Peer-review started: August 11, 2016
First decision: September 13, 2016
Revised: September 26, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: January 8, 2017
Processing time: 148 Days and 17.4 Hours
To investigate shear wave (SW) propagation velocity in patients with untreated hepatitis C and patients with sustained virological response (SVR).
A total of 136 hepatitis C patients [85 patients who had not received antiviral therapy (naïve group) and 51 patients who had received antiviral therapy and subsequently achieved SVR of at least 24 wk (SVR group)] and 58 healthy volunteers and outpatients without liver disease (control group) underwent evaluation of liver stiffness by SW elastography (SWE). Various parameters were evaluated in the chronic hepatitis C patients at the time of SWE.
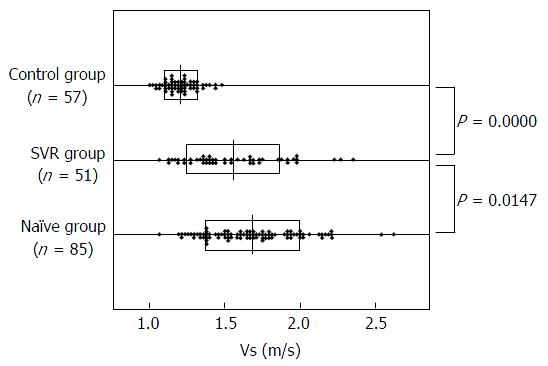
SW propagation velocity (Vs) was 1.23 ± 0.14 m/s in the control group, 1.56 ± 0.32 m/s in the SVR group, and 1.69 ± 0.31 m/s in the naïve group. Significant differences were seen between the control group and the SVR group (P = 0.0000) and between the SVR group and the naïve group (P = 0.01417). All four fibrosis markers were higher in the naïve group than in the SVR group. In the naïve group, Vs was positively correlated with alanine aminotransferase (ALT) (r = 0.5372), α feto protein (AFP) (r = 0.4389), type IV collagen (r = 0.5883), procollagen III peptide (P-III-P) (r = 0.4140), hyaluronic acid (r = 0.4551), and Mac-2 binding protein glycosylation isomer (M2BPGi) (r = 0.6092) and negatively correlated with albumin (r = -0.4289), platelets (r = -0.5372), and prothrombin activity (r = -0.5235). On multiple regression analysis, Vs was the most strongly correlated with ALT (standard partial regression std β = 0.4039, P = 0.00000). In the SVR group, Vs was positively correlated with AFP (r = 0.6977), type IV collagen (r = 0.5228), P-III-P (r = 0.5812), hyaluronic acid (r = 0.5189), and M2BPGi (r = 0.6251) and negatively correlated with albumin (r = -0.4283), platelets (r = -0.4842), and prothrombin activity (r = -0.4771). On multiple regression analysis, Vs was strongly correlated with AFP (standard partial regression std β = 0.5953, P = 0.00000) and M2BPGi (standard partial regression std β= 0.2969, P = 0.03363).
In hepatitis C patients, liver stiffness is higher in treatment-naïve patients than in those showing SVR. SWE may be a predictor of hepatocarcinogenesis in SVR patients.
Core tip: This study is the first to compare liver stiffness in a group of hepatitis C patients in whom the virus was eliminated with antiviral therapy and a group of untreated hepatitis C patients using shear wave elastography. The liver stiffness value was higher in the untreated group than in the group in which the virus had been eliminated, which is thought to be due hepatitis activity. This study also suggests the possibility that liver stiffness measurements with shear wave elastography can be used as predictors of hepatocarcinogenesis in patients in whom the virus has been eliminated.
- Citation: Suda T, Okawa O, Masaoka R, Gyotoku Y, Tokutomi N, Katayama Y, Tamano M. Shear wave elastography in hepatitis C patients before and after antiviral therapy. World J Hepatol 2017; 9(1): 64-68
- URL: https://www.wjgnet.com/1948-5182/full/v9/i1/64.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i1.64
Shear wave elastography (SWE) is a new technology that gauges liver stiffness by measuring the propagation velocity of shear waves generated in liver tissue. At the same time, images are observed in real time using a normal B-mode ultrasound probe. The velocity of laterally propagated shear waves (lateral waves) is measured. SWE is useful for a diagnosis of breast tumor[1], thyriod tumor[2], muscle stiffness[3] as well as liver stiffness. SWE resembles acoustic radiation force impulse[4], but it is new another technology.
Liver stiffness measurements with SWE are reported to be useful in diagnosing fibrosis in hepatitis C[5]. In studies using transient elastography, liver stiffness was affected not only by liver fibrosis but also by necroinflammatory activity[6,7]. Therefore, the meaning of liver stiffness is predicted to differ in untreated patients with hepatitis activity and patients whose hepatitis has subsided with antiviral therapy.
The purpose of this study was to investigate the significance of SW propagation velocity in patients with untreated hepatitis C and patients with sustained virological response.
This prospective study was reviewed and approved by the Ethics Committee of Dokkyo Medical University Koshigaya Hospital, and written, informed consent was obtained from all participants and healthy volunteers. This study conformed to the ethical guidelines of the 2008 Declaration of Helsinki.
The subjects were 136 chronic hepatitis C patients who were diagnosed in the Department of Gastroenterology of Dokkyo Medical University Koshigaya Hospital from April to October, 2015. The 136 patients included 85 patients in a naïve group who had not received antiviral therapy and 51 patients who had received antiviral therapy, either interferon-based therapy or direct-acting antiviral agent therapy (daclatasvir/asunaprevir), and subsequently achieved sustained virological response (SVR) of at least 24 wk (SVR group). Patients with decompensated liver cirrhosis, hepatocellular carcinoma, autoimmune disease, collagen disease, or chronic heart disease were excluded. Patients with a history of drinking ≥ 20 g alcohol per day and those diagnosed with obvious fatty liver on abdominal ultrasound were also excluded.
To obtain a standard liver stiffness value, SWE was performed in a total of 58 people including healthy volunteers and outpatients without liver disease (control group).
Measurement of liver stiffness by shear wave elastography was performed using a LOGIQ E9 (GE Healthcare, Milwaukee, WI). The right lobe of the liver was visualized through an intercostal space while the patient was lying in a supine position with the right arm in maximum abduction. Measurements were taken while subjects held their breath during spontaneous breathing. The visual depth of the system was fixed at 8 cm, and the region of interest was 1-2 cm below the surface of the liver. The system was adjusted so that sample volume depth was 4 cm or less. Liver stiffness was automatically calculated by the apparatus, and the results are expressed as the velocity of shear wave velocity (Vs) (m/s). Measurements were performed by two investigators (Suda T and Tamano M) who have measurement experience of SWE more than 100 patients. They shot 10 to 12 times on liver segment 5, and the result was considered reliable only when 10 successful shots and a measurement success rate > 80% were obtained.
The following clinical parameters were determined in chronic hepatitis C patients at the time SWE was performed: Age; aspartate aminotransferase (AST); alanine aminotransferase (ALT); total bilirubin, serum albumin; white blood cell (WBC) count; platelet count; prothrombin activity; α fetoprotein (AFP); hyaluronic acid; type IV collagen; procollagen III peptide (P-III-P); Mac-2 binding protein glycosylation isomer (M2BPGi) measurements; and the Fib-4 index.
Continuous data for Vs and other clinical parameters are expressed as means ± SD. The Mann-Whitney U test was used for between-group comparisons. Correlations between Vs and other parameters were assessed using Spearman’s rank correlation coefficient and multiple regression analysis. Values of P < 0.05 were regarded as statistically significant.
Figure 1 shows Vs (m/s) measured by SWE in each group. Vs was 1.23 ± 0.14 m/s in the control group, 1.56 ± 0.32 m/s in the SVR group, and 1.69 ± 0.31 m/s in the naïve group. Significant differences were seen between the control group and the SVR group (P = 0.0000) and between the SVR group and the naïve group (P = 0.01417).
Table 1 shows the characteristics of the naïve group and the SVR group. Compared with the SVR group, the naïve group had significantly higher AST and ALT values (P = 0.00001) and a significantly lower serum albumin value (P = 0.01049). No significant differences were seen between the two groups in total bilirubin, WBC, platelet count, or prothrombin activity. AFP was significantly higher in the naïve group than in the SVR group (P = 0.00773). All four fibrosis markers were higher in the naïve group than in the SVR group. No significant differences were seen in type IV collagen or hyaluronic acid, but significant differences were seen in P-III-P (P = 0.00215) and M2BPGi (P = 0.00546). The FIB-4 index tended to be higher in the naïve group than in the SVR group, but the difference was not significant (P = 0.37443).
| Characteristics | Naïve group(n = 85) | SVR group(n = 51) | P value |
| Age (yr) | 63.5 ± 13.7 | 64.9 ± 10.2 | 0.9726 |
| Sex (male/female) | 43/42 | 27/24 | 0.79039 |
| AST (IU/L) | 55.4 ± 38.7 | 29.2 ± 16.8 | 0.00001 |
| ALT (IU/L) | 61.9 ± 50.1 | 23.9 ± 18.0 | 0.00001 |
| Total bilirubin (mg/dL) | 0.89 ± 0.36 | 0.95 ± 0.33 | 0.14736 |
| Serum albmin (g/dL) | 4.10 ± 0.46 | 4.32 ± 0.44 | 0.01049 |
| WBC (× 103/mm3) | 5.21 ± 1.83 | 5.24 ± 1.56 | 0.79015 |
| Hb (g/dL) | 13.9 ± 1.6 | 14.1 ± 1.5 | 0.47879 |
| Platelet (× 104/mm3) | 14.5 ± 6.4 | 14.5 ± 5.7 | 0.89078 |
| Prothrombin activity (%) | 97.5 ± 12.1 | 96.9 ± 13.1 | 0.98341 |
| AFP (g/dL) | 9.6 ± 10.6 | 5.1 ± 3.3 | 0.00773 |
| Type IV collagen (ng/mL) | 180.5 ± 71.8 | 179.2 ± 71.6 | 0.62913 |
| P-III-P (U/mL) | 0.92 ± 0.27 | 0.81 ± 0.31 | 0.00215 |
| Hyaluronic acid (ng/mL) | 191.5 ± 290.3 | 102.1 ± 93.5 | 0.24908 |
| M2BPGi (COI) | 3.67 ± 4.41 | 2.03 ± 2.49 | 0.00546 |
| FIB-4 index | 4.44 ± 4.21 | 3.39 ± 2.63 | 0.37443 |
Vs was positively correlated with ALT (r = 0.5372), AFP (r = 0.4389), type IV collagen (r = 0.5883), P-III-P (r = 0.4140), hyaluronic acid (r = 0.4551), and M2BPGi (r = 0.6092). Vs was negatively correlated with albumin (r = -0.4289), platelets (r = -0.5372), and prothrombin activity (r = -0.5235). A multiple regression analysis was performed with the five parameters of ALT, platelets, prothrombin activity, type IV collagen, and M2BPGi that had correlation coefficients (r) ≥ 0.5, and the results showed that Vs was the most strongly correlated with ALT in the naïve group (standard partial regression std β = 0.4039, P = 0.00000) (Table 2).
| Coefficient (β) | SE(β) | Std β | t-value | df | P value | |
| ALT | 0.00261 | 0.00052 | 0.4069 | 5.02998 | 62 | 0.00000 |
| Plt | -0.0121 | 0.00436 | -0.27300 | 0.27300 | 62 | 0.00740 |
| PT % | -0.0044 | 0.00245 | -0.1712 | 1.79923 | 62 | 0.07685 |
| Type IV collagen | 0.00061 | 0.00040 | 0.1578 | 1.51030 | 62 | 0.13605 |
| M2BPGi | 0.00844 | 0.00709 | 0.1361 | 1.19098 | 62 | 0.23820 |
Vs was positively correlated with AFP (r = 0.6977), type IV collagen (r = 0.5228), P-III-P (r = 0.5812), hyaluronic acid (r = 0.5189), and M2BPGi (r = 0.6251). Vs was negatively correlated with albumin (r = -0.4283), platelets (r = -0.4842), and prothrombin activity (r = -0.4771). A multiple regression analysis was performed with the five parameters of AFP, type IV collagen, P-III-P, hyaluronic acid, and M2BPGi that had correlation coefficients (r) ≥ 0.5, and the results showed that Vs was strongly correlated with two parameters, AFP (standard partial regression std β = 0.5953, P = 0.00000) and M2BPGi (standard partial regression std β = 0.2969, P = 0.03363) (Table 3).
| Coefficient (β) | SE (β) | Std β | t-value | df | P value | |
| AFP | 0.05564 | 0.0098 | 0.5953 | 5.6797 | 28 | 0.00000 |
| Type IV Collagen | -0.0003 | 0.00066 | -0.0626 | 0.42483 | 28 | 0.67420 |
| P-III-P | 0.13859 | 0.17013 | 0.146 | 0.81462 | 28 | 0.42217 |
| Hyaluronic acid | 0.00053 | 0.00053 | 0.1658 | 0.99713 | 28 | 0.32724 |
| M2BPGi | 0.03554 | 0.01591 | 0.2969 | 2.23421 | 28 | 0.03363 |
The extent of hepatic fibrosis has classically been evaluated by histological procedures. However, the accuracy of this evaluation of hepatic fibrosis is limited by both sampling variability and inter-observer variability between pathologists[8,9]. In addition, liver biopsy is associated with patient discomfort and a risk of serious complications[10].
Transient elastography (TE) has attracted attention as a noninvasive, objective diagnostic tool, and liver stiffness measured by TE is reported to be useful in diagnosing fibrosis in hepatitis C[11]. TE is useful in diagnosing non-alcoholic fatty liver disease[12] and in predicting carcinoma development in viral hepatitis patients[13]. However, TE is a test that is done blindly using a special probe in the right hepatic lobe confirmed with B mode, as a result of which measurement results are imprecise if vessels or other structures are present in the measured region. SWE is built into ultrasonic diagnostic equipment, and reliable measurements are possible in a short time under observation with normal B mode[14].
The results of SWE measurements are expressed as the SW propagation velocity Vs (m/s). In this investigation, the Vs was 1.23 ± 0.14 m/s in healthy livers, 1.69 ± 0.31 m/s in the naïve group, and 1.56 ± 0.32 m/s in the SVR group. The naïve group had a significantly higher Vs than the SVR group, suggesting that Vs decreases with virus elimination in hepatitis C patients. In this study, however, the naïve group and SVR group were different populations, and Vs measurements over time in the same population will be needed to accurately compare Vs before and after treatment.
Vs is determined not only by tissue elasticity (fibrosis), but it is also affected by viscosity. Thus, in cases of active hepatitis, propagation is expected to become faster due to increased tissue viscosity from increased exudate into the interstitium and cell infiltration, and in acute hepatitis that trend is marked[15,16]. In the naïve group, Vs was most strongly correlated with ALT. This is thought to be because the naïve group included many patients with active hepatitis with high ALT levels. Good positive correlations were seen between Vs and the liver fibrosis markers of hyaluronic acid, type IV collagen, P-III-P, and M2BPGi in the naïve group. Thus, in the naïve group, Vs is thought to reflect both hepatic activity (viscosity) and fibrosis (elasticity).
In the SVR group, hepatitis had subsided for six months or more, and, in fact, the correlation between Vs and ALT in the SVR group in this study was very low. Therefore, Vs in the SVR group is presumed to almost purely reflect liver fibrosis (elasticity), and thus it is thought to have better correlations with fibrosis markers. Among the four different fibrosis markers, M2BPGi had the strongest positive correlation with Vs. M2BPGi is a new liver fibrosis marker that quantitatively measures changes in the carbohydrate structure of Mac-2 binding protein[17], and it is also considered useful in predicting carcinogenesis in hepatitis C patients[18-20].
In hepatitis C patients, AFP is a useful indicator of hepatocarcinogenesis following interferon therapy[21]. Although AFP had positive correlations in both the naïve group and the SVR group, a stronger correlation was seen in the SVR group. In the naïve group, AFP reflects inflammation and necrosis of hepatocytes and the accompanying hepatocyte regeneration. In the SVR group, on the other hand, AFP has a strong element as a surrogate marker of hepatocellular carcinoma, as mentioned previously. A very interesting finding in the SVR group in the present study was the strong correlations between Vs and AFP and between Vs and M2BPGi, which suggest the possibility that liver stiffness measurements with SWE may be used as predictors of hepatocarcinogenesis in hepatitis C patients following SVR.
In hepatitis C patients, liver stiffness with SWE was higher in the naïve group than in the SVR group, presumably due to hepatitis activity. In the SVR group, liver stiffness measurements with SWE may be a predictor of hepatocarcinogenesis.
Shear wave elastography (SWE) is a new technology that gauges liver stiffness by measuring the propagation velocity of shear waves generated in liver tissue. At the same time, images are observed in real time using a normal B-mode ultrasound probe. The velocity of laterally propagated shear waves (lateral waves) is measured.
The results of SWE measurements are expressed as the SW propagation velocity Vs (m/s). In this investigation, the Vs was 1.23 ± 0.14 m/s in healthy livers, 1.69 ± 0.31 m/s in the naïve group, and 1.56 ± 0.32 m/s in the SVR group. The naïve group had a significantly higher Vs than the SVR group, suggesting that Vs decreases with virus elimination in hepatitis C patients. In this study, however, the naïve group and sustained virological response (SVR) group were different populations, and Vs measurements over time in the same population will be needed to accurately compare Vs before and after treatment.
A very interesting finding in the SVR group in the present study was the strong correlations between Vs and α feto protein and between Vs and Mac-2 binding protein glycosylation isomer, which suggest the possibility that liver stiffness measurements with SWE may be used as predictors of hepatocarcinogenesis in hepatitis C patients following SVR.
In the SVR group, liver stiffness measurements with SWE may be a predictor of hepatocarcinogenesis.
This manuscript by Suda et al points to assess the changes of shear wave velocity in patients with untreated chronic hepatitis C and patients who received antiviral therapy and obtained SVR. The authors found significant differences between shear wave velocity between patients with SVR and those untreated.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pawlowska M, Pekgoz M, Stanciu C S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Zhang Q, Xiao Y, Dai W, Suo J, Wang C, Shi J, Zheng H. Deep learning based classification of breast tumors with shear-wave elastography. Ultrasonics. 2016;72:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 2. | Gangadhar K, Hippe DS, Thiel J, Dighe M. Impact of Image Orientation on Measurements of Thyroid Nodule Stiffness Using Shear Wave Elastography. J Ultrasound Med. 2016;35:1661-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Du LJ, He W, Cheng LG, Li S, Pan YS, Gao J. Ultrasound shear wave elastography in assessment of muscle stiffness in patients with Parkinson’s disease: a primary observation. Clin Imaging. 2016;40:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Osakabe K, Ichino N, Nishikawa T, Sugiyama H, Kato M, Shibata A, Asada W, Kawabe N, Hashimoto S, Murao M. Changes of shear-wave velocity by interferon-based therapy in chronic hepatitis C. World J Gastroenterol. 2015;21:10215-10223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Okuda S, Tsuji N, Imayoshi Y, Yasuda E. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: Comparison of liver fibrosis indices. Hepatol Res. 2015;45:E122-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Lupşor M, Badea R, Stefănescu H, Grigorescu M, Sparchez Z, Serban A, Branda H, Iancu S, Maniu A. Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C. Results from a cohort of 324 patients. J Gastrointestin Liver Dis. 2008;17:155-163. [PubMed] |
| 7. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 509] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 9. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1398] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 10. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 804] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 11. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 12. | Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res. 2011;41:965-970. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Ferraioli G, Parekh P, Levitov AB, Filice C. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med. 2014;33:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Kim SU, Han KH, Park JY, Ahn SH, Chung MJ, Chon CY, Choi EH, Kim DY. Liver stiffness measurement using FibroScan is influenced by serum total bilirubin in acute hepatitis. Liver Int. 2009;29:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 17. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M. A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Tamaki N, Kurosaki M, Kuno A, Korenaga M, Togayachi A, Gotoh M, Nakakuki N, Takada H, Matsuda S, Hattori N. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol Res. 2015;45:E82-E88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Sasaki R, Yamasaki K, Abiru S, Komori A, Nagaoka S, Saeki A, Hashimoto S, Bekki S, Kugiyama Y, Kuno A. Serum Wisteria Floribunda Agglutinin-Positive Mac-2 Binding Protein Values Predict the Development of Hepatocellular Carcinoma among Patients with Chronic Hepatitis C after Sustained Virological Response. PLoS One. 2015;10:e0129053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 21. | Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H. α-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |