Peer-review started: April 6, 2016
First decision: May 17, 2016
Revised: June 24, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: January 8, 2017
Processing time: 275 Days and 15.1 Hours
To assess the accuracy of shear wave elastography (SWE) alone and in combination with aminotransferase platelet ratio index (APRI) score in the staging of liver fibrosis.
A multicenter prospective study was conducted to assess the accuracy of SWE (medians) and APRI to predict biopsy results. The analysis focused on distinguishing the different stages of liver disease, namely, F0 from F1-4, F0-1 from F2-4, F0-2 from F3-4 and F0-3 from F4; F0-F1 from F2-F4 being of primary interest. The area under the receiver operating characteristic (AUROC) curve was computed using logistic regression model. The role of age, gender and steatosis was also assessed.
SWE alone accurately distinguished F0-1 from F2-4 with a high probability. The AUROC using SWE alone was 0.91 compared to 0.78 for using the APRI score alone. The APRI score, when used in conjunction with SWE, did not make a significant contribution to the AUROC. SWE and steatosis were the only significant predictors that differentiated F0-1 from F2-4 with an AUROC of 0.944.
Our study validates the use of SWE in the diagnosis and staging of liver fibrosis. Furthermore, the probability of a correct diagnosis is significantly enhanced with the addition of steatosis as a prognostic factor.
Core tip: The gold standard in the diagnosis and staging of liver fibrosis is an invasive liver biopsy. The accuracy of non-invasive tools such as ultrasound shear wave elastography either alone or in combination with the use of the aspartate transaminase platelet ratio index score compared to histology to guide management of liver fibrosis is not known. We addressed this question in a multicenter trial in patients with chronic progressive liver disease in a low to middle income country.
- Citation: Sande JA, Verjee S, Vinayak S, Amersi F, Ghesani M. Ultrasound shear wave elastography and liver fibrosis: A Prospective Multicenter Study. World J Hepatol 2017; 9(1): 38-47
- URL: https://www.wjgnet.com/1948-5182/full/v9/i1/38.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i1.38
Liver fibrosis is a progressive condition that if diagnosed early and staged accurately, allows early clinical intervention that may arrest or slow down progression to end stage decompensated cirrhosis. The spectrum of chronic liver disease and fibrosis that leads to end stage decompensated cirrhosis, is an important cause of morbidity and mortality in the world[1]. Early diagnosis, accurate staging and re-evaluation of liver fibrosis is aimed at avoiding the progression from normal to minimal to significant fibrosis and timely management of patients with advanced disease.
There are several chronic progressive liver diseases that lead to liver fibrosis. Non-alcoholic fatty liver disease (NAFLD) is one of the most common. NAFLD is closely associated with obesity and insulin resistance. Pathological changes in the biochemical profile of the liver that lead to liver fibrosis also occur due to chronic metabolic conditions such as diabetes and degenerative conditions like atherosclerosis[2,3]. Other common causes of liver fibrosis include infections such as chronic viral hepatitis and human immunodeficiency virus. Drugs and other toxins also play an important role. This list is not exhaustive but this paper focuses on the last four causes. Liver fibrosis is characterized by excessive accumulation of extracellular matrix due to the release of inflammatory mediators and free radicals to cause oxidative stress and liver fibrogenesis. During this process hepatic stellate cells activation occurs. Platelet derived growth factor, tumor necrosis factor α, transforming growth factor β or reactive oxygen species play a role in the progression to liver fibrosis. Several phenotypic alterations occur with the end result being irreversible[4].
The current gold standard in the diagnosis and staging of liver fibrosis is liver biopsy. Liver biopsy and more recently ultrasound guided liver biopsy only evaluates 1/50000 of the liver parenchyma. It is invasive, has a complication rate (albeit small,) and is subject to intra and inter-observer variability[5]. Because of the imperfect nature of liver biopsies, over the last several years there has been a growing trend to validate non-invasive tools to diagnose and stage liver fibrosis. Alkaline aminotransferase platelet ratio index (APRI) is a laboratory marker that has been shown to have some value but is inferior to liver biopsy. Ultrasound and magnetic resonance have been used for elasticity imaging. Magnetic resonance elastography, even though promising, has some disadvantages. Aside from the significant cost of the study, it cannot be performed in a liver with iron overload because of signal-to-noise limitations; has longer examination times compared to ultrasound elastography, and is subject to respiratory artifact[6]. Ultrasound elastography has been validated and has been shown in many studies to have similar sensitivity and specificity to liver biopsies[5,7].
Ultrasound elastography measures the liver stiffness/elasticity by assessing at least 100 times the proportion of the liver that a biopsy does. Transient elastography (TE) has been validated in multiple studies[8] but shear wave elastography (SWE) may be preferred because unlike transient elastography, which consists of a vibrator producing shear waves, the latter can perform a conventional ultrasound at the same time. The technique is integrated into an ultrasound system. The principle behind the interpretation of shear wave elastography is that shear waves produced by a focused ultrasound beam are directly related to the stiffness of the liver from where they are generated[5,7,8]. SWE is also reportedly more accurate than TE in assessing significant fibrosis (≥ F2)[8,9]. The use of shear wave elastography in the diagnosis and staging of liver fibrosis has been increasing. Being a non-invasive technique proves advantageous because repeat measurements can be obtained in patients with chronic progressive liver diseases. However, this non-invasive procedure does have some pitfalls. It is subject to intra- and inter-observer variability, validated cut-offs have mainly only been demonstrated in hepatitis C; Acute hepatitis can have false positives. In patients with a high body mass index, erroneous values may be obtained. A very practical pitfall is confounding factors such as edema, inflammation, cholestasis and congestion. All these must be put in context and a multidisciplinary clinical approach used in the interpretation of the results[5,7,8,10].
A limitation of prior studies is the lack of integration of the accuracy and limitations of elastography. No prior study has combined elastography with the use of APRI and histology to guide management of liver fibrosis. This study addresses the gaps and makes practical inferences that focus on accurate early diagnosis and staging. The focus in our study is “interpretation within a clinical context”. This multi-institutional study performed in Kenya aims to capture and highlight factors based on the disease burden in this region. Ultrasound elastography has only recently been made available in East Africa. The findings, therefore, could be of wider benefit because of the high burden of other etiologies of liver disease such as hepatitis B in the region. Most studies thus far have been carried out in the West with the disease burden focused on hepatitis C. In addition, the literature largely reports data from middle-high economic areas whereas adherence to clinical guidelines may not be as feasible in poor/resource challenged facilities.
The primary objective was to analyze the accuracy of shear wave elastography in comparison to liver biopsy in differentiating the various stages of liver fibrosis. The secondary objective was to evaluate whether the addition of the APRI score to SWE would improve the accuracy of this differentiation. With these, illustrate the role of the shear wave elastography, APRI score, and biopsy solely and or in combination in the diagnostic algorithm of accurate quantification of liver fibrosis. We also sought to assess the role of other covariates, namely, age, gender and steatosis, and their influence on the relative importance of SWE and the APRI score in predicting the extent of liver fibrosis.
Three hospitals were included in this prospective study: Aga Khan University (AKU) Hospital, Kenyatta Teaching and Referral Hospital and St Mary’s Mission Hospital. Approval was obtained from the relevant Scientific and Ethics committees. All consecutive patients referred for an ultrasound guided liver biopsy at all three institutions were subject to recruitment based on the inclusion and exclusion criteria as well as informed written consent. The study included patients above eighteen years of age with chronic progressive diffuse liver disease. Patients were excluded from the study if they did not have any of the three diagnostic tests, i.e., liver biopsy, APRI score or SWE.
Consecutive patients were recruited by the principle investigator in three ways: (1) referral for an ultrasound guided liver biopsy at the AKU Radiology Department; (2) referral from St Mary’s Hospital with biopsy performed by the AKU Radiology department and pathological analysis performed at AKU department; and (3) referral for a liver biopsy request to be analyzed at the Pathology department of Kenyatta National Hospital.
At recruitment, a study file was opened for each patient by the principle investigator at the AKU. Routine liver function tests, platelet counts, and demographic information related to confounding factors of chronic liver disease, including information on alcohol use, Human Immunodeficiency Virus status, viral load and CD4 levels, hepatitis B and C status was collected. Men who had been drinking more than 30 g of alcohol per day and women who had been drinking more than 20 g of alcohol per day were considered current drinkers. Patients who had stopped drinking completely for more than six months before the biopsy were considered ex-drinkers[11].
At the AKU routine ultrasound of the liver was performed to qualitatively record presence (grade 0-3) or absence of steatosis using established criteria published by Lupşor-Platon et al[12]. Any other diffuse or focal lesions were documented followed by SWE. At AKU elastography measurements were taken from the right lobe[13] of the liver with the patients holding their breath. Measurements were considered successful using validated criteria established by Castéra et al[14]: “(1) 10 valid shots; (2) a ratio of valid shots to the total number of shots of 60% or higher; and (3) variability of measurements less than 30% of the median value of liver stiffness measurements”. Philips iU22 ultrasound machine with its C5-1 curvilinear transducer was used. The units for SWE readouts (liver stiffness) were kilopascals (kPa). Four sonologists each with more than 5 years’ experience in routine liver scanning and validated ultrasound elastography experience from uniform training performed each exam independently. The median stiffness (used to grade the fibrosis), average stiffness and standard deviation of measurements generated by the software were recorded and interpreted by the four sonologists independently[7,10]. Each patient had one liver biopsy specimen taken from the right lobe[13] after the ultrasound elastography which was graded histologically for fibrosis based on the Metavir classification system[15,16]. This was done by two experienced histopathologists at the AKU and KNH Pathology departments. Each specimen was evaluated by the two histopathologists from each respective pathology department. Discrepancies were resolved by consensus between the two. The histopathologists from the two sites were full-time faculty, certified by the Kenya Medical Practitioners and Dentists Board, practicing in University Hospitals each with greater than ten years’ experience in liver biopsy assessment for fibrosis. In each patient the time interval between ultrasound, elastography and histology was not more than one month. The pathologists and sonologists were blinded to clinical data and elastography or histology grade.
Interpretation of liver fibrosis by shear wave elastography in kPa divided the entity into no fibrosis (F0), mild fibrosis (F1), severe fibrosis (F2), significant fibrosis (F3) and cirrhosis (F4). Automatic median value generated by the ultrasound software was used to establish the elastography grade as follows < 4.6 = F0, 4.6-5.6 = F1, 5.7-7.0 = F2, 7.1-12.0 = F3 and > 12 = F4[10,17-19]. APRI score was calculated using a formula proposed by original study of Wai et al[20]: APRI = [(AST level/ULN)/platelet counts (109/L)] × 100. A score of < 0.5 was graded as F0, 0.5-1.5 as F1-3 and > 1.5 as F4. The corresponding histology grade was assessed[21,22].
The sample size was determined with the aim to keep the standard error of the AUROC at 0.05. This set the difference between the upper and lower 95%CI limits to 0.20 (± 2 standard errors). From previous publications the range for the AUROC for significant fibrosis, as determined from non-invasive tests, is approximately 0.69 to 0.89; for cirrhosis the range is from 0.81 to 0.98. Assuming the AUROC to be approximately 0.8[20] the sample size of 110 patients would yield a standard error of 0.05 (Table 1).
| AUROC | Total (n) | % Positive | # Positive | # Negative | SE | Confidence interval | |
| Lower | Upper | ||||||
| 0.8 | 110 | 30 | 33 | 77 | 0.050 | 0.701 | 0.899 |
| 0.8 | 130 | 30 | 39 | 91 | 0.046 | 0.709 | 0.891 |
Shear wave elastography has been shown to have a lower operator error technique than transient elastography (3%-16%)[23-25]. As a precautionary measure we raised the sample size from 110 to 130.
The statistical review of the study was performed by a biomedical statistician. Logistic regression models with backward elimination, using SAS version 9.3, were utilized to assess the significance of SWE median, the APRI score and the covariates age (categorized as below and above the median), gender and steatosis. Besides the P-values, the analysis provided the ORs and their respective 95%CI limits. The sensitivity and the specificity were computed based on the variables included in the model. This in turn enabled the ROCs and the AUROC to be determined. Summary statistics and correlation coefficients, where appropriate, were computed.
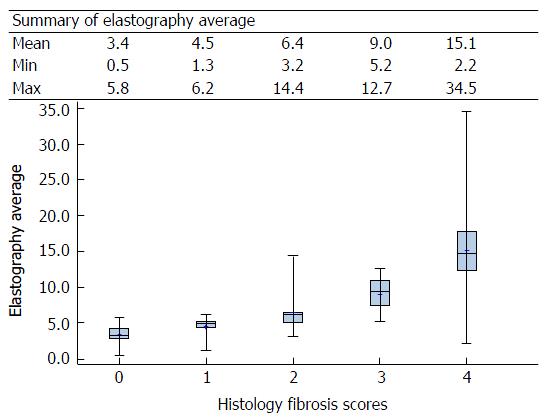
One hundred and twenty-eight patients were recruited for the study. AKU, KNH and St. Mary’s contributed 54 (42.2%), 53 (41.4%) and 21 (16.4%) patients, respectively. The most prevalent viral infection was hepatitis B that was noted in 30 (23.4%) patients. This was followed by human immunodeficiency virus (HIV) with 18 (14.1%) and hepatitis C with 13 (10.2%) patients (Appendix 1); fifteen patients had 2 or more infections. Sixty-three (49.2%) of the patients had a steatosis score of 0 and 61 (47.7%) of the patients had a histology fibrosis score of 0. Eighty-one (63.3%) patients fell in the histology fibrosis score subgroup F0-1; the remaining 47 (36.7%) fell in the F2-F4 subgroup. Fifty percent of the patients had an elastography score of F0 and 59 (46%) of the patients the APRI score of F0 (Appendix 2). The elastography median scores were the lowest among HIV subjects followed by those with hepatitis B and then a hepatitis C infection. The highest scores were from those with multiple viral infections. The APRI scores also follow the pattern described above for the elastography median scores (Appendix 3). There appears to be a good correlation between the elastography median scores and the histology fibrosis scores (Appendix 4). The elastography and APRI fibrosis score are statistically significantly correlated with the histology fibrosis scores (Appendix 5) (Figure 1 and Table 2).
| Histology fibrosis scores | Total | χ2 | |||||
| 0 (n = 61) | 1 (n = 20) | 2 (n = 20) | 3 (n = 10) | 4 (n = 17) | (n = 128) | P value | |
| Elastography fibrosis score | |||||||
| F0 | 51 (83.6) | 7 (35.0) | 5 (25.0) | 0 (0.0) | 1 (5.9) | 64 (50.0) | < 0.0001 |
| F1 | 10 (16.4) | 10 (50.0) | 2 (10.0) | 2 (20.0) | 1 (5.9) | 25 (19.5) | |
| F2 | 0 (0.0) | 3 (15.0) | 9 (45.0) | 1 (10.0) | 0 (0.0) | 13 (10.2) | |
| F3 | 0 (0.0) | 0 (0.0) | 3 (15.0) | 7 (70.0) | 1 (5.9) | 11 (8.6) | |
| F4 | 0 (0.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 14 (82.4) | 15 (11.7) | |
| APRI fibrosis score | |||||||
| 0 | 45 (73.8) | 6 (30.0) | 6 (30.0) | 1 (10.0) | 1 (5.9) | 59 (46.1) | < 0.0001 |
| 1 to 3 | 14 (23.0) | 13 (65.0) | 9 (45.0) | 9 (90.0) | 8 (47.1) | 53 (41.4) | |
| 4 | 2 (3.3) | 1 (5.0) | 5 (25.0) | 0 (0.0) | 8 (47.1) | 16 (12.5) | |
| Steatosis | |||||||
| Grade 0 | 48 (78.7) | 8 (40.0) | 2 (10.0) | 1 (10.0) | 4 (23.5) | 63 (49.2) | < 0.0001 |
| Grade 1 | 6 (9.8) | 8 (40.0) | 5 (25.0) | 1 (10.0) | 1 (5.9) | 21 (16.4) | |
| Grade 2 | 6 (9.8) | 2 (10.0) | 7 (35.0) | 4 (40.0) | 0 (0.0) | 19 (14.8) | |
| Grade 3 | 1 (1.6) | 2 (10.0) | 6 (30.0) | 4 (40.0) | 12 (70.6) | 25 (19.5) | |
Table 3 summarizes some of the key results that stemmed from the analysis of the SWE median and APRI score data using logistic regression. Both variables show a high degree of statistical significance in their individual ability to distinguish between the lower stages of fibrosis compared to the higher stages. This is true across all possible partitions of the Metavir fibrosis scores. However, the AUROCs for SWE medians are much higher than those for APRI score.
| F0-3 vs F4 | F0-2 vs F3-4 | F0-1 vs F2-4 | F0 vs F1-4 | ||
| SWE median | P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| OR | 1.708 | 1.789 | 2.983 | 2.683 | |
| 95%CI | 1.379, 2.115 | 1.432, 2.236 | 1.839, 4.838 | 1.789, 4.025 | |
| AUROC | 0.926 | 0.929 | 0.908 | 0.879 | |
| APRI score | P value | 0.0202 | 0.039 | 0.0005 | 0.0008 |
| OR | 1.511 | 1.404 | 3.482 | 4.651 | |
| 95%CI | 1.067, 2.141 | 1.018, 1.938 | 1.727, 7.018 | 1.895, 11.416 | |
| AUROC | 0.812 | 0.784 | 0.780 | 0.803 | |
| SWE and APRI1 | AUROC | 0.927 | 0.931 | 0.920 | 0.890 |
| APRI influence | 0.001 | 0.002 | 0.012 | 0.011 |
The SWE median differentiates the Metavir fibrosis subgroups F0-1 and F2-4 with an AUROC of 0.908 compared to 0.780 for the APRI score. These results imply that the SWE on its own is a better predictor of the differentiating the subgroups than the APRI score. When we utilize both variables simultaneously, the increase in the AUROC attributed to the APRI score is less than 1.2% higher than that predicted by SWE median. This amounts to about 13% of the 9% not predicted correctly by the SWE median. The results for other partitions of the histology fibrosis scores mimic those described above with the AUROC for SWE median being around 0.9 and that of the APRI score being about 0.8.
Additional logistic regression models incorporated several other variables in the analysis to evaluate their impact on the AUROC. The variables considered, in addition to the SWE median and the APRI score were the covariates age (categorized as below and above the median), gender and steatosis score. The results of the logistic regression analysis (Table 4) with all of the aforementioned variables in the model showed that SWE and the steatosis score were the only two variables that made significant contributions to the predictive power of the model.
| Variable | DF | Coefficient estimate | Standard error | Wald χ2 | Pr > χ2 | OR estimate | Lower 95%CI limit for OR | Upper 95%CI limit for OR | |
| Intercept | 1 | -6.6482 | 1.3510 | 24.2158 | < 0.0001 | ||||
| Age category | LE median | 1 | 0.0971 | 0.3219 | 0.0910 | 0.7629 | 1.214 | 0.344 | 4.288 |
| APRI score | 1 | 0.1946 | 0.3873 | 0.2526 | 0.6153 | 1.215 | 0.569 | 2.595 | |
| Elastography median | 1 | 0.9041 | 0.2513 | 12.9464 | 0.0003 | 2.470 | 1.509 | 4.042 | |
| Sex | Female | 1 | 0.3330 | 0.3551 | 0.8797 | 0.3483 | 1.947 | 0.484 | 7.830 |
| Steatosis | 1 | 1.1317 | 0.3462 | 10.6843 | 0.0011 | 3.101 | 1.573 | 6.112 |
Using the backward elimination method, all of the variables that were not making a significant contribution at the 0.1 level were dropped from the model. The results of these analysis (Table 5) show that for the primary objective of differentiating between F0-1 and F2-4 is accomplished quite well with an AUROC of 0.944; the two variables that made a significant contribution were SWE and steatosis. The steatosis score adds significantly to the prediction model that tries to identify the fibrosis group that a patient belongs to (Table 5); this is true in every case except for the F0-3 vs F4 partition. The APRI score on the other hand makes a significant contribution to only the partition F0-2 vs F3-4. Given that the APRI score appears in only one partition as an important predictor, an additional analysis was performed by dropping the APRI score from the model. This resulted in adding a few additional observations to the data set used for analysis since missing APRI scores had contributed to a slightly reduced sample size. In addition, the steatosis score was added to the F0-3 vs F4 model in order to have a unique set of predictors across all partitions. These results are presented in Table 6. The fact that the results from Tables 5 and 6 are very similar implies that the missing data points did not influence the outcome in any meaningful way.
| F0-3 vs F4 | F0-2 vs F3-4 | F0-1 vs F2-4 | F0 vs F1-4 | |
| SWE median | < 0.0001 | < 0.0001 | 0.0003 | 0.0002 |
| APRI score | NS1 | 0.0404 | NS | NS |
| Age | NS | NS | NS | NS |
| Gender | NS | NS | NS | NS |
| Steatosis | NS | 0.0263 | 0.0002 | 0.0007 |
| AUROC | 0.926 | 0.962 | 0.944 | 0.902 |
| Variable | Pr > χ2 | OR estimate | Lower 95%CI limit for OR | Upper 95%CI limit for OR |
| F0-3 vs F4 | ||||
| Intercept | < 0.0001 | |||
| Elastography median | < 0.0001 | 1.681 | 1.347 | 2.099 |
| Steatosis | 0.6529 | 1.187 | 0.563 | 2.502 |
| AUROC = 0.936 | ||||
| F0-2 vs F3-4 | ||||
| Intercept | < 0.0001 | |||
| Elastography median | < 0.0001 | 1.684 | 1.353 | 2.097 |
| Steatosis | 0.0568 | 1.846 | 0.982 | 3.467 |
| AUROC = 0.954 | ||||
| F0-1 vs F2-4 | ||||
| Intercept | < 0.0001 | |||
| Elastography median | 0.0003 | 2.397 | 1.500 | 3.828 |
| Steatosis | 0.0002 | 3.135 | 1.703 | 5.772 |
| AUROC = 0.944 | ||||
| F0 vs F1-4 | ||||
| Intercept | < 0.0001 | |||
| Elastography median | 0.0002 | 2.221 | 1.463 | 3.370 |
| Steatosis | 0.0007 | 2.496 | 1.473 | 4.230 |
| AUROC = 0.902 |
The F0-2 vs F3-4 data shows that the AUROC obtained with the use of the SWE median and the steatosis score is 0.954 (Table 6). By adding the APRI score (Table 5) the AUROC increase of 0.008 or 0.8%. This represents a decrease of about 13% (0.008 of 0.046) in the error rate. On the other hand, adding steatosis to the model after including the SWE median and the APRI score, the AUROC increase from 0.931 (Table 3) to 0.962 (Table 5), an increase of 0.031 or 3.1%. This represents a decrease of about 44.9% (0.031 of 0.069) in the error rate.
The infection with the highest prevalence was hepatitis B. There were also patients with HIV, hepatitis C and the co-infections in this cohort. While the prevalence of hepatitis B, C and HIV in Sub-Saharan Africa has not been conclusively established[26], it has been postulated that hepatitis B is relatively more prevalent than hepatitis C as compared to the western world where hepatitis C is more prevalent[27]. Most studies on liver fibrosis quantification have been carried out in the western world therefore it is important to have the same studies carried out in Sub-Saharan Africa where the epidemiology of the viral infections is likely to be different. The elastography median scores were the lowest among HIV subjects followed by those with hepatitis B infection; the next highest score was for patients with a hepatitis C infection. The lowest score was from those with multiple infections. The APRI scores also follow the pattern described above for the elastography scores. This pattern corresponds with what has previously been described. Hepatitis C most likely has higher levels of quantified liver fibrosis because of the three viruses it has the most indolent and chronic clinical course. The mortality from these infections is correlated to chronic liver disease and not due to progression of the virus due to the success of antiretroviral therapy[27,28].
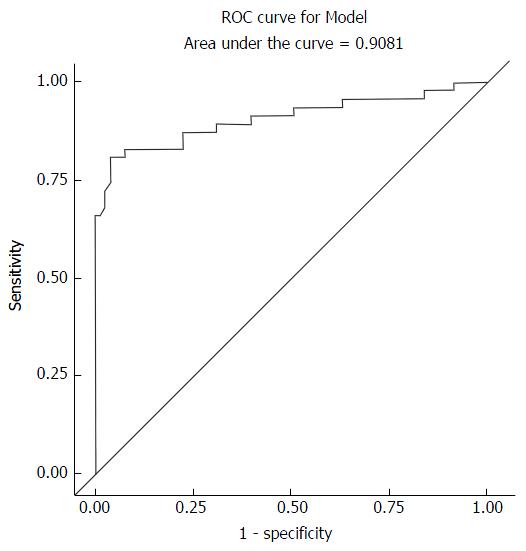
Analysis categorized fibrosis as, F0 vs F1-4, F0-1 vs F2-4, F0-2 vs F3-4, F0-3 vs F4. The results focus on F0-1 vs F2-4 which is of most clinical significance (no and non-significant fibrosis vs significant fibrosis that demands intervention). There is a good correlation between the elastography median scores and the histology fibrosis scores. The OR of 3.0 implies that the elastography median scores are 3 times more likely to correctly identify a fibrosis score of F2-4 compared to F0-1. The upper and lower limits for the OR with 95%CI do not cross 1 which strengthens this result. However, the limits are wide 1.8-5. This may be due to the small sample size and because close to 50% of the sample at F0. If F0 were to be eliminated from the analysis this may reduce the limits with a questionable effect on clinical significance. The accuracy of the elastography median depends on the sensitivity and specificity. This is clear from the raw data used to generate the ROC curve. An elastography median of approximately 3.8 is the point at which you get the best sensitivity matched with specificity. The ROC curve for model, which depicts sensitivity and specificity, illustrates how for a very high sensitivity, specificity is low and as sensitivity reduces; one arrives at a point where specificity is acceptable clinically. That is, one can identify disease with a high sensitivity and be correct (specifically know that you are also picking the non-diseased). The AUROC for the elastography median was 0.91. The elastography and APRI fibrosis score are statistically significantly correlated with the histology fibrosis scores. However, APRI score in itself or when combined with elastography median score does not significantly increase the accuracy of elastography in the differentiation of non-significant vs significant fibrosis. APRI had an AUROC of 0.78. APRI and elastography median had an AUROC of 0.92. Therefore APRI does not have a statistically significant effect on the prediction of F0-1 from F2-4 when added to elastography. And when used alone it is significantly less accurate than elastography. However in patients with chronic progressive liver fibrosis who need repeated analysis to categorize and monitor the progress of liver fibrosis APRI does have a clinically significant role in the management algorithm of liver fibrosis.
Previous studies vary on the accuracy of elastography. The sensitivity, specificity and diagnostic accuracy of shear wave elastography in the determination of liver stiffness compared with biopsy results is comparable to[8-10]. The accuracy of elastography mirrors those depicted by these studies albeit a slightly higher accuracy in this study. This may be due to the difference in grouping of the fibrosis scores for analysis. Also, if the F0 of this study are removed from the analyses this may lead to more similar figures since the F0 constitute approximately 50%. The diagnostic accuracy of shear wave elastography and APRI score in the determination of liver stiffness has not been reported before[8-10].
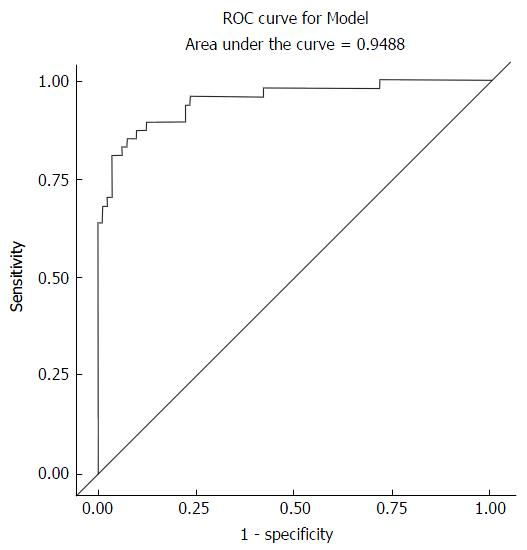
Ordinal regression and backward elimination was used to analyze significance of HIV, hepatitis B, alcohol use, steatosis, age and gender. It showed that steatosis has a significant OR and P-value in the analysis for fibrosis. Ferraioli et al[10] showed that steatosis does not affect the performance of elastography. The challenge as stated in this paper is the confounding effect of various pathologies in the diagnosis and staging of liver fibrosis. This is particularly relevant in the generation and given the wide use of reference ranges for all modalities used in the diagnosis and staging of liver fibrosis. It is for this reason that to date several studies have used variable reference ranges for F0-F4[8,10,17-19]. Our results highlight the potential effect of the presence of steatosis (EF-S_1_10_3_Logistic_All Variables_F0-F1VsF2-F4 document) on the diagnosis and characterization of liver fibrosis. Of note from this result is the increase in the AUROC from 0.91 (Figure 2) to 0.95 (Figure 3) in the logistic regression backward elimination analysis that is attributable to the elastography median and steatosis each with a significant P-value and OR (Table 4). This is an area that needs further study, especially since steatosis was measured subjectively in this study. The data on HIV and alcohol use as variables were not adequately powered to add to this analysis.
This study is timely because of the growing use of elastography in the developed world. In the developing world the use of this diagnostic tool needs to grow via an evidence based approach that is tailored to the local disease burden. Most research in the west has focused on hepatitis C and alcohol or nonalcoholic steatosis. The wider disease burden covered in this study is thus a more homogenous representation of chronic liver fibrosis pathology. The complimentary use of APRI score is especially relevant in resource limited setups.
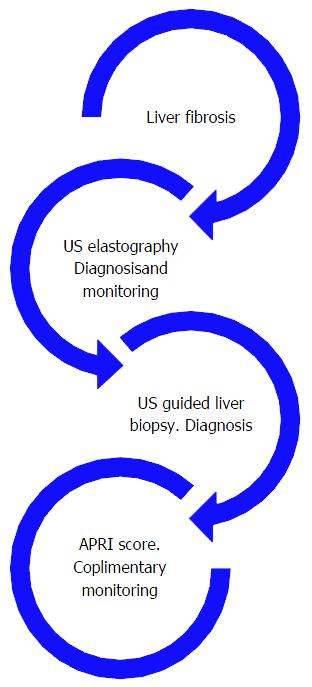
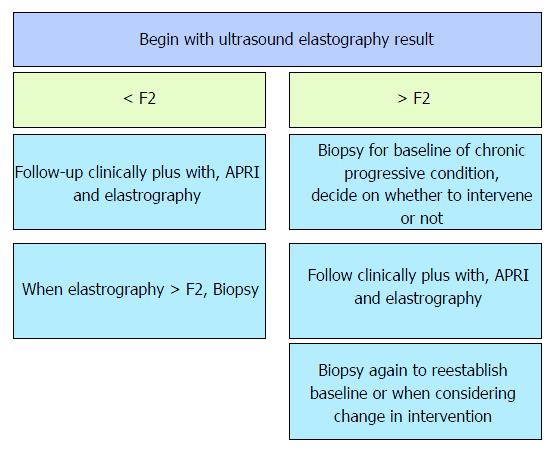
This study brought to light theoretical implication of the need to standardize the diagnosis and accurate staging and follow-up of liver fibrosis. Figure 4 depicts the link between all tests used in the evaluation of liver fibrosis (Figure 4).
The use of all tests should be complimentary and histology has its place but the noninvasive tests may be more logical in the beginning and for progressive monitoring. Inference from the results generates a flowchart below describing a management algorithm (Figure 5).
Practical unique and reported challenges exist. Care needs to be taken in interpretation and training for elastography. Technical and interpretation skill for elastography specifically when choosing the ten values to include in the report; this includes excluding/ignoring far outliers, though these outliers may represent focal areas of different fibrotic stages! Use of the median to give a final conclusion of the ten chosen readings is appropriate especially in the setting of variable readings. But, care must be exercised. There should be a low threshold to recommend a biopsy (still the gold standard) to confirm the findings especially if the mean elastography reading is in keeping with a diagnosis of no fibrosis yet the standard deviation is high in the presence of individual readings of cirrhosis[5]. The challenge of the heterogeneously fibrotic liver or presence of lesions, e.g., metastasis causing heterogeneity of the liver rendering fibrosis assessment questionable in terms of using just the median to conclude on the level of fibrosis especially when there is a big difference in the individual stiffness values must be remembered. These issues red flag the danger of not allowing room to vary an impression and advise further evaluation with an United States guided liver biopsy to correlate[5]. Indeed, one must also bear in mind that the suggestion to further evaluate with a liver biopsy is inherently flawed because of the attend pitfalls of the tool. Chronic progressive liver fibrosis needs accurate early diagnosis and interval monitoring. Elastography is a validated tool. APRI can be used as a complimentary tool though its effect is not of statistical significance but clinical significance. Biopsy remains the gold standard. We propose a flow chart at diagnosis. Further, the probability of a correct diagnosis is significantly enhanced with the addition of steatosis as a prognostic factor (Figure 5).
The number of ultrasound guided liver biopsies for the assessment of liver fibrosis has continued to reduce because of the increasing use of elastography which is noninvasive and without the side effects associated with liver biopsy. This diagnostic trend is reinforced by the continued validation of elastography[5,7,10,12]. Therefore during this study the recruitment rate was low. As such participants were recruited from three different sites to meet the sample size requirement to adequately power the results. The ultrasound and elastography where all performed at the same site (AKU). Biopsy results were analyzed by pathologists at two of the three sites (AKU and KNH). This may have potentially led to variable histological inter-observer variability. To counter this each sample was read by each pathologist at each respective site and discrepant values were resolved by consensus. However, major and minor discrepancy analyses of histology for inter-observer discrepancies were not assessed.
There are limitations associated with elastography, including the confounding effects of inflammatory activity, and to a lesser extent, steatosis[13], on liver stiffness evaluation. There is also reduced accuracy observed in lower fibrosis stages (F0-F2). Furthermore, the incidences of failed and unreliable scans have been reported to be approximately 3% to 16% in transient elastography but less in shear wave elastography (figures not reported yet)[22]. The sample size was inflated by 5% to cater for this. A typical liver biopsy covers 1/10000th of the liver while elastography covers a larger area. Matching the two sites covered by the two exams may not have been 100%.
The information sort in the data collection form to analyze the secondary objectives was sensitive in nature including queries about alcohol use and HIV status. This precluded complete disclosure from participants and led to inadequate data on related parameters. This led to a reduction in the power of inferences regarding the role of alcohol and HIV.
This has been an East African experience: Unique challenges and similar differences to those published. More comprehensive analysis needs to be done to further reveal the extent of confounding factors affecting the use of elastography in the diagnosis and staging of liver fibrosis. The role of steatosis needs further objective assessment. Further work needs to be done to describe the in cooperation of magnetic resonance elastography in the diagnostic algorithm of liver fibrosis.
Our study validates the use of ultrasound shear wave elastography in the diagnosis and staging of fibrosis within the context of liver disease in a LMIC.
We would like to thank the Aga Khan United States Research funding body and research support team for funding this study and their non-wavering technical support. Many thanks to the Aga Khan Research team for support and to all patients and staff that were involved in this endeavor.
Chronic progressive liver diseases cause liver fibrosis whose end result is decompensated liver failure. Liver fibrosis that results from these diseases can be reversed if diagnosed early. The current gold standard in the diagnosis of liver fibrosis is a liver biopsy preferably ultrasound guided, which is an invasive procedure with limitations and risks. Recent research have validated the use of shear wave ultrasound based liver elastography which is a non-invasive imaging based tool that has a sensitivity and specificity that almost parallels histological diagnosis from a liver biopsy. The staging of liver fibrosis at diagnosis uses a Metavir scoring system that has been adapted by elastography. Aminotransferase to platelet ratio index is a liver function test that has some usefulness in the diagnosis of liver fibrosis. The combined use of histology, elastography and aminotransferase to platelet ratio index has not been elucidated.
Previous studies have shown that ultrasound based elastography can substitute liver biopsy in the accurate diagnosis of liver fibrosis.
This is the first study to evaluate the combined role of ultrasound based elastography, histology and aminotransferase to platelet ratio index in a low to middle income country for the management of progressive liver fibrosis.
The use of the three tests should be complimentary and histology has its place but the noninvasive tests may be more logical in the beginning and for progressive monitoring. More comprehensive analysis needs to be done to further reveal the extent of confounding factors affecting the use of elastography in the diagnosis and staging of liver fibrosis. Further work needs to be done to describe the in cooperation of magnetic resonance elastography in the diagnostic algorithm of liver fibrosis.
Elastography is radiological based software that can diagnose and quantify the degree of liver fibrosis. It is either ultrasound or magnetic resonance based. Ultrasound bases elastography uses sound have to assess for and quantify liver stiffness that is directly related to liver fibrosis. Aminotransferase to platelet ratio is a laboratory parameter derived from part of the routine liver function tests and platelet count.
The authors have validated the use of ultrasound shear wave elastography in the diagnosis and staging of fibrosis within the context of liver disease in a low to middle income country. Practical management algorithms that in cooperate the use of ultrasound based elastography, histology and aminotransferase to platelet ratio index have been demonstrated. More comprehensive analysis needs to be done to further reveal the extent of confounding factors affecting the use of ultrasound or magnetic resonance based elastography.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Kenya
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jiang ZG, Peng JY, Yuan YF S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4116] [Article Influence: 205.8] [Reference Citation Analysis (3)] |
| 2. | Fan Y, Fang X, Tajima A, Geng X, Ranganathan S, Dong H, Trucco M, Sperling MA. Evolution of hepatic steatosis to fibrosis and adenoma formation in liver-specific growth hormone receptor knockout mice. Front Endocrinol (Lausanne). 2014;5:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Kochan K, Marzec KM, Chruszcz-Lipska K, Jasztal A, Maslak E, Musiolik H, Chłopicki S, Baranska M. Pathological changes in the biochemical profile of the liver in atherosclerosis and diabetes assessed by Raman spectroscopy. Analyst. 2013;138:3885-3890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Dong D, Yin L, Qi Y, Xu L, Peng J. Protective Effect of the Total Saponins from Rosa laevigata Michx Fruit against Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Nutrients. 2015;7:4829-4850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Lissandrin R, Filice G, Filice C, Above E, Barbarini G, Brunetti E. Performance of liver stiffness measurements by transient elastography in chronic hepatitis. World J Gastroenterol. 2013;19:49-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 6. | Lee JE, Lee JM, Lee KB, Yoon JH, Shin CI, Han JK, Choi BI. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis B viral infection using magnetic resonance elastography. Korean J Radiol. 2014;15:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Ferraioli G, Parekh P, Levitov AB, Filice C. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med. 2014;33:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Frulio N, Trillaud H. Ultrasound elastography in liver. Diagn Interv Imaging. 2013;94:515-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 10. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Dal Bello B, Filice G, Filice C. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787-4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2343] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 12. | Lupşor-Platon M, Stefănescu H, Mureșan D, Florea M, Szász ME, Maniu A, Badea R. Noninvasive assessment of liver steatosis using ultrasound methods. Med Ultrason. 2014;16:236-245. [PubMed] |
| 13. | Beland MD, Brown SF, Machan JT, Taliano RJ, Promrat K, Cronan JJ. A pilot study estimating liver fibrosis with ultrasound shear-wave elastography: does the cause of liver disease or location of measurement affect performance? AJR Am J Roentgenol. 2014;203:W267-W273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1796] [Cited by in RCA: 1848] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 15. | Fung J, Lai CL, Seto WK, Yuen MF. The use of transient elastography in the management of chronic hepatitis B. Hepatol Int. 2011;5:868-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kim BK, Kim HS, Park JY, Kim do Y, Ahn SH, Chon CY, Park YN, Han KH, Kim SU. Prospective validation of ELF test in comparison with Fibroscan and FibroTest to predict liver fibrosis in Asian subjects with chronic hepatitis B. PLoS One. 2012;7:e41964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Suh CH, Kim SY, Kim KW, Lim YS, Lee SJ, Lee MG, Lee J, Lee SG, Yu E. Determination of Normal Hepatic Elasticity by Using Real-time Shear-wave Elastography. Radiology. 2014;271:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Suh C, Kim SY, Kim KW. Estimation of reference values for liver elasticity in biopsy-proven normal liver using Supersonic Shear Wave imaging: measurement reliability and effect of steatosis. Euro Soc Radiol. 2013;. [DOI] [Full Text] |
| 19. | Sporea I, Bota S, Gradinaru-Taşcău O, Sirli R, Popescu A, Jurchiş A. Which are the cut-off values of 2D-Shear Wave Elastography (2D-SWE) liver stiffness measurements predicting different stages of liver fibrosis, considering Transient Elastography (TE) as the reference method? Eur J Radiol. 2014;83:e118-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3242] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 21. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1070] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 22. | Jang HW, Kim SU, Park JY, Ahn SH, Han KH, Chon CY, Park YN, Choi EH, Kim do Y. How many valid measurements are necessary to assess liver fibrosis using FibroScan® in patients with chronic viral hepatitis? An analysis of subjects with at least 10 valid measurements. Yonsei Med J. 2012;53:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, Fedchuk L, Sattonnet F, Pais R, Lebray P. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013;58:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Osaki A, Kubota T, Suda T, Igarashi M, Nagasaki K, Tsuchiya A, Yano M, Tamura Y, Takamura M, Kawai H. Shear wave velocity is a useful marker for managing nonalcoholic steatohepatitis. World J Gastroenterol. 2010;16:2918-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Apata IW, Averhoff F, Pitman J, Bjork A, Yu J, Amin NA, Dhingra N, Kolwaite A, Marfin A. Progress toward prevention of transfusion-transmitted hepatitis B and hepatitis C infection--sub-Saharan Africa, 2000-2011. MMWR Morb Mortal Wkly Rep. 2014;63:613-619. [PubMed] |
| 27. | Clausen LN, Lundbo LF, Benfield T. Hepatitis C virus infection in the human immunodeficiency virus infected patient. World J Gastroenterol. 2014;20:12132-12143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Rosenthal E, Roussillon C, Salmon-Céron D, Georget A, Hénard S, Huleux T, Gueit I, Mortier E, Costagliola D, Morlat P. Liver-related deaths in HIV-infected patients between 1995 and 2010 in France: the Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalité 2010 survey. HIV Med. 2015;16:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |