Published online May 18, 2017. doi: 10.4254/wjh.v9.i14.657
Peer-review started: November 17, 2016
First decision: February 4, 2017
Revised: February 11, 2017
Accepted: April 23, 2017
Article in press: April 24, 2017
Published online: May 18, 2017
Processing time: 181 Days and 15 Hours
To retrospectively evaluate the diagnostic performance of free-breathing diffusion-weighted imaging (FB-DWI) with modified imaging parameter settings for detecting hepatocellular carcinomas (HCCs).
Fifty-one patients at risk for HCC were scanned with both FB-DWI and respiratory-triggered DWI with the navigator echo respiratory-triggering technique (RT-DWI). Qualitatively, the sharpness of the liver contour, the image noise and the chemical shift artifacts on each DWI with b-values of 1000 s/mm2 were independently evaluated by three radiologists using 4-point scoring. We compared the image quality scores of each observer between the two DWI methods, using the Wilcoxon signed-rank test. Quantitatively, we compared the signal-to-noise ratios (SNRs) of the liver parenchyma and lesion-to-nonlesion contrast-to-noise ratios (CNRs) after measuring the signal intensity on each DWI with a b-factor of 1000 s/mm2. The average SNRs and CNRs between the two DWI methods were compared by the paired t-test. The detectability of HCC on each DWI was also analyzed by three radiologists. The detectability provided by the two DWI methods was compared using McNemar’s test.
For all observers, the averaged image quality scores of FB-DWI were: Sharpness of the liver contour [observer (Obs)-1, 3.08 ± 0.81; Obs-2, 2.98 ± 0.73; Obs-3, 3.54 ± 0.75], those of the distortion (Obs-1, 2.94 ± 0.50; Obs-2, 2.71 ± 0.70; Obs-3, 3.27 ± 0.53), and the chemical shift artifacts (Obs-1, 3.38 ± 0.60; Obs-2, 3.15 ± 1.07; Obs-3, 3.21 ± 0.85). The averaged image quality scores of RT-DWI were: Sharpness of the liver contour (Obs-1, 2.33 ± 0.65; Obs-2, 2.37 ± 0.74; Obs-3, 2.75 ± 0.81), distortion (Obs-1, 2.81 ± 0.56; Obs-2, 2.25 ± 0.74; Obs-3, 2.96 ± 0.71), and the chemical shift artifacts (Obs-1, 2.92 ± 0.59; Obs-2, 2.21 ± 0.85; Obs-3, 2.77 ± 1.08). All image quality scores of FB-DWI were significantly higher than those of RT-DWI (P < 0.05). The average SNR of the normal liver parenchyma by FB-DWI (11.0 ± 4.8) was not significantly different from that shown by RT-DWI (11.0 ± 5.0); nor were the lesion-to-nonlesion CNRs significantly different (FB-DWI, 21.4 ± 17.7; RT-DWI, 20.1 ± 15.1). For all three observers, the detectability of FB-DWI (Obs-1, 43.6%; Obs-2, 53.6%; and Obs-3, 45.0%) was significantly higher than that of RT-DWI (Obs-1, 29.1%; Obs-2, 43.6%; and Obs-3, 34.5%) (P < 0.05).
FB-DWI showed better image quality and higher detectability of HCC compared to RT-DWI, without significantly reducing the SNRs of the liver parenchyma and lesion-to-nonlesion CNRs.
Core tip: This retrospective study evaluated the image quality of free-breathing diffusion-weighted imaging (FB-DWI) of the liver and its diagnostic performance for hepatocellular carcinoma compared with respiratory-triggered DWI. The free-breathing technique is widely believed to be inappropriate for body DWI because motion artifact causes decreased image quality. However, after a modification of imaging parameters, FB-DWI showed better image quality without significantly reducing the signal-to-noise ratio of the normal liver parenchyma and the lesion-to-nonlesion contrast-to-noise ratio compared to respiratory-triggering-DWI. As a result, the improvement of the image quality of FB-DWI contributed to an increased rate of detection of hepatocellular carcinoma.
- Citation: Takayama Y, Nishie A, Asayama Y, Ishigami K, Kakihara D, Ushijima Y, Fujita N, Shirabe K, Takemura A, Honda H. Image quality and diagnostic performance of free-breathing diffusion-weighted imaging for hepatocellular carcinoma. World J Hepatol 2017; 9(14): 657-666
- URL: https://www.wjgnet.com/1948-5182/full/v9/i14/657.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i14.657
Diffusion-weighted imaging (DWI) has been widely adopted as a magnetic resonance imaging (MRI) method in clinical practice[1,2]. DWI can be used for the detection and characterization of malignant and nonmalignant lesions[3-5]. Liver DWI has been applied to quantify the degrees of chronic liver disease and fibrosis, and to detect and characterize liver lesions[2,6-9]. DWI can provide additional information that can be used to differentiate malignant liver lesions from benign liver lesions and to estimate the histological grade of hepatocellular carcinomas (HCCs)[6,7,10]. The combination of DWI and dynamic contrast-enhanced (DCE)-MRI has shown higher diagnostic performance compared to DWI or DCE-MRI alone[11-13]. Although DWI has an essential role to play in the assessment of HCCs on liver MRI, its sensitivity for detecting HCCs is thought to be low compared to that of DCE-MRI[2,14].
DWI suffers from image distortion and/or chemical shift artifacts related to the echo planar imaging (EPI) technique and to motion and susceptibility artifacts[15-17]. With the goal of overcoming these issues, a previous study investigated MR parameter settings to obtain high spatial resolution and less artifacts without losing a significant level of the signal-to-noise ratio on liver MRI[18]. With this method, DWI with MR parameter settings provides improved image quality and detections of malignant liver tumors such as HCCs and metastatic liver tumors[18]. Here we hypothesized that DWI with modified MR parameter settings for the improvement of image quality might result in further improvement in the detectability of HCCs.
The purpose of this retrospective study was to evaluate the image quality and the detectability of HCCs in patients with chronic liver disease on DWI with modified MR parameter settings.
This study was approved by the Institutional Review Board of our institute. The requirement for written informed consent was waived due to the retrospective nature of the study. From November 2010 to September 2011, 468 consecutive patients who underwent liver MRI at our institute were enrolled. The inclusion criteria were: (1) patients who were admitted to the Department of Surgery and were suspected to have HCCs due to chronic liver disease; (2) patients who underwent gadoxetic acid-enhanced MRI (Gd-EOB-MRI) on the same MR scanners; and (3) patients who underwent treatments such as surgical resection, transcatheter arterial infusion chemotherapy (TAI) or transcatheter arterial chemoembolization (TACE). The exclusion criteria were: (1) patients with other malignant liver tumors, such as cholangiocellular carcinoma (ICC) and metastatic liver tumor; and (2) patients in whom follow-up computed tomography (CT) and/or MRI were not performed. Finally, 51 patients (age range: 26-82; mean: 63.8 years; male/female ratio: 33/18; Child-Pugh grades: A = 31, B = 16 and C = 4) were enrolled.
MR protocol: MR examinations were performed on a clinical whole-body 3.0 Tesla MR system (Achieva 3.0 T TX; Philips Healthcare, Best, the Netherlands) using a 32-channel cardiac phased-array coil. For the comparison of imaging modalities, each patient was scanned with two different types of DWI. One type was DWI with modified MR parameter settings for the improvement of images referring to the literature[18]; we called this type of DWI “free-breathing (FB)-DWI” in this study because the FB technique was applied. The other type was DWI without modified MR parameter settings using a navigator-echo-based, real-time respiratory-gating and respiratory-triggering technique which we refer to as RT-DWI in this study. A navigator-echo-based technique was not applied for respiratory-triggering (RT)-DWI.
The details of the MR parameters of the two DWI methods are summarized in Table 1. An apparent diffusion coefficient (ADC) map was developed for each DW image, by referring to the signal intensity decay on the DW image with b-values of 0, 500 and 1000 s/mm2.
| Imaging technique | FB-DWI | RT-DWI |
| Spin echo single-shot EPI | Spin echo single-shot EPI | |
| SENSE factor | 2 | 2 |
| TR/TE (ms) | 6250/56 | 1877/55 |
| Flip angle (degree) | 90° | 90° |
| Field of view (mm2) | 380 × 299 | 380 × 299 |
| Matrix (frequency × phase) | 112 × 176 | 112 × 68 |
| Slice thickness (mm) | 7 | 7 |
| Slice gap (mm) | 1 | 1 |
| No. of slice | 25 | 25 |
| No. of excitations | 2 | 2 |
| b-value (s/mm2) | 0.500 and 1.000 | 0.500 and 1.000 |
| Respiratory compensation | Free-breathing without navigator echo | Respiratory-triggered with navigator echo |
| Fat-suppression | SPAIR | SPIR |
| SPAIR delay (ms) | 100 | |
| SPAIR TR (ms) | 250 | |
| Frequency offset (Hz) | 250 | 180 |
| EPI factor | 75 | 25 |
| Band width (Hz/pixel) | 4050.4 | 4438.5 |
| Scan time (min:s) | 3:32 | 3:201 |
Other imaging sequences included an axial T2-weighted single-shot turbo spin echo, axial dual-echo T1-weighted fast field echo, and a gadoxetic acid-enhanced dynamic study. For the gadoxetic acid-enhanced dynamic study, a multiphase dynamic study including arterial, portal, late and hepatobiliary phases was performed using axial enhanced T1 high-resolution isotropic volume excitation (eTHRIVE). First, pre-contrast images were scanned. Gadoxetic acid (Primovist; Bayer, Osaka, Japan) at 0.1 mL/kg was injected through the antecubital vein for 5 s at a variable injection rate using a power injector, followed by a bolus administration of 20 mL of saline at the same injection rate. The timing of the arterial dominant phase was determined with a test injection of 0.5 mL of gadoxetic acid. The scanning of the portal, late and hepatobiliary phases began at the arterial phase +30 s, 180 s and 20 min after the injection of the contrast agent, respectively.
CT protocol: On the follow-up CT examination, the scanning was performed before and after 100 mL of iodinated contrast medium (Iopamiron 370: Bayer Schering Pharma, Osaka, Japan; or Omnipaque 350: Daiichi-Sankyo, Tokyo) was administered, using a 64-MDCT scanner (Aquilion 64, Toshiba Medical, Tokyo). The contrast was intravenously administered at a rate of 3 mL/s. Contrast-enhanced images were obtained during the arterial phase (43 s after the initiation of the injection), the portal venous phase (70 s), and the delayed phase (240 s). The imaging acquisition parameters were as follows: Voltage, 120 kV; electric current, automatic; collimation, 0.5 mm; image reconstruction thickness, 5 mm; and helical pitch, 53.
Qualitatively, the sharpness of the liver contour, the image noise and the chemical shift artifacts on FB-DWI and RT-DWI with b-values of 1000 s/mm2 were independently evaluated by three radiologists (Daisuke Kakihara, Yasuhiro Ushijima, and Nobuhiro Fujita, with 17, 17 and 12 years of experience in interpreting liver MRI, respectively) who were blinded to the imaging information and clinical data, using 4-point scoring. The details of the 4-point scoring are shown in Table 2. Quantitatively, the SNRs of the liver parenchyma and the lesion-to-nonlesion CNRs between the liver parenchyma and HCCs were calculated after drawing polygonal regions of interest (ROIs) on each DWI with a b-factor of 1000 s/mm2; this procedure was performed by one radiologist using a commercially available PACS workstation (SYNAPSE; Fujifilm Medical, Tokyo). The SNRs and CNRs were calculated using the following equations, as described in detail elsewhere[19,20]:
| Score | Sharpness of the liver contour | Distortion and chemical shift artifacts |
| 1 | Unclear liver contour | Severe distortion or artifacts compromise the diagnostic capability of DWI in the whole liver |
| 2 | The liver contour is partially unclear | Distortion or artifacts are moderate, and they compromise the diagnostic capability of DWI in 50% or more of the liver |
| 3 | The liver contour is mostly clear | Distortion or artifacts are mild, and they compromise the diagnostic capability of DWI in less than 50% of the liver |
| 4 | The entire liver contour is clear | No distortion or artifacts; the diagnostic capability of DWI is not compromised |
SNR of the liver parenchyma = SIliver/SDliver, Lesion to nonlesion CNR = |SIliver − SItumor|/SDliver
Where SIliver is the signal intensity of the liver parenchyma, SItumor the signal intensity of the tumor, and SDliver the standard deviation of the SI of the liver parenchyma. The SD of the liver parenchyma was taken as the estimated local noise for the calculation. In parallel imaging, noise is not distributed homogeneously throughout the image, and thus it is better to estimate noise in close proximity to the site of SI measurement[21]. The SNR cannot be calculated as a characteristic of the entire image but rather is calculated as a local property that characterizes the signal quality with respect to local noise levels[21].
Three ROIs were made as large as possible on the normal liver parenchyma to avoid major vessels, tumors, and artifacts for each patient. The same ROIs were duplicated on each DW image. The range and averaged areas of ROIs of the normal liver parenchyma were 102.1-1414.0 mm2 and 385.4 mm2, and the corresponding values for the hepatic lesions were 146.7-1237.4 mm2 and 502.7 mm2. The measurements of the SNR of normal liver parenchyma and a lesion-to-nonlesion CNR were repeated three times for each subject. The same ROIs were duplicated at the same slice and position for the two DWI methods.
A final total of 105 HCCs (size range: 5-140 mm; mean: 17.1 mm; location, left lobe/right lobe: 52/53) was used for the assessment of the detectability of HCCs by the two different types of DWI. The number and location of HCCs were determined by one coordinator (Yukihisa Takayama, with 15 years of experience in interpreting liver MRI) who was a coordinator of this study and had knowledge of the clinical data of each patient. In the 38 patients who underwent surgery, 38 HCCs were identified using pathological reports after surgical resection. Of the other 67 HCCs in the 13 patients who underwent TAI or TACE, the HCCs were clinically defined using the enhancement in the early phase and hypointensity in the hepatobiliary phase of Gd-EOB-MRI and the nodular accumulation of emulsion of iodized oil (Lipiodol Ultrafluid; Terumo, Tokyo) on follow-up CT performed 2 wk after the TAI or TACE[22-24].
The 3-mo follow-up Gd-EOB-MRI or DCE-CT confirmed the absence of HCCs in other liver parenchyma. The treated HCCs and hepatic hemangiomas were diagnosed based on the lack of early enhancement on Gd-EOB-MRI and DCE-CT and high signal intensity on T2-weighted imaging at an least 6-mo follow-up Gd-EOB-MRI or DCE-CT[24-26]. Liver tumors other than hemangiomas were not identified in 51 patients.
The detectability of HCC by each type of DWI was analyzed by three radiologists (Daisuke Kakihara, Yasuhiro Ushijima, and Nobuhiro Fujita, with 17, 17 and 12 years of experience in interpreting liver MRI, respectively) who independently interpreted the sets of DWI with b-values of 0, 500 and 1000 s/mm2 and the ADC map at a 1-mo interval in random order. They were blinded to the clinical data and other imaging results such as those obtained by T1- and T2-weighted imaging, Gd-EOB-MRI, and DCE-CT.
On each type of DWI, HCC was diagnosed as a lesion showing mild-to-moderate hyperintensity compared to the liver parenchyma on DW images at a b-value of 0 s/mm2 and restricted diffusion (i.e., the lesion remained hyperintense) at a b-value of 500 and/or 1000 s/mm2, with an ADC value visually lower or equal to that of the surrounding liver parenchyma. Liver hemangiomas were diagnosed by referring to the hyperintensity on DWI with a b-value of 0 s/mm2 and an ADC value visually higher than that of the surrounding liver parenchyma. Each observer recorded the location of each HCC by placing an arrow on the image. The detectability of HCC was calculated on a tumor-by-tumor basis, and the detectability provided by the two types of DWI were compared. If liver hemangiomas were interpreted by each observer as HCC, those lesions were considered false-positives and were excluded from the assessment of detectability by the coordinator. In addition, the relationship between detectability and the sizes of the HCCs was analyzed.
Image quality scores of the sharpness of the liver contour, the distortion, and the chemical shift artifacts between FB-DWI and RT-DWI were compared with the Wilcoxon signed-rank test. The SNRs of normal liver parenchyma and lesion-to-nonlesion CNRs between the two types of DWI were compared with a paired t-test. The detectability provided by the two DWI methods was compared using McNemar’s test. A P-value < 0.05 was considered to indicate a significant difference for each analysis. Statistical analyses were performed using IBM SPSS statistics 18.0 software (IBM Japan, Tokyo). The statistical methods of this study were reviewed by Akihiro Nishie from the Department of Clinical Radiology, Graduate School of Medical Sciences, Kyushu University and Junji Kishimoto from the Center for Clinical and Translational Research, Kyushu University.
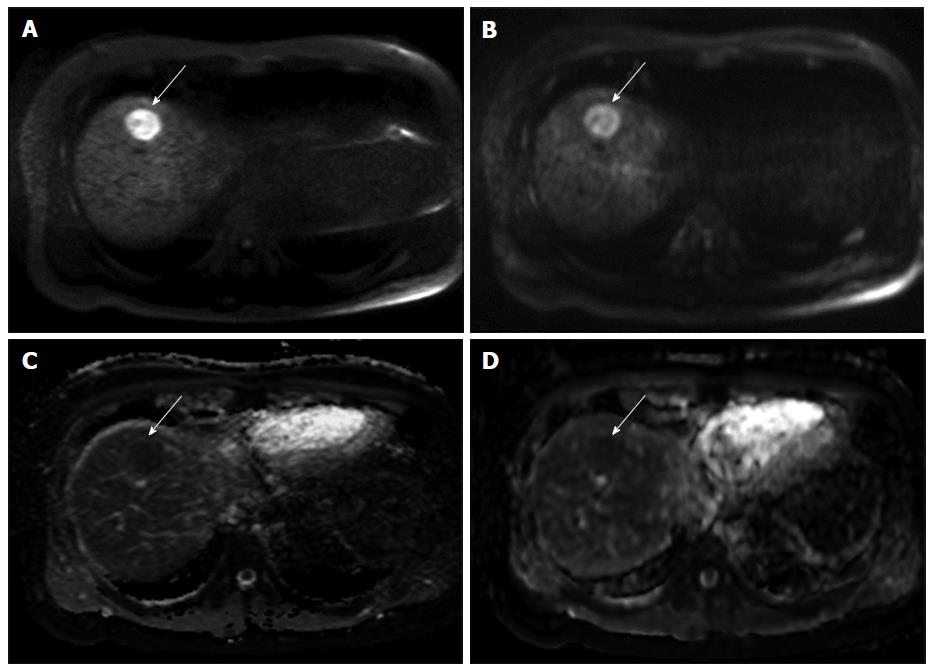
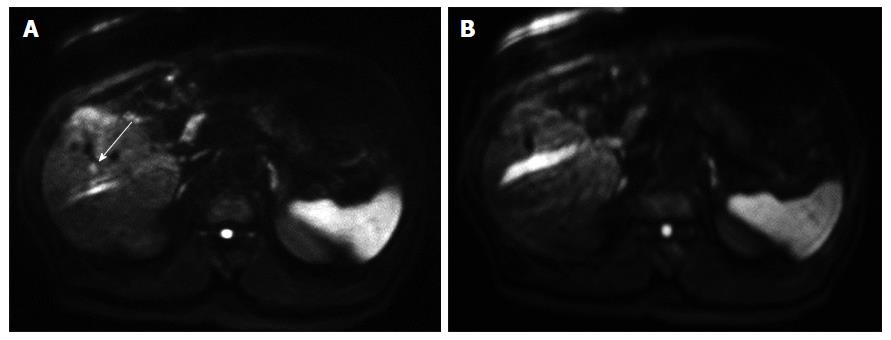
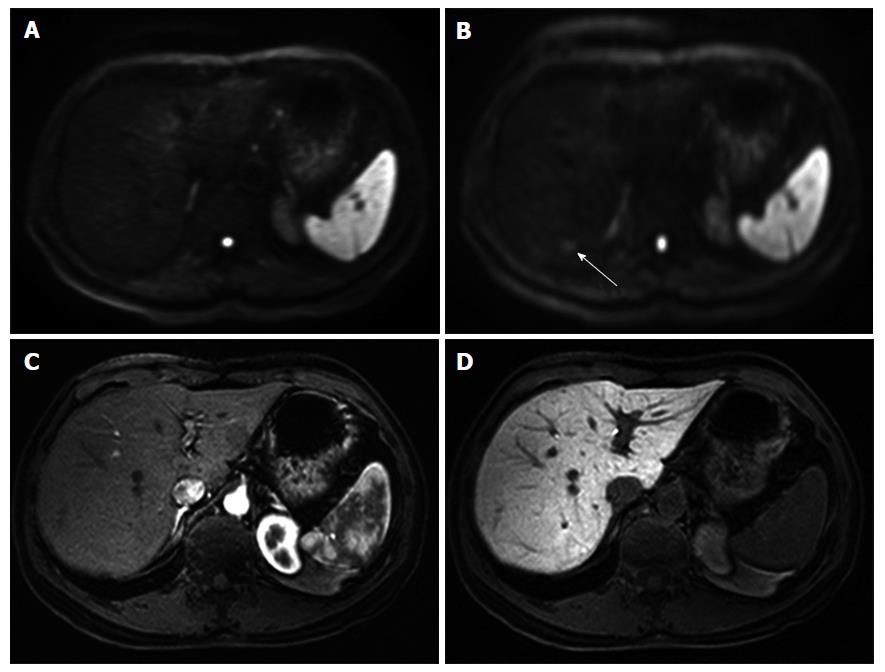
The results of the qualitative assessment by the three observers are shown in Table 3. For all three observers, the average image quality scores of the sharpness of the liver contour, the distortion, and the chemical shift artifacts of FB-DWI were significantly higher than those of RT-DWI (P < 0.05). There were no significant differences between FB-DWI and RT-DWI in the average or SDs of the SNR of the normal liver parenchyma (FB-DWI, 11.0 ± 4.8; RT-DWI, 11.0 ± 5.0) or the lesion-to-nonlesion CNR (FB-DWI, 21.4 ± 17.7; RT-DWI, 20.1 ± 15.1). Three representative cases are shown in Figures 1-3.
| Sharpness of the liver contour | Distortion | Chemical shift artifacts | ||||
| FB-DWI | RT-DWI | FB-DWI | RT-DWI | FB-DWI | RT-DWI | |
| Observer 1 | 3.08 ± 0.81 | 2.33 ± 0.65a | 2.94 ± 0.50 | 2.81 ± 0.56a | 3.38 ± 0.60 | 2.92 ± 0.59a |
| Observer 2 | 2.98 ± 0.73 | 2.37 ± 0.74a | 2.71 ± 0.70 | 2.25 ± 0.74a | 3.15 ± 1.07 | 2.21 ± 0.85a |
| Observer 3 | 3.54 ± 0.75 | 2.75 ± 0.81a | 3.27 ± 0.53 | 2.96 ± 0.71a | 3.21 ± 0.85 | 2.77 ± 1.08a |
In Figure 1, the HCC is more clearly described as hyperintensity on FB-DWI than on RT-DWI. In Figure 2, the HCC was detected by FB-DWI, whereas it was concealed by a chemical shift artifact on RT-DWI. A pseudolesion caused by a chemical shift artifact from fat tissue between the liver parenchyma and diaphragm is shown in Figure 3.
For all three observers, the sensitivity of FB-DWI [observer (Obs)-1, 43.6%; Obs-2, 53.6%; and Obs-3, 45.0%] was significantly higher than that of RT-DWI (Obs-1, 29.1%; Obs-2, 43.6%; and Obs-3, 34.5%) (P < 0.05). Regarding the relationship between detectability and the size of the HCCs, the detectability of the two types of DWI was significantly different when the tumor size was 5-22 mm. FB-DWI showed significantly higher detectability of these HCCs (Obs-1, 36.0%; Obs-2, 48.3%; and Obs-3, 33.7%) compared to RT-DWI (Obs-1, 18.0%; Obs-2, 36.0%; and Obs-3, 24.7%) (P < 0.05). There was no significant difference in the detectability of HCCs between the two types of DWI when the tumor size was > 22 mm.
The results of the present study revealed that, compared to RT-DWI, FB-DWI showed better image quality without significantly reducing the SNR of the normal liver parenchyma and the lesion-to-nonlesion CNR. The improvement of the image quality of FB-DWI might contribute to an increased detection rate of HCCs. The free-breathing technique was applied to FB-DWI of liver MRI even though it is widely believed that this technique is inappropriate due to its susceptibility to sensitive motion artifacts, which results in decreased SNRs and CNRs and image blurring[3,27]. However, our present findings demonstrated that FB-DWI had better image quality and equivalent SNRs of the liver parenchyma and lesion-to-nonlesion CNRs compared to RT-DWI.
Other studies have obtained similar results using DWI with a free-breathing technique, such as good image quality, good reproducibility of ADC values of liver tumors, and good diagnostic performance for liver lesions[28-31]. There are several possible reasons for the good image quality provided by DWI with a free-breathing technique; one is that an increased number of excitations contributes to a reduction in motion artifacts. However, FB-DWI used only two excitations, and thus the results showing good image quality, SNR and CNR cannot be attributed to a multiplicity of excitations.
Another possible reason is the advances in MR technology in recent years (such as statistic and gradient magnetic fields, a 32-channel torso-cardiac phased-array coil, and dual-source parallel radiofrequency excitation and transmission technology) that address problems related to the EPI technique[9,18,32]. In particular, the rapid image acquisition of the EPI sequence allows minimization of the blurring from T2* signal intensity decay during the gradient-echo train[9,18,32], and it is insensitive to the effects of macroscopic patient motion because of the very fast readout of the complete image data (within approximately 100 ms)[9]. This may account for the good image quality of FB-DWI in the present study.
Homogeneous fat suppression is essential for DWI, to avoid the image degradation caused by chemical shifts when using EPI[27]. For the FB-DWI in the present series, the SPAIR technique was applied because this technique is minimally affected by B1 inhomogeneity and is effective for obtaining homogeneous fat-suppression[33]. Chemical shift artifacts on DWI can be reduced by providing homogeneous fat-suppression as well. Fewer chemical artifacts on DWI are advantageous for the depiction of liver lesions. The present study’s findings related to the reduction of chemical shift artifacts occasionally mimicked a hepatic tumor. We thus conclude that FB-DWI will be advantageous for the depiction of HCCs.
In this study, we found that the detectability of HCC by FB-DWI was superior to that by RT-DWI. In fact, the detection of HCC by FB-DWI was equivalent to the reported results using breath-hold or respiratory-triggered techniques (45%-55%)[2,34,35]. That is, FB-DWI provides better spatial resolution, fewer chemical shift artifacts, and a comparable SNR and lesion-to-nonlesion CNR compared to RT-DWI[18]. This improvement in the detectability of HCCs, especially that of small-sized HCCs, is also probably due to the better spatial resolution and fewer chemical shift artifacts of FB-DWI.
The lower detectability of HCCs on DWI alone is a limitation because of the difficulty of differentiating a tumor from surrounding cirrhotic liver due to their similar diffusion properties and ADC values, and to the tumor grades of HCCs[2,7,14]. In contrast, two earlier meta-analyses showed that DWI had high sensitivity (81% and 93%) for detecting HCCs[13,36]. We speculate that the reasons for the difference in the rate of sensitivity between our results and those of the two prior meta-analyses might be related to patient selection bias, the background of liver parenchyma in patients with chronic liver disease (cirrhotic or not), and/or the tumor characteristics (e.g., tumor size and malignant grade)[13,36].
Generally, DCE-MRI is the first choice for the evaluation of liver tumors, and DWI is not used alone. However, it is known that the combination of DCE-MRI and DWI improves the detectability and provides additional information to characterize liver lesions[10-12,37,38]. DWI thus plays an important role in the assessment of liver tumors for patients with contrast-agent contraindications, such as renal failure or a history of adverse reaction to a contrast agent[38,39]. DWI has also been applied for other evaluations of HCCs, such as for assessment of the treatment effects of TACE and molecular target therapy[40,41]. FB-DWI can also contribute to the detection or treatment assessment of HCCs in combination with DCE-MRI.
There are several limitations of this study. First, most of the HCCs examined in this study were confirmed by imaging findings, although some HCCs were confirmed by histological results after surgical resection. Therefore, we did not check the histological subtypes of the HCCs, such as whether they were well-, moderately or poorly differentiated. The more aggressive HCCs (i.e., poorly differentiated HCCs) are known to show more water molecule restriction within the tumor compared to the well-differentiated HCCs. In other words, it was difficult to detect well-differentiated HCCs on DWI. In addition, there was a risk to include small ICCs which showed hypervascular tumor like HCCs. However, in this study, we did not focus on the differential diagnosis or characterization of liver tumors. The results of our study were not influenced even if a few ICCs were included in the subjects.
Second, several cases showed severe artifacts at the lateral segment of the liver because of cardiac and respiratory motions. In such cases, the artifacts hampered the visualization of the HCCs. The reduction of motion artifact at the lateral segment of the liver remains a problem to be solved.
In conclusion, FB-DWI provided better image quality and showed higher detectability of HCCs in patients with chronic liver disease compared to RT-DWI, without significantly reducing the SNR of the normal liver parenchyma or the lesion-to-nonlesion CNR. FB-DWI was better at detecting HCCs in patients with chronic liver disease compared to RT-DWI. Free-breathing diffusion-weighted imaging with modified MR parameter settings is advantageous in the diagnosis of HCCs.
We thank Dr. Junji Kishimoto, Associate Professor, Center for Clinical and Translational Research, Kyushu University for providing guidance for the statistical methods of this study.
Diffusion-weighted imaging (DWI) is widely adopted as a magnetic resonance imaging (MRI) method in clinical practice because it is useful for the detection and characterization of benign and malignant lesions. DWI is also important for liver MRI to evaluate hepatocellular carcinomas (HCCs) in patients with chronic liver disease. Although dynamic contrast-enhanced MRI has shown higher diagnostic performance for HCCs, DWI can be used as a substitute for the detection and characterization of HCCs for patients who have a contraindication for contrast agents. However, liver DWI occasionally suffers from image distortion and/or chemical shift artifacts related to the echo planar imaging technique and to motion and susceptibility artifacts. To overcome these issues, a previous study investigated the ideal MR parameter settings for obtaining high spatial resolution and fewer artifacts without losing a significant portion of the signal-to-noise ratio or contrast-to-noise ratio on liver MRI. Although DWI with modified MR parameter settings for the improvement of image quality might result in further improvement in the detectability of HCCs, no previous study has investigated this, to our knowledge. In this study, the authors evaluated the image quality and the detectability of HCCs in patients with chronic liver disease on DWI with modified MR parameter settings.
DWI is an important diagnostic imaging tool for the detection and characterization of liver tumors, including HCC in patients with chronic liver disease. Especially in the case of patients who are contraindicated for a contrast agent, DWI plays an important role for the evaluation of HCCs. Nonetheless, few prior reports have analyzed free-breathing DWI for the liver. The results of the present study may help clarify the clinical utility of free-breathing DWI for the diagnosis of HCC in patients with chronic liver disease.
In this study, the authors report the clinical utility of FB-DWI with modified MR parameter settings for the improvement of image quality for diagnosing HCC in patients with chronic liver disease. The free-breathing technique is generally avoided in liver DWI because it is hampered by image distortion and/or chemical shift artifacts related to the echo planar imaging technique and to motion and susceptibility artifacts. They evaluated the clinical impacts of previously reported modified MR parameter settings to overcome these issues with FB-DWI. FB-DWI with modified MR parameter settings provided better image quality without reducing the SNR of the normal liver parenchyma and the lesion-to-nonlesion CNR. In addition, the improvement of the image quality of FB-DWI might help increase the detection of HCCs.
These findings indicate that FB-DWI with modified MR parameter settings is especially useful for patients who are contraindicated for contrast agents and have difficulty holding their breath during the MRI scan. The improvement of image quality helps increase the detection of HCCs without reducing the SNR of the normal liver parenchyma and the lesion-to-nonlesion CNR.
FB-DWI: Diffusion-weighted imaging using free-breathing technique during the scan; RT-DWI: Diffusion-weighted imaging using a navigator-echo-based, real-time respiratory-gating and respiratory-triggering technique during the scan.
This paper aims to show modified FB-DWI be to detect HCC than conventional MR sequence and patients who are contraindicated for contrast agents.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chuang WL, Jia NY S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Kanematsu M, Goshima S, Watanabe H, Kondo H, Kawada H, Noda Y, Moriyama N. Diffusion/perfusion MR imaging of the liver: practice, challenges, and future. Magn Reson Med Sci. 2012;11:151-161. [PubMed] |
| 3. | Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Parikh T, Drew SJ, Lee VS, Wong S, Hecht EM, Babb JS, Taouli B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology. 2008;246:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 5. | Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275-282. [PubMed] |
| 6. | Hwang J, Kim YK, Jeong WK, Choi D, Rhim H, Lee WJ. Nonhypervascular Hypointense Nodules at Gadoxetic Acid-enhanced MR Imaging in Chronic Liver Disease: Diffusion-weighted Imaging for Characterization. Radiology. 2015;277:309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Nishie A, Tajima T, Asayama Y, Ishigami K, Kakihara D, Nakayama T, Takayama Y, Okamoto D, Fujita N, Taketomi A. Diagnostic performance of apparent diffusion coefficient for predicting histological grade of hepatocellular carcinoma. Eur J Radiol. 2011;80:e29-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Nishie A, Tajima T, Ishigami K, Ushijima Y, Okamoto D, Hirakawa M, Nishihara Y, Taketomi A, Hatakenaka M, Irie H. Detection of hepatocellular carcinoma (HCC) using super paramagnetic iron oxide (SPIO)-enhanced MRI: Added value of diffusion-weighted imaging (DWI). J Magn Reson Imaging. 2010;31:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Kwon HJ, Byun JH, Kim JY, Hong GS, Won HJ, Shin YM, Kim PN. Differentiation of small (≤ 2 cm) hepatocellular carcinomas from small benign nodules in cirrhotic liver on gadoxetic acid-enhanced and diffusion-weighted magnetic resonance images. Abdom Imaging. 2015;40:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Piana G, Trinquart L, Meskine N, Barrau V, Beers BV, Vilgrain V. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011;55:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Vandecaveye V, De Keyzer F, Verslype C, Op de Beeck K, Komuta M, Topal B, Roebben I, Bielen D, Roskams T, Nevens F. Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. Eur Radiol. 2009;19:2456-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Wu LM, Xu JR, Lu Q, Hua J, Chen J, Hu J. A pooled analysis of diffusion-weighted imaging in the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Gastroenterol Hepatol. 2013;28:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 446] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Dietrich O, Biffar A, Baur-Melnyk A, Reiser MF. Technical aspects of MR diffusion imaging of the body. Eur J Radiol. 2010;76:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Kuhl CK, Textor J, Gieseke J, von Falkenhausen M, Gernert S, Urbach H, Schild HH. Acute and subacute ischemic stroke at high-field-strength (3.0-T) diffusion-weighted MR imaging: intraindividual comparative study. Radiology. 2005;234:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Mürtz P, Krautmacher C, Träber F, Gieseke J, Schild HH, Willinek WA. Diffusion-weighted whole-body MR imaging with background body signal suppression: a feasibility study at 3.0 Tesla. Eur Radiol. 2007;17:3031-3037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Takayama Y, Nishie A, Asayama Y, Ishigami K, Kakihara D, Ushijima Y, Fujita N, Yoshiura T, Takemura A, Obara M. Optimization and Clinical Feasibility of Free-breathing Diffusion-weighted Imaging of the Liver: Comparison with Respiratory-Triggered Diffusion-weighted Imaging. Magn Reson Med Sci. 2015;14:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. AJR Am J Roentgenol. 2009;192:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Taouli B, Sandberg A, Stemmer A, Parikh T, Wong S, Xu J, Lee VS. Diffusion-weighted imaging of the liver: comparison of navigator triggered and breathhold acquisitions. J Magn Reson Imaging. 2009;30:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169-184. [PubMed] |
| 22. | Bruix J, Sherman M; American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 23. | Silva AC, Evans JM, McCullough AE, Jatoi MA, Vargas HE, Hara AK. MR imaging of hypervascular liver masses: a review of current techniques. Radiographics. 2009;29:385-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Takayama Y, Nishie A, Asayama Y, Ushijima Y, Fujita N, Shimamoto D, Yoshiura T, Obara M, Takemura A, Yoneyama M. Three-dimensional T2-weighted imaging for liver MRI: clinical values of tissue-specific variable refocusing flip-angle turbo spin echo imaging. J Magn Reson Imaging. 2015;41:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Goshima S, Kanematsu M, Watanabe H, Kondo H, Shiratori Y, Onozuka M, Moriyama N. Hepatic hemangioma and metastasis: differentiation with gadoxetate disodium-enhanced 3-T MRI. AJR Am J Roentgenol. 2010;195:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Tamada T, Ito K, Ueki A, Kanki A, Higaki A, Higashi H, Yamamoto A. Peripheral low intensity sign in hepatic hemangioma: diagnostic pitfall in hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI of the liver. J Magn Reson Imaging. 2012;35:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Kwee TC, Takahara T, Ochiai R, Katahira K, Van Cauteren M, Imai Y, Nievelstein RA, Luijten PR. Whole-body diffusion-weighted magnetic resonance imaging. Eur J Radiol. 2009;70:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Chen X, Qin L, Pan D, Huang Y, Yan L, Wang G, Liu Y, Liang C, Liu Z. Liver diffusion-weighted MR imaging: reproducibility comparison of ADC measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology. 2014;271:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 29. | Choi JS, Kim MJ, Chung YE, Kim KA, Choi JY, Lim JS, Park MS, Kim KW. Comparison of breathhold, navigator-triggered, and free-breathing diffusion-weighted MRI for focal hepatic lesions. J Magn Reson Imaging. 2013;38:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Inchingolo R, De Gaetano AM, Curione D, Ciresa M, Miele L, Pompili M, Vecchio FM, Giuliante F, Bonomo L. Role of diffusion-weighted imaging, apparent diffusion coefficient and correlation with hepatobiliary phase findings in the differentiation of hepatocellular carcinoma from dysplastic nodules in cirrhotic liver. Eur Radiol. 2015;25:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Jerome NP, Orton MR, d’Arcy JA, Collins DJ, Koh DM, Leach MO. Comparison of free-breathing with navigator-controlled acquisition regimes in abdominal diffusion-weighted magnetic resonance images: Effect on ADC and IVIM statistics. J Magn Reson Imaging. 2014;39:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Poustchi-Amin M, Mirowitz SA, Brown JJ, McKinstry RC, Li T. Principles and applications of echo-planar imaging: a review for the general radiologist. Radiographics. 2001;21:767-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Lauenstein TC, Sharma P, Hughes T, Heberlein K, Tudorascu D, Martin DR. Evaluation of optimized inversion-recovery fat-suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging. 2008;27:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Hardie AD, Kizziah MK, Boulter DJ. Diagnostic accuracy of diffusion-weighted MRI for identifying hepatocellular carcinoma with liver explant correlation. J Med Imaging Radiat Oncol. 2011;55:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Lewis S, Kamath A, Chatterji M, Patel A, Shyknevsky I, Dyvorne HA, Kuehn B, Taouli B. Diffusion-weighted imaging of the liver in patients with chronic liver disease: comparison of monopolar and bipolar diffusion gradients for image quality and lesion detection. AJR Am J Roentgenol. 2015;204:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Chen ZG, Xu L, Zhang SW, Huang Y, Pan RH. Lesion discrimination with breath-hold hepatic diffusion-weighted imaging: a meta-analysis. World J Gastroenterol. 2015;21:1621-1627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Hardie AD, Kizziah MK, Rissing MS. Can the patient with cirrhosis be imaged for hepatocellular carcinoma without gadolinium?: Comparison of combined T2-weighted, T2*-weighted, and diffusion-weighted MRI with gadolinium-enhanced MRI using liver explantation standard. J Comput Assist Tomogr. 2014;35:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Kim YK, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn Reson Imaging. 2014;32:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Okigawa T, Utsunomiya D, Tajiri S, Okumura S, Sasao A, Wada H, Oda S, Arimura H, Hayashida E, Urata J. Incidence and severity of acute adverse reactions to four different gadolinium-based MR contrast agents. Magn Reson Med Sci. 2014;13:1-6. [PubMed] |
| 40. | Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, Charu V, Schernthaner R, Wang Z, Tacher V. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273:746-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Corona-Villalobos CP, Halappa VG, Geschwind JF, Bonekamp S, Reyes D, Cosgrove D, Pawlik TM, Kamel IR. Volumetric assessment of tumour response using functional MR imaging in patients with hepatocellular carcinoma treated with a combination of doxorubicin-eluting beads and sorafenib. Eur Radiol. 2015;25:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |