/or prominent periportal edema.
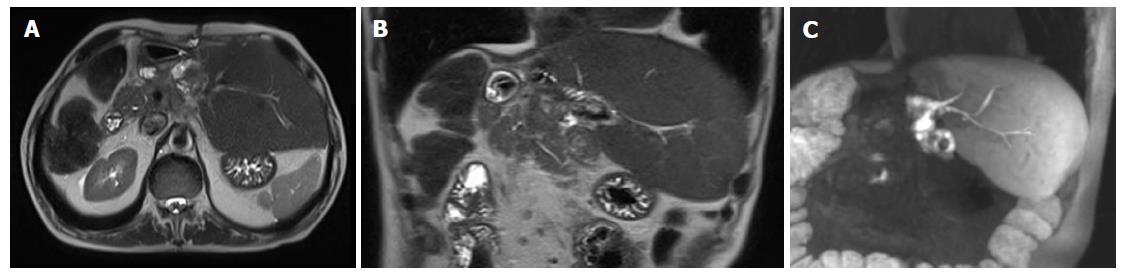
FLR and DH are often surrounded by a thin rim of free fluid, which is usually more prominent around the DH when the endobag is on site. Of note, thin walls make the endobag usually not directly visible on images. Small air bubbles are frequently mixed within the perihepatic fluid, sometimes at a larger extent along the line of hepatectomy (Figure 5). It is crucial not to misdiagnose this normal finding with an infected collection, which is usually larger, lenticular or round in shape and sometimes well-encapsulated on contrast-enhanced images. Mild periportal edema is commonly present as a thin hypodense (on MDCT) or hyperintense (on T2-weighted MRI images) halo surrounding the intrahepatic portal branches.
Except for the portal branches of the DH, the vascular supply to the liver is preserved, with the hepatic artery for the DH appearing slightly hypertrophic compared to the baseline examination to compensate for portal occlusion. No biliary dilation should be observed, in both the DH and FLR.
Main complications: Postoperative complications of ALPPS include bleeding, bile leakage, fluid or bile collections, biliary fistula, cholangitis, portal vein thrombosis (PVT), hepatic vein and hepatic arterial thrombosis, hepatic dysfunction, liver failure, persistent postoperative ascites, pleural effusion, prolonged ileus, coagulation disorders, cardiovascular, respiratory, and renal system dysfunction, encephalopathy and infection[5,53]. Clinical presentation is often challenging, since patient’s signs and symptoms tend to be non-specific. They include fever, abdominal pain, jaundice, ascites, pleural effusion, abnormal liver tests and bleeding or bile within the drains[54]. Post-hepatectomy liver failure has been specifically defined according to so called 50-50 criteria (prothrombin time < 50% and total serum bilirubin > 50 mmol/L on postoperative day 5 or after)[55]. Imaging is recommended in symptomatic patients to rule-out vascular, biliary or parenchymal causes. The most common ALPPS complications encountered on abdominal cross-sectional imaging are collections, hemorrhage and vascular thrombosis.
Collections are represented by hematoma (up to 50% of cases), biloma (25%) and infected collections (25%)[56]. Collections tend to origin from the resection surfaces, i.e., (assuming right trisectionectomy) in the subphrenic space if originating from the FLR, and within the endobag if originating from DH. Small bilomas and/or transient hematomas are common during the first post-operative days, being rapidly reabsorbed or showing no tendency to increase. On the contrary, collections with large size or increasing in volume over a few days should be regarded as pathological (Figure 6). Bilomas are virtually indistinguishable from serous collections on MDCT, since they present homogeneous fluid content (< 30 HU) without contrast-enhancement. Active biliary leakage can be shown on gadoxetic acid-enhanced MRCP because of contrast extravasation from bile ducts or liver surface into the collection[57,58]. Early diagnosis of biliary leakage is important to prevent biliary sepsis. In this case, stage 2 might be anticipated before the FLR is sufficiently hypertrophied, even if at risk of subsequent insufficient liver function. Hematomas usually show more heterogeneous content than bilomas, with mixed internal areas of low and high attenuation (> 30 HU) on MDCT reflecting the presence of fibrin septa and clots. On MRI, bilomas appears as fluid collections with hypointensity on T1-weighted images and hyperintensity on T2-weightd images, whereas hematomas show typical hyperintensity on T1-weighted fat suppressed images. Treatment options for collections include drainage under sonographic or MDCT guidance, as well as surgical toilette in more extensive cases[3,59]. Infected collections typically show small air bubbles from anaerobic bacteria, and may be surrounded by thickened contrast-enhancing walls of peripheral inflammatory tissue.
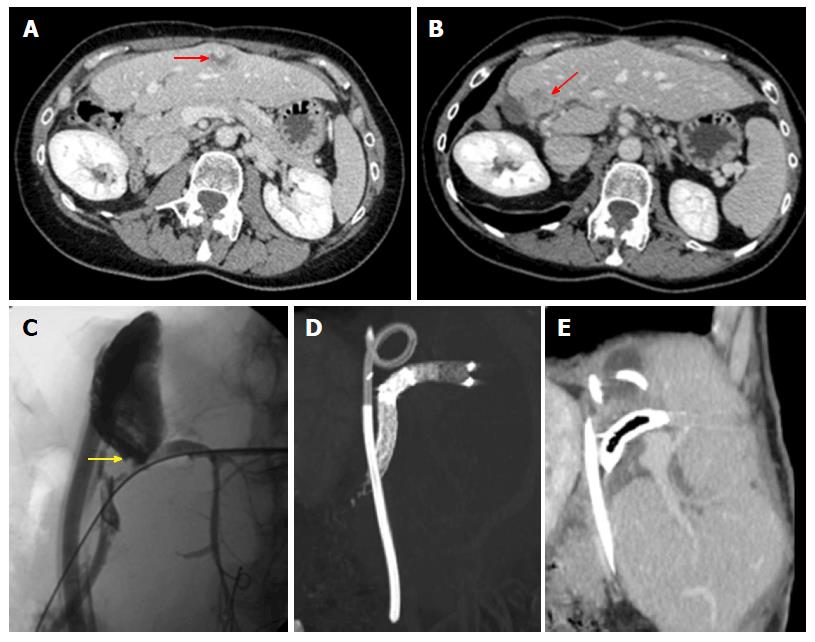
Postoperative hemorrhage generally arises within 48 h from intervention, commonly originating from the resection margins (e.g., because of an arterial branch truncation or congestion of the hepatic vein due to stenosis or ligation), incomplete intraoperative hemostasis or dehiscence of vascular sutures[3]. MDCT with angiographic phase should be promptly performed to identify the site of bleeding and guide embolization or surgery.
The most threatening vascular complication after stage 1 is portal thrombosis. This rare condition may affect the portal trunk and/or the FLR branch, thus affecting the hypertrophy process. Not surprisingly, patients showing extensive PVT are at high risk of liver failure and death[7,26,28]. Color Doppler US has a primary role in detecting thrombosis. Similarly to other postoperative scenarios[41], thrombosis manifests with absent flow, with our without direct demonstration of an intraluminal echogenic thrombus on B-mode. Although no specific data on ALPPS have been reported, to our knowledge, contrast-enhanced US is supposedly of help in confirming absent contrast arrival in thrombotic vessels[41]. Post-contrast MDCT and/or MRI acquired on venous and delayed phases are useful to confirm color Doppler findings, as well as to map the extent of thrombosis (portal trunk and/or FLR main branch and/or intrahepatic branches) and the degree of occlusion (partial or complete filling defects). Contrast enhancement of vascular walls is an additional findings of thrombosis, likely representing contrast engorgement within dilated vasa vasorum[60,61]. Partial thrombosis may benefit from medical therapy, whereas complete thrombosis requires thrombolysis.
Goals: Early cross-sectional imaging is usually not required in the case of an uncomplicated clinical course. Chest X-ray and abdominal US with color Doppler interrogation of major vessels are usually sufficient to monitor the patient in the first weeks after the intervention. MDCT and/or MRI should be ordered in the case of suspicious complications and/or inconclusive findings on US. On the contrary, cross-sectional imaging has a major role in the delayed postoperative period, mainly in assessing tumor recurrence and/or late complications with or without prior US.
Recommended imaging follow-up includes US and MDCT or MRI scan after 3 and 6-12 mo from surgery, respectively[54]. However, there is no definite schedule for imaging controls, which should be tailored to patients according to the type and extent of the operated tumor, concomitant chemotherapy and history of major complications after surgical stage 1 and/or 2. MRI is reserved to cases of suspicious biliary complications or for characterizing ambiguous CT findings.
Normal findings: Asymptomatic, small amounts of intra-abdominal air or small fluid collections are common findings in the postoperative phase. Air is usually reabsorbed early, whereas collections can persist up to two months after surgery[56]. Another frequent finding is represented by a hypoattenuating linear band adjacent to liver raw surface (about 30%-50% of cases), which has been related to the effects of parenchymal devascularization or bile/blood accumulation[56]. No vascular or biliary abnormalities should be found (Figure 7).
Of note, transitory splenic enlargement is commonly encountered within 6 mo from hepatectomy. The degree of splenomegaly is generally proportional to the volume of liver resection, with average increase in splenic volume of about 40% compared to the preoperative period[62-64].
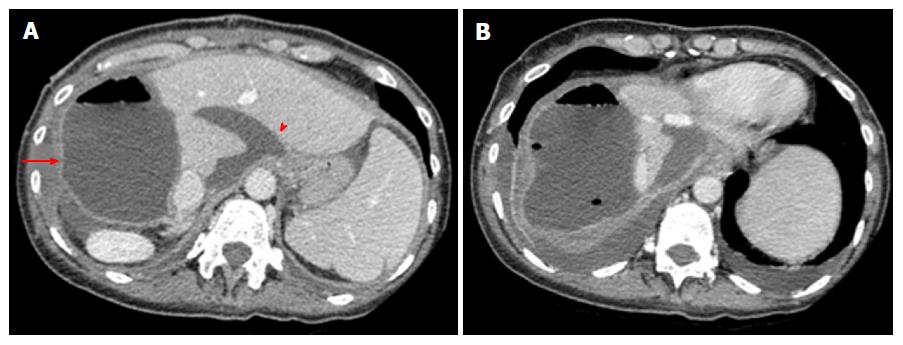
Main complications: Complications after stage 2 may be classified into early and late, depending on the onset from surgery. Early complications occur within a few weeks from stage 2, and manifest with a clinical and radiological spectrum similar to that following stage 1 surgery. Thus, hematomas/bilomas (Figure 8), bleeding, vascular thrombosis and pleural effusion represent main expected findings, presenting as described above. Late complications are stage 2 specific, and tend to occur from 3 to about 6 mo after this surgical step. The most frequent and relevant ones are tumor recurrence and biliary complications. The treatment of late complications may be challenging, especially if further surgery is needed. Indeed, additional interventions may in turn increase the risk of morbidity (Figure 9).
The International ALPSS registry[27] reported disease-free survival of 73% and 59% at 1 and 2 years after ALPSS, respectively, with median survival of 14 mo. Recurrence appears with MDCT and/or MRI signs of the original tumor, though recurrence can manifest with pleomorphic, nonspecific appearance in our experience (Figure 9). Suspicious solid lesions should be regarded as tumor recurrence, regardless of the fact they mimic preoperative lesions or not.
Late biliary complications include stricture and fistula. Because of the recent introduction of ALPSS, it is difficult to quantify the prevalence of these complications, which are generally rare in experienced centers. Strictures are multifactorial in origin, having been related to mechanical stress from FLR enlargement and rotation, as well as to iatrogenic causes (inaccurately placed clips, injury, periductal bile leakage and ischemia due to injured FLR hepatic artery)[65,66].
MRCP is the elective tool to assess the site of obstruction, which appears as a focal zone of absent signal on fluid-sensitive images, as well as the degree of proximal biliary dilation[42]. Strictures of the bilioenteric anastomosis should be evaluated with gadoxetic acid-based MRCP, which shows lack of contrast flow from the biliary tree to the anastomotic bowel loop[65,67]. This technique is of help also in identifying the site of bile extravasation when chronic biliary fistula is suspected. Similarly to other clinical scenarios[42], MRCP is electively ordered in patients with low pre-test probability of biliary complications, since a negative result is reliable enough to avoid invasive procedures of direct cholangiography. On the other hand, MRCP is effective also in patients with high pre-test probability of disease, since it provides a panoramic and detailed representation of pathological findings, i.e., an accurate road-map for planning the most appropriate interventional approach. Most bilomas and strictures are treated with endoscopic sphincterotomy and balloon dilation followed by endoprothesis placement.
An overall view of normal postoperative findings and complications after both surgical stages 1 and 2 is provided in Table 3.
| Normal findings | Abnormal findings | ||
| Postoperative phase | Goals of ALPPS | Findings not to be confused with pathological aspects | prompting intervention |
| After surgical stage 1 | Hypertrophic FLR (≥ 40% of baseline preoperative volume) | Thin rim of free fluid around both FLR and DH | Large, persisting collections (hematoma, bilomas, infected collections) |
| Air bubbles within the perihepatic fluid, especially on the hepatectomy line | Bleeding | ||
| Biliary dilation | |||
| Mild periportal edema | Bile leakage/fistula | ||
| Hypertrophy of hepatic artery for the DH | Portal vein thrombosis | ||
| After surgical stage 2 | Uncomplicated appearance of the FLR (e.g., no relapsing focal liver lesions) | Thin rim of free fluid around FLR | Early complications |
| Air bubbles | see surgical stage 1 | ||
| Hypoattenuating linear band adjacent to liver raw surface | Late complications (3-6 mo) | ||
| Biliary stricture | |||
| Rotation of hypertrophic FLR | Biliary fistula | ||
| Transitory splenomegaly | Tumor recurrence | ||
ALPSS is an increasingly popular two-stage hepatectomy technique associated with portal ligation aimed to obtain rapid and adequate FLR hypertrophy, thus extending operability in patients with massive primary or secondary neoplastic liver involvement.
Cross-sectional imaging, especially MDCT, plays a key role in planning ALPPS procedure and monitoring different surgical stages. In particular, MDCT is the main instrument to provide liver volumetry, which is of special importance in assessing technique feasibility and assessing variation in volume of the FLR between surgical stages. MDCT also confirm a clinical or sonographic suspicion of complications, including collections, bilomas, hematomas, post-surgical bleeding, PVT, and tumor recurrence. MRI should be used as a problem-solving tool in both preoperative and postoperative phases, whereas MRCP has an elective role in assessing biliary complications.
The Authors thank Dr. Iliana Bednarova’, MD (Institute of Radiology, University of Udine, Italy) for having revised English language.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shan YF, Richter B S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Torres OJ, Fernandes ES, Herman P. ALPPS: past, present and future. Arq Bras Cir Dig. 2015;28:155-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983-7991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 172] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (3)] |
| 4. | Schnitzbauer A, Lang S A, Fichtner-Feigl S, Loss M, Kroemer A, Goessmann H, Farkas SA, Kirchner G, Jung EM, Scherer MN. In situ split with portal vein ligation induces rapid left lateral lobe hypertrophy enabling two-staged extended right hepatic resection. Berlin: Oral Presentation 2010; 35. |
| 5. | Herman P, Krüger JA, Perini MV, Coelho FF, Cecconello I. High Mortality Rates After ALPPS: the Devil Is the Indication. J Gastrointest Cancer. 2015;46:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Bertens KA, Hawel J, Lung K, Buac S, Pineda-Solis K, Hernandez-Alejandro R. ALPPS: challenging the concept of unresectability--a systematic review. Int J Surg. 2015;13:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Zhang GQ, Zhang ZW, Lau WY, Chen XP. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new strategy to increase resectability in liver surgery. Int J Surg. 2014;12:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G, Aloia T. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525-536, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777-785. [PubMed] |
| 10. | Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, Croome KP. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery. 2015;157:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-1049; discussion 1049-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 934] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 14. | Wiederkehr JC, Avilla SG, Mattos E, Coelho IM, Ledesma JA, Conceição AF, Wiederkehr HA, Wiederkehr BA. Associating liver partition with portal vein ligation and staged hepatectomy (ALPPS) for the treatment of liver tumors in children. J Pediatr Surg. 2015;50:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 16. | Sala S, Ardiles V, Ulla M, Alvarez F, Pekolj J, de Santibañes E. Our initial experience with ALPPS technique: encouraging results. Updates Surg. 2012;64:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Torres OJ, Moraes-Junior JM, Lima e Lima NC, Moraes AM. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new approach in liver resections. Arq Bras Cir Dig. 2012;25:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 19. | Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015;22:3109-3120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Li L, Ewald F, Gulati A, Nashan B. Associating liver partition and portal vein ligation for staged hepatectomy: From technical evolution to oncological benefit. World J Gastrointest Surg. 2016;8:124-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg. 2013;17:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Torres OJ, Fernandes Ede S, Oliveira CV, Lima CX, Waechter FL, Moraes-Junior JM, Linhares MM, Pinto RD, Herman P, Machado MA. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig. 2013;26:40-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Peteja M, Pelikan A, Vavra P, Lerch M, Ihnat P, Zonca P and Janout V. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: A Contemporary Surgical Oncology Conundrum. J Gastrointest Dig Syst. 2015;5:3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-836; discussion 836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 28. | Tschuor Ch, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, Slankamenac K, Clariá RS, de Santibaňes E, Hernandez-Alejandro R, Clavien PA. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Björnsson B, Gasslander T, Sandström P. In situ split of the liver when portal venous embolization fails to induce hypertrophy: a report of two cases. Case Rep Surg. 2013;2013:238675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Jackson T, Siegel KA, Siegel CT. Rescue ALPPS: Intraoperative Conversion to ALPPS during Synchronous Resection of Rectal Cancer and Liver Metastasis. Case Rep Surg. 2014;2014:487852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Vyas SJ, Davies N, Grant L, Imber CJ, Sharma D, Davidson BR, Malago M, Fusai G. Failure of portal venous embolization. ALPPS as salvage enabling successful resection of bilobar liver metastases. J Gastrointest Cancer. 2014;45 Suppl 1:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Edmondson MJ, Sodergren MH, Pucher PH, Darzi A, Li J, Petrowsky H, Campos RR, Serrablo A, Jiao LR. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): Many routes to the summit. Surgery. 2016;159:1058-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, van Delden OM. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 34. | Vennarecci G, Laurenzi A, Santoro R, Colasanti M, Lepiane P, Ettorre GM. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg. 2014;38:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg. 2013;65:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Schadde E, Malagó M, Hernandez-Alejandro R, Li J, Abdalla E, Ardiles V, Lurje G, Vyas S, Machado MA, de Santibañes E. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery. 2015;157:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | de Santibañes M, Alvarez FA, Santos FR, Ardiles V, de Santibañes E. The associating liver partition and portal vein ligation for staged hepatectomy approach using only segments I and IV as future liver remnant. J Am Coll Surg. 2014;219:e5-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Petrowsky H, Györi G, de Oliveira M, Lesurtel M, Clavien PA. Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg. 2015;261:e90-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Brustia R, Scatton O, Soubrane O. Variation on a Theme: Alternative to Plastic Bag in ALPPS Procedures: Feasibility and Clinical Safety of COVA+™ Membrane in ALPPS Procedures. World J Surg. 2015;39:3023-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Dokmak S, Belghiti J. Which limits to the “ALPPS” approach? Ann Surg. 2012;256:e6; author reply e16-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Girometti R, Como G, Bazzocchi M, Zuiani C. Post-operative imaging in liver transplantation: state-of-the-art and future perspectives. World J Gastroenterol. 2014;20:6180-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Fulcher AS, Turner MA. Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology. 1999;211:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Ito K, Siegelman ES, Stolpen AH, Mitchell DG. MR imaging of complications after liver transplantation. AJR Am J Roentgenol. 2000;175:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Girometti R, Cereser L, Bazzocchi M, Zuiani C. Magnetic resonance cholangiography in the assessment and management of biliary complications after OLT. World J Radiol. 2014;6:424-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Palmucci S. Focal liver lesions detection and characterization: The advantages of gadoxetic acid-enhanced liver MRI. World J Hepatol. 2014;6:477-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 47. | Matos AP, Velloni F, Ramalho M, AlObaidy M, Rajapaksha A, Semelka RC. Focal liver lesions: Practical magnetic resonance imaging approach. World J Hepatol. 2015;7:1987-2008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 48. | Valls C, Ruiz S, Martinez L, Leiva D. Radiological diagnosis and staging of hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:115-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 49. | Catalano OA, Singh AH, Uppot RN, Hahn PF, Ferrone CR, Sahani DV. Vascular and biliary variants in the liver: implications for liver surgery. Radiographics. 2008;28:359-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN; Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 51. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 52. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 53. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ; Edinburgh Liver Surgery and Transplantation Experimental Research Group (eLISTER). The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 54. | Vivarelli M, Vincenzi P, Montalti R, Fava G, Tavio M, Coletta M, Vecchi A, Nicolini D, Agostini A, Ahmed EA. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review. PLoS One. 2015;10:e0144019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 56. | Mulé S, Colosio A, Cazejust J, Kianmanesh R, Soyer P, Hoeffel C. Imaging of the postoperative liver: review of normal appearances and common complications. Abdom Imaging. 2015;40:2761-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Melamud K, LeBedis CA, Anderson SW, Soto JA. Biliary imaging: multimodality approach to imaging of biliary injuries and their complications. Radiographics. 2014;34:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 58. | Hoeffel C, Azizi L, Lewin M, Laurent V, Aubé C, Arrivé L, Tubiana JM. Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. Radiographics. 2006;26:1603-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Yamashita Y, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, Sugimachi K. Bile Leakage After Hepatic Resection. Annals of Surgery. 2001;233:45-50. |
| 60. | Tirumani SH, Shanbhogue AK, Vikram R, Prasad SR, Menias CO. Imaging of the porta hepatis: spectrum of disease. Radiographics. 2014;34:73-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Parvey HR, Raval B, Sandler CM. Portal vein thrombosis: imaging findings. AJR Am J Roentgenol. 1994;162:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Petrovai G, Truant S, Langlois C, Bouras AF, Lemaire S, Buob D, Leteurtre E, Boleslawski E, Pruvot FR. Mechanisms of splenic hypertrophy following hepatic resection. HPB (Oxford). 2013;15:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Jacobs KE, Visser BC, Gayer G. Changes in spleen volume after resection of hepatic colorectal metastases. Clin Radiol. 2012;67:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Yin S, Wang H, Park O, Wei W, Shen J, Gao B. Enhanced liver regeneration in IL-10-deficient mice after partial hepatectomy via stimulating inflammatory response and activating hepatocyte STAT3. Am J Pathol. 2011;178:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Ward J, Sheridan MB, Guthrie JA, Davies MH, Millson CE, Lodge JP, Pollard SG, Prasad KR, Toogood GJ, Robinson PJ. Bile duct strictures after hepatobiliary surgery: assessment with MR cholangiography. Radiology. 2004;231:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 67. | Boraschi P, Donati F. Biliary-enteric anastomoses: spectrum of findings on Gd-EOB-DTPA-enhanced MR cholangiography. Abdom Imaging. 2013;38:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |