Published online Mar 28, 2016. doi: 10.4254/wjh.v8.i9.421
Peer-review started: May 7, 2015
First decision: September 8, 2015
Revised: March 6, 2016
Accepted: March 14, 2016
Article in press: March 16, 2016
Published online: March 28, 2016
Processing time: 330 Days and 10.5 Hours
Hepatocellular carcinoma (HCC) is the leading cause of deaths in cirrhotic patients and the third cause of cancer related deaths. Most HCC are associated with well known underlying risk factors, in fact, HCC arise in cirrhotic patients in up to 90% of cases, mainly due to chronic viral hepatitis and alcohol abuse. The worldwide prevention strategies are conducted to avoid the infection of new subjects and to minimize the risk of liver disease progression in infected patients. HCC is a condition which lends itself to surveillance as at-risk individuals can readily be identified. The American and European guidelines recommended implementation of surveillance programs with ultrasound every six months in patient at-risk for developing HCC. The diagnosis of HCC can be based on non-invasive criteria (only in cirrhotic patient) or pathology. Accurately staging patients is essential to oncology practice. The ideal tumour staging system in HCC needs to account for both tumour characteristics and liver function. Treatment allocation is based on several factors: Liver function, size and number of tumours, macrovascular invasion or extrahepatic spread. The recommendations in terms of selection for different treatment strategies must be based on evidence-based data. Resection, liver transplant and interventional radiology treatment are mainstays of HCC therapy and achieve the best outcomes in well-selected candidates. Chemoembolization is the most widely used treatment for unresectable HCC or progression after curative treatment. Finally, in patients with advanced HCC with preserved liver function, sorafenib is the only approved systemic drug that has demonstrated a survival benefit and is the standard of care in this group of patients.
Core tip: Liver cancer is the fifth leading cause of cancer worldwide, and the third-leading cause of cancer death. Altouhg some risk factors have been classically associated with development of hepatocellular carcinoma (HCC), in the last years, also, some protective factors have been described, like coffee drink, and drugs like statins and beta-blockers. The current European Association for the Study of Liver and American Association for the Study of Liver Diseases guidelines recomended the barcelona clinic liver cancer classification as staging system for prognosis prediction and treatment allocation The therapeutic approach in patients with HCC depends on factors such as liver function, tumour extension and comorbidities existence. Available treatments are: Surgical treatments, percutaneous ablation, chemoembolization, radioembolization and systemic treatment.
- Citation: Pascual S, Herrera I, Irurzun J. New advances in hepatocellular carcinoma. World J Hepatol 2016; 8(9): 421-438
- URL: https://www.wjgnet.com/1948-5182/full/v8/i9/421.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i9.421
Hepatocellular carcinoma (HCC) is one of the leading cancer in the world. It is an important health problem especially in high incidence areas. Nowadays the global incidence is still growing, but with the development of hepatitis B vaccine and the new therapies in hepatitis C virus (HCV), a gradual decline in the incidence is expected in the next decades. Another important issue is the high mortality of the patients with this tumour. In spite of well established surveillance programs in patients with chronic liver disease, most tumours are diagnosed in intermediate-advanced stage, and only palliative measured can be applied.
In the next pages we will review the risk factors associated with the development of HCC, the new advances in diagnosis imaging, the main prognosis classification and finally the therapeutic approach.
Liver cancer is the fifth-leading cause of cancer diagnosed in men worldwide[1], and the seventh cause of cancer in women, representing about 7% of the total number of cancer diagnoses. Globally, liver cancer is the third-leading cause of cancer death, after lung and stomach[2,3]. The annual incidence of HCC is similar to the deaths per year that it generates, which point out the aggressiveness of this disease[1].
The HCC incidence increases progressively with advancing age in population with a peak at the age of 70-year-old[4]. In Chinese and black African population, mainly infected with hepatitis B virus (HBV), the patient are younger, and in Sub-Saharan Africa (an area with a high incidence of HBV infection) can appear in the third decade of life[5,6].
The incidence of HCC is highest in men, with a male to female ratio of 2.4 and this difference is even higher in populations with a high incidence of HCC, with an average of 3.7 to 1[3]. The differences in the geographical distribution of HCC reflects the differences in exposure to the hepatitis viruses and different environmental pathogens, so the incidence is highest in East Asia, Sub-Saharan Africa and Melanesia, with 85% of the total number of cases[2,3], while in most industrialized countries the incidence is low, except in the South of Europe[7]. Globally there is a growing incidence of the number cases of HCC, even in United States and Europe, mainly due to the high number of people infected with the virus of HCV in these areas[3]. The universal vaccination against HBV in children born after 1980 in some endemic countries has decrease the rate of HCC in children and it is expected a reduction of the incidence of this tumour in the future in these areas[8,9].
Multiple risk factors have been associated with the development of HCC, being the most frequent chronic viral hepatitis (B and C), alcohol abuse, and exposure to aflatoxins, however, this can occur in people without any known risk factor[10].
Geographically in Africa and East Asia, the most frequently risk factor associated with HCC is chronic HBV infection, while in Western countries, HCV infection is the main risk factor[2]. Overall 54% of cases could be attributed to HBV infection, 31% to HCV infection and 15% to other causes. Cirrhosis is the main risk factor for the development of HCC and about 30%-35% of all cirrhotic patients will develop HCC in the course of their disease, which may be due to chronic viral hepatitis, alcohol, hereditary metabolic diseases, or autoimmune and non-alcoholic fatty liver disease[11]. It is estimated that the annual risk of developing HCC in the cirrhotic patients is between 1%-8% according to the aetiology[12]. The risk of developing HCC increases progressively in male patients, with advanced age, low platelet count, and oesophageal varices[13], as well as it has also been associated with increasing pressure portal[14], or with the degree of liver stiffness measured with transient elastography[15-17].
HBV and HCV Chronic infection are the main risk factor for the development of HCC[18-21]. The higher prevalence of HBV infection occurs in China, Southeast Asia and Sub-Saharan Africa[8,21]. Globally, it is estimated that 54% of all liver cancers are attributable to HBV infection[22]. The prevalence of HCV infection is higher in Egypt, Japan and the South of Italy[21].
The development of HCC associated with HBV infection usually occurs in patients with cirrhosis, but it can appear in patients without cirrhosis[5,23-28]. So screening for HCC will be recommended in this group of patients. Some risk factors for the development of HCC have been identified in patients with chronic HBV infection: The presence of hepatitis virus e antigen (as an indicator of viral replication)[28], high viral load[29], genotype C (which is the most prevalent in Asia)[30] and infection in early childhood or perinatal period[31-33]. Several studies have demonstrated that the treatment of chronic HBV hepatitis with interferon or nucleotide analogues (suppressing viral load) reduces the relative risk of developing HCC[31,34-43], but these benefits have not been observed in patients who develop resistance to the treatment. Some studies suggest that patients co-infected by HBV and HCV have greater risk of developing HCC[44-46].
There is a very well known association between HCV chronic infection and the development of HCC, in fact, the risk of developing HCC in these patients increase between 20 and 30 times[21,47-49]. In very few cases it may occur in patients with HCV infection and lower grades of hepatic fibrosis[13,50]. High viral loads and HCV genotype 1b infection have been associated with higher risk of HCC occurrence[51]. The levels of inflammatory markers of oxidative stress are higher in patients infected with HCV and HCC[52] and the immune response can be another cofactor in the progression from cirrhosis to HCC in HCV infected patient[53]. In patients with HVC infection who achieve sustained viral response after treatment, there is a decrease in the risk of HCC[54,55]. The universal analysis of blood donations for anti-HCV has resulted in a substantial decrease in the number of cases of hepatitis C in blood donors and the use of needles and disposable syringes and other changes in medical procedures have substantially reduced new infections by HCV. As well as HCV and HBV co-infection may increase the risk of developing cirrhosis and HCC[56], the HIV infection appears to be a cofactor that increases the risk of developing HCC in cirrhotic patients with viral hepatitis[57].
The infection by trematode in blood is endemic in tropical areas of Africa, the Caribbean, Asia, and South America. The species of Schistosoma japonicum, already identified as possible human carcinogen, has been associated with risk of developing HCC in infected by HBV and HCV patients[58,59].
The ingestion of food contaminated with aflatoxin B1 (fungi Aspergillus flavus and Aspergillus parasiticus), which can be found at staple foods of tropical and subtropical areas, is a co-factor of risk in the development of HCC, especially in some regions of Africa and Asia, associated with infection by HBV[60,61]. Several studies have shown increased HCC mortality in some rural Chinese areas associated with drinking water potentially contaminated with toxins of some algae (microcystins), with hepatotoxic effect[62,63]. Other studies have established a relationship between the consumption of betel nut, very common in Asia, with an increasing risk of developing cirrhosis and HCC[64,65].
Many studies have associated chronic alcohol consumption with the development of liver cirrhosis and HCC[66-72], although quantity of alcohol ingestion and duration of consumption that supposes a significant risk for developing HCC is unknown. It has been described a relationship between genetic polymorphisms of the enzymes involved in the metabolic pathway of ethanol and increased risk of HCC in excessive drinkers. An increased risk of HCC in heavy alcohol drinkers has been associated to the polymorphism of the aldehyde dehydrogenase and the dysfunction of the enzyme Glutatión S-transferasa[73,74]. Some studies have established that smoking is a significant co-factor in the development of HCC[66,75,76].
The obesity, diabetes and dyslipidemia have also been identified as cofactors of risk in the development of HCC, although the pathophysiological mechanisms have not been clarified. It is believed that the deposit of fat in the liver could alter some metabolic functions in patients with diabetes mellitus[77,78]. In these patients, liver steatosis can lead to a nonalcoholic fatty hepatitis, whose pathogenesis is unclear but it have been related to chronic inflammation, oxidative stress, insulin resistance and lipotoxicity, constituting a cofactor for the development of liver cirrhosis and HCC[79-82].
The metabolic syndrome, which is defined by the presence of central obesity, dyslipemia, hypertension, and impaired glucose metabolism, has also been associated with an increased risk of developing HCC[83].
Patients with hemochromatosis may develop HCC by up 45% cases, according to some studies, iron overload can lead to the development of cirrhosis and HCC in these patients[84]. The protein alpha-1-antitrypsin deficiency is a documented risk factor in the development of cirrhosis and HCC that also could be without cirrhosis[85]. Occasionally, patients with cirrhosis secondary to Wilson’s disease, autoimmune hepatitis or primary biliary cirrhosis can develop HCC[86-88]. Several studies suggest that porphyria may increase the risk of developing HCC, even in patients without cirrhosis[89-97].
A meta-analysis showed an increase of significant risk of any primary liver cancer, and also of HCC in patients with cholelithiasis[98]. The oral anticonceptive (OC) consumption has been rarely associated with the emergence of benign tumours of the liver in young women, like hepatic haemangioma, focal nodular hyperplasia and specially hepatocellular adenoma[99]. Some cases of malignant transformation of liver adenomas in women taking OC have been described[100,101], but subsequent studies did not corroborate these results[102]. Some studies have suggested that the excessive consumption of saturated fats and meat may increase the risk of HCC[103,104]. Although others authors have not found this association[105]. Nitrogenous compounds (used in smoked fish, cheeses, bacon, sausages and other foods) may increase the risk of liver disease and cancer[106].
In an American study, individuals with a family history of first degree with liver cancer, had up to four times more likely to develop liver cancer than the general population, suggesting that certain shared genetic and environmental factors would influence the risk of developing liver cancer[107]. There is some evidence that there might be an association between a polymorphism of the gene of epidermal growth factor and the risk of developing HCC, although these data require further investigation[108-115].
The use of statins has been associated with a decrease in the risk of developing HCC[116,117]. In a meta-analysis, including 10 studies, the risk of developing HCC was lower in people taking statins[118].
A recent retrospective, observational study establishes the hypothesis that treatment with propranolol may reduce the risk of HCC in cirrhotic patients[119].
The consumption of fish, vegetables and omega-3 fatty acids has been associated with a lower risk of developing HCC in different studies[107,120,121]. Similarly, the increased consumption of vitamin E has also been associated with lower risk of HCC rate[122]. The Mediterranean diet, characterized by high consumption of vegetables, olive oil and cereals, with moderate wine consumption and fish, and low consumption of meat, is associated with a lower risk of HCC[123].
Surveillance is cost effective in high risk cirrhotic patient, with an expected annual incidence of HCC exceeding 1%-5% per year, and in some cases of non-cirrhotic patients with HBV chronic infection. The problem is that most of the studies of surveillance of HCC in chronic liver disease have been developed in endemic Asian countries with high incidence of HBV infection. In fact, the only prospective study has been developed in China, exclusively in patients with HBV infection. In this study, the mortality related to HCC was lower in patients under HCC surveillance[127]. Other retrospective studies conducted in Europe and America also have showed a better prognosis in patients diagnosed in surveillance programs[128-130]. Both, European American and Asian guidelines recommended that patient with high risk of developing HCC should be entered into surveillance programs. This should be performed using ultrasonography every six months[131-133].
According to the latest consensus conferences and practice guidelines, nowadays, to get to a definitive diagnosis of HCC, will not be necessary to perform a liver biopsy if the tumour is higher than 1 cm in diameter and the typical imaging features are present in a contrast enhanced study [dynamic computed tomography (CT) scan or magnetic resonance (MR)]. Thus, to properly documented the existence of HCC is required that the tumour enhances more intensely in the arterial phase than the surrounding liver and less than the surrounding liver in the venous phase. But these rules are only applicable if the patient has well diagnosed cirrhosis or a HBV chronic hepatitis. In any other cases (patient with typical lesion but without liver disease or patient with atypical lesion and cirrhosis), a liver biopsy must be performed to establish the diagnosis. The serum alphafetoprotein level has no longer be used for diagnosis of HCC, because is insufficiently sensitive or specific for use as a surveillance assay[130,131].
In order to reduce the variability in liver lesion interpretation and standardize the report from CT and MR information, the American College of Radiology has developed a new classification: Liver Imaging-Reporting and Data System (LI-RADS). The LI-RADS assigns imaging findings to one of five categories, allowing radiologist to stratify individual observations according to the level of concern HCC. So LR-1 is an observation definitively benign and LR-5 is definitively HCC. The intermediate stages correlates with probably benign (LR-2), intermediate possibility of being HCC (LR-3) and probably HCC (LR-4) according to radiological features, lesion diameter and contrast enhanced behaviour[134]. As has been described recently, the nodules both LI-RADS category 4 and category 5 have high specificity for HCC diagnosis, and in addition, a relevant proportion of lesions categorized as LI-RADS category 2 and 3 could be HCC and a liver biopsy should be recommended in such patients[135]. A consensus is necessary between different organizations in order to optimize reporting of CT and MR imaging features in the patients at risk for HCC[136].
The main prognosis predictors of survival in patients with HCC are: Liver function, tumour burden (size and number of HCC nodules, vascular invasion), serum alpha-fetoprotein level and performance status. Nowadays, there is no universally adopted staging system for HCC. The most widely and accepted staging system in oncology, the classification of malignant tumours (TNM), has been adapted for HCC by the American Joint Committee on Cancer. Currently, the United Network for Organ Sharing, the organ allocation administration in United States of America, allocates donors organs for liver transplantation for the treatment of HCC based on the revised TNM classification. The problem of this system is that it does not incorporate any measure of liver function reserve, which is critical in HCC. Prognosis for HCC is impacted by local spread and hepatic dysfunction, and any staging system in HCC should include parameters that represent both aspects because an advanced liver disease can contraindicate any therapeutic approach as much as an advanced and extended HCC. The first staging system specifically designed for HCC was the Okuda classification[137], but other staging systems have been described in the last decades: Cancer of the Liver Italian Program[138], French classification[139], Barcelona clinic liver cancer classification (BCLC)[140], Chinese University Prognosis Index[141], the Japan Integrated Staging[142], which has been redefined including biomarkers and the Taipei Integrated Scoring System, based on total tumour volume[143]. In Table 1 are represents the parameters included in these staging system. Some of these classifications have been externally validated in separated groups.
| Staging system | Size | Nodules | Met | PVT | AFP | CH | Alb | Bil | ALP | Ascites | PS |
| TNM | Yes | Yes | Yes | No | No | No | No | No | No | No | No |
| Okuda | Yes | No | No | No | No | No | Yes | Yes | No | Yes | No |
| CLIP | Yes | No | No | Yes | Yes | Yes | No | No | No | No | No |
| FRENCH | No | No | No | Yes | Yes | No | No | Yes | Yes | No | Yes |
| BCLC | Yes | Yes | Yes | Yes | No | Yes | No | Yes | No | No | Yes |
| JIS | Yes | Yes | Yes | No | No | Yes | No | No | No | No | No |
| CUPI | Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | No |
The current European Association for the Study of Liver (EASL)-EORTC GP guidelines and the American Association for the Study of Liver Diseases (AASLD) guidelines endorse the BCLC classification and recommend the use of this staging system for prognosis prediction and treatment allocation[132,133]. The BCLC classification divides HCC patients in five stages, from (0, A, B, C, D) according to pre-established prognosis variables: Size and number of nodules, vascular invasion, performance status and Child-Pugh stage. The five stages are: 0 very early stage, A early, B intermediate, C advanced and D terminal and each stage represents the first approach to the evaluation of the patients with expected prognosis and initial treatment option to be considered. Early stage patients may be treated with potential curative treatment: Percutaneous ablation, surgery or liver transplant (LT). Intermediate stage patients may be treated with chemoembolization, advanced stages may be treated with systemic therapy (sorafenib) and in terminal patients only best supportive approach can be applied. But, as in all recommendations, the final treatment indication should take into account a detail evaluation of additional characteristics of the patients that imply a personalized decision making. So, a young patient with Child C and a small tumour should be considered for LT, not for best supportive care.
The therapeutic approach in patients with HCC depends on several factors such as liver function, size and number of nodules, tumour extension, age and comorbidities existence. Currently, available treatments can be divided into surgical treatments (resection or transplantation), percutaneous ablation (Chemistry: Acid ethanol acetic or thermal: Microwave, laser, radiofrequency and cryoablation), chemoembolization, radioembolization and systemic treatment. The goal of curative treatments should be to obtain a complete response, according to modified RECIST radiological criteria[144,145]. The recommendation of selection for different treatment strategies are based on evidence-based data and local experience and capacities. Is advisable that any decision of treatment should be adopted by multidisciplinary HCC teams including hepatologist, oncologist, surgeons, radiologist and interventional radiologist. Properly allocate each treatment in each case is a crucial decision and is mandatory to warrant a good results in terms of survival, treatment morbidity and mortality and recurrence.
As in any tumour, the surgical resection should be the first option to be considered in patients with HCC. The problem is the limitation that supposes the presence of liver cirrhosis, hypertension portal, coagulopathy, or hepatic dysfunction associated, that may contraindicate any surgery and resection of the tumour. The results of surgery to make appropriate estimated that survival at 5 years should reach 60% and 5 years tumour recurrence 70%, peri-operative mortality must be 2%-3% and less than 10% of transfusion requirements. Anatomic resection aiming 2 cm margins provides better results and survival but only could be applied in patients with preserved liver function. Adequate selection of patients for surgery involves a correct assessment of liver function, using Model End Stage Liver Disease punctuation, Child-Pugh class or more sophisticated estimation with the measurement of indocyanine green retention rate or hepatic venous pressure gradient (HVPG). Portal hypertension is an independent prognosis factor in patients undergoing resection and the extensive assessment is recommended before surgery using the component of portal hypertension: Platelet counts, splenomegaly, esophageal varices, and/or HVPG. In practice, BCLC recommendation is to avoid surgery in patient with advanced liver insufficiency, hypertension portal or high bilirubin[146].
If the patient is properly selected, with preserved liver function and no clinically significant portal hypertension, the next step is to evaluate tumour extension: Size and number of nodules, vascular invasion and presence of microsatellites. Tumour size, multinodularity and vascular invasion, are well known predictors of recurrence and survival. Characteristically, microscopic vascular invasion is related to tumour size and involves 20% of tumours of 2 cm, 30%-60% of tumours 2-5 cm and up to 60%-90% of tumours up to 5 cm[147]. With all of this in mind, hepatic resection should be considered for small solitary tumours (and multifocal only if technically possible) with adequate hepatic function. In BCLC staging system, surgery is reserved for patient in the very/early stage, with well preserved liver function and a single tumour less than 2 cm, without portal hypertension and normal bilirubin.
Since Mazafferro described the Milan criteria in 1996 (solitary tumour less than 50 mm in diameter or less than 3 tumours, and 30 mm in diameter each one, in the absence of extrahepatic vascular spread), numerous studies have validated the results of the initial study, both in terms of 5-year survival and recurrence of the tumour (Table 2)[148-155]. This study also allowed that transplantation became a feasible option for treatment in these patients, and also showed that to achieve acceptable rates of survival (i.e., similar to that of the patients transplanted without HCC), the size and number of tumour should be limited. The situation of treatment of HCC has changed dramatically in the last decades. A better knowledge about the tumour behaviour, improvement in surgical techniques and radiological therapies together with a better selection of potential candidates to each treatment have allowed to improve the survival of patients with HCC. The optimisation of the criteria as well as the management of patient already listed for LT remains a source of debate. Important questions, like the expansion of eligibility criteria for LT beyond Milan criteria, the role of down-staging as a bridge to LT or the possible need of adjuvant therapies in patient in waiting list in order to avoid tumour progression and eventual drop-out, are still unresolved.
Alternative eligibility criteria beyond Milan criteria have been proposed, and some of them have been incorporated into clinical practice. The main aim of all these new approaches is to permit the fair allocation of liver graft between more potential recipient with similar survival and tumour recurrences. Having in mind the recognised predictors of recurrence (size and number of nodules, presence of bi-lobar disease, tumour differentiation and presence of micro or macro vascular invasion or tumour satellites), some groups have proposed different expensive criteria. In fact, the limitation of some of the studies have been the used of pathological examination of the explants to determine the tumour burden (data that obviously is only disposable after the LT) instead of radiological staging, as it is showed in Table 3[155-162]. This fact, hinders the correct interpretation of the results a consequently the clinical application of the results. The University of California, San Francisco criteria constitutes a well recognised extension to Milan criteria and have been applied in clinical practice[151]. First published in 2001, demonstrated that patients with a single tumour less 65 mm in diameter, or 2-3 tumours each with less 45 mm diameter, with a total tumour diameter less than 80 mm, had similar survival than patients inside Milan criteria[155]. Subsequent studies (both prospective and retrospective) have reported favourable results with expanded criteria. A recent retrospective and multicentre study by Mazzaferro et al[155], have been performed introducing “up to seven” criteria: the sum of the number of tumour nodules and the diameter of the largest nodule (in centimetres) being less than 7[154]. These results have been externally validated in an independent cohort[162,163]. The international consensus conference for liver transplantation for HCC recommended to consider the LT in patients with HCC inside Milan criteria and only a modest expansion of the number of potential candidates may be considered outside Milan criteria[164].
| Ref. | Patients MC/EC | HCC criteria | Staging method | Design | 5-yr survival (%) MC/EC |
| Yao et al[158] UCSF criteria | 46/14 | 1 < 6 cm | Explant | Retro | 72 |
| 2-3 > 4, 5 cm | |||||
| Sum diameter < 8 cm | |||||
| Herrero et al[154] Navarra Criteria | 35/12 | 1 < 6 cm | Rx | Pros | |
| 2-3 < 5 cm | |||||
| Kneteman et al[157] | 19/21 | 1 < 7.5 cm | Explant | Pros | 87/83 (4-yr) |
| 18/9 | Multinodular < 5 cm | Rx | 92/77 | ||
| Yao et al[152] | 130/38 | 1 < 6 cm | Rx | Pros | 90/93 |
| 2-3 > 4, 5 cm | |||||
| Sum diameter < 8 cm | |||||
| Silva et al[159] Valencia Criteria | 231/26 | 1 < 5 cm | Explant | Retro | 62/69 |
| 254/27 | 2-3 < 5 cm | Rx | |||
| Sum diameter 10 cm | |||||
| Herrero et al[156] | 59/26 | 1 < 6 cm | Explant | Pros | 70/56 |
| 2-3 < 5 cm | Rx | 66/68 | |||
| Mazzaferro et al[155] Metroticket | 444/283 | Sum nodules/size | Explant | Retro | 73/71 |
| 7 cm | |||||
| Fan et al[160] Shanghai Criteria | 394/176 | 1 < 9 cm | Explant | Retro | 51/65 |
| 2-3 < 5 cm | |||||
| Sum diameter 9 cm | |||||
| Guiteau et al[161] | 363/82 | 1 < 6 cm | Rx | Pros | 73/71 (3-yr) |
| 2-3 < 5 cm | |||||
| Sum diameter 9 cm |
Another important question is the role of downstaging in patients with HCC exceeding Milan criteria, using locoregional therapies: Radiofrequency ablation (RFA), transarterial chemoembolization (TACE), transarterial radioembolization or surgery. The objective of these therapies should be to decrease tumour size or number of tumours in order to achieve a pre-established locally criteria acceptable for LT. Some of the studies have reported successfully results with this strategy achieving 5 years survival similar to that of patients with HCC who meet Milan criteria without requiring downstaging[165,166]. Nevertheless, there are some unresolved issues. The defined upper limit for size and number of nodules eligibility for downstaging and the possible role of alpha-fetoprotein has not been well defined. The assessment of adequate response is variable in the different reports, although the recommendation should be to consider the amount of available tumour according to modified RECIST criteria. Otherwise, the acceptable criteria previously defined as successful downstaging in each study, has been different, as well as the observation period recommended after the tumour has been downstaged, before considering for LT. The recommendation of Consensus Conference was that LT may be considered after successful downstaging, without evidence for preferring a specific locoregional therapy and using criteria including size and number of viable tumour[164].
HCC is the tumour that takes the greatest advantage from interventional radiology therapies for several reasons: Not only surgical difficulties in cirrhotic patients, but also ablative and endovascular treatments have demonstrated high response rates and survival benefits.
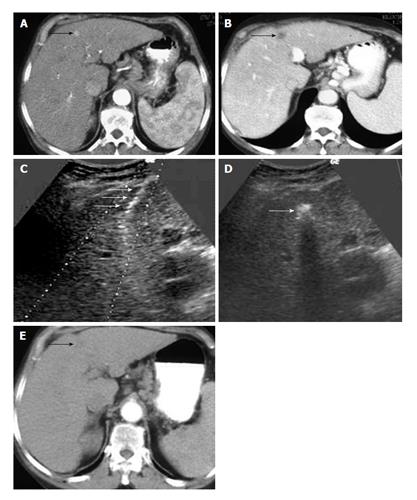
Among all chemical ablative treatments, percutaneous ethanol injection (PEI) has a widespread use, although it has more difficulties to treat encapsulated tumours against other substances as acetic acid. PEI has been the most used ablative therapy until 1999[167], but it has been disregarded after the emergence of more sophisticated techniques. Despite it has also evolved with multi-pronged needles that minimize some PEI disadvantages as the need of multiple sessions[168], they have a limited use and nowadays PEI use is reserved for the treatment of HCC < 2 cm with unfavorable RFA locations (Figure 1).
Among 2000-2010 numerous cohort studies and some randomized control trials (RCTs) and metanalisis[169] demonstrated that RFA gets better control of the disease compared to PEI. It has the ability to create bigger necrosis, including a peripheral ring to the tumour, and therefore higher complete necrotic rates - even sustained necrosis - particularly in tumours < 3 cm, where ablation is more effective.
Initial complete response has demonstrated a positive impact on survival, although there still will be high recurrence rates, comparable to surgical resection. HCC usually appears in the setting of underlying chronic hepatic disease and this conditioned the appearance of new nodules, but there are also same segment recurrence nodules as a result of the growth of small peritumoral satellites or vascular microinvasion out of the ablated zone.
There are some researches[170,171] with specimen from surgery, about the distance of microsatellites depending on tumour size that come to the conclusion that a reasonable limit of RFA is 2, 5-3 cm in order to create a security margin of 5 mm. This makes us use RFA needles 1 or 2 numbers of ablation greater than the tumour diameter. Other strategies to increase the ablation zone are overlapping techniques or multi-pronged needles, but their clinical use is difficult and not widespread.
RFA creates a complete necrosis area with a predictable diameter, whenever is not affected by nearby medium-large-sized vessels that could condition the perfusion-mediated tissue cooling, known as the heat sink effect. This limitation and the presence of non-treated microsatellites make up their main theoretical limitations, but there are also others that limit their clinical use: Ultrasound visualization of the nodule within liver parenchyma (difficult at fatty liver, macronodular cirrhosis, VIII segment nodules…) and the risk of damage of nearby organs (yuxtahiliar, gallbladder, stomach, duodenum, large intestine). This potential damage contraindicates RFA if we are not able to isolate them with sterile water instillation (spacing technique). Last, sub capsular tumours are not good indication of RFA due to the risk of tumoral seeding.
BCLC protocol last review[140] considered RFA as the first therapy at HCC < 2 cm, when a patient is not candidate to LT. This stage is also known as very early stage 0 or carcinoma in situ. RFA is also considered an alternative curative treatment at early stage (A) (single or 3 nodules ≤ 3 cm), with survival benefit up to 70%.
Microwave ablation is emerging as an alternative to RFA with several advantages. It is able to induce greater intratumoral temperature and bigger ablation area during less time than RFA. Thus, it is less dependent from tissue impedance and less influenced by heat-sink effect. Nowadays, it has less scientific evidence than RFA and there is lack of comparative papers between both techniques, but it seems logical to use it at HCC nearby to large hepatic vessels.
Irreversible electroporation is the technique more expensive, less used in clinical practice and with less evidence, although it is not affected by heat-sink effect and it doesn’t damage adjacent structures. Therefore, its use seems useful to treat complex location lesions[144,172].
TACE has been established by a meta-analysis of RCTs[173] as the standard of care for nonsurgical patients with large or multinodular noninvasive HCC isolated to the liver and with preserved liver function, known as intermediate stage HCC.
It is frequently used to control tumour progression (palliative treatment) as primary therapy or while waiting for liver transplantation, but some considerations has to be remarked. Intermediate stage is actually a heterogeneous group of patients and TACE benefit should be assessed in subgroups of patients as it has already been remarked[174]. Moreover, large series treated by TACE reported patients with single nodule stage A HCC[175,176].
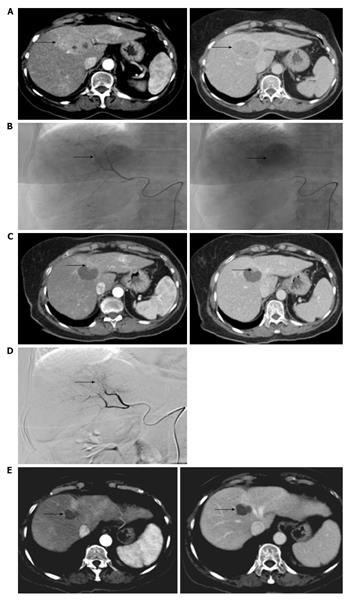
This would be justified by the recent concept of treatment stage migration: If a subject in a given stage is not candidate to the recommended treatment, we should consider the treatment of the more advance stage[140]. In our experience more than 1/3 of patient candidates to RFA, due to ablation difficulties, were treated by TACE (Figure 2), as has also been remarked in the literature[177].
Thus, early stage HCCs have been treated with TACE with reported maintained complete responses and it has been suggested to include TACE as an alternative curative intention therapy (stage A), in selected patients and performed with a concrete technique[178].
TACE technique is an interesting underestimate debate. There are different accepted techniques to perform endovascular HCC treatments with no enough evidence to determine the best option and this implies huge difficulties to standardize the results. Bland embolization or simple chemoinfusion have evolved to combined techniques of intra-arterial chemotherapy followed by ischemic changes after intra-arterial embolic materials (TACE).
Conventional TACE involves the selective injection of a chemotherapeutic agent (usually Doxorrubicine) emulsified in a viscous carrier (lipiodol), followed by embolic material into the feeding arteries of the tumour.
It has been the most common way to perform TACE since the beginning of the century-validated with level 1 of evidence[173] - and is still acceptable with widespread use, above all in eastern countries. There are different ways to perform it regarding on how to mix lipiodol and contrast, being more or less selective and types of lipiodol aggregation. The optimal way should include filling of the “rear door of the tumour”, i.e., small portal drainage veins[179].
An alternative way to perform TACE is widespread in the clinical practice, known as drug-eluting beads-TACE (DEB-TACE). It concerns performed microspheres loaded with chemotherapeutic agents which allows the delivery of large amounts of drugs to the tumour for a prolonged period of time (improve antitumoral efficacy), thereby decreasing plasma levels of the chemotherapeutic agent and potentially systemic effects (better tolerance).
A prospective multi-institutional RCT (Precision V)[178] demonstrated significant better tolerance compared to cTACE, but only improved response in advanced disease (Child-Pugh B). Later several cohort studies and some RCTs favors DEB-TACE vs cTACE in response rates and survival, but nowadays it is a usual debate in HCC symposiums because more evidence is needed to evaluate the two modalities of TACE. Actually, DEB-TACE has implemented in the clinical practice of western countries based on some clear rationale: Maximize drug delivery, long lasting effect/slow and sustained release, tumour effect vs systemic side effects and better reproducibility.
Technical recommendations to perform it have been published to improve its efficacy, helping reproducibility and constitute clear working tendencies[180-182]: (1) Must use microcatheter with super-selective injection at feeding arteries; (2) Use angio-CT system technology for tumour targeting; (3) Mix beads with contrast 3-4:1 to increase visibility; (4) Avoid complete stasis (endpoint near stasis); (5) Inject slowly (1 mL/min) trying to introduce as much Doxorubicin as possible inside the tumour (maximum 150 mg); (6) Use of small size microspheres to increase penetrability. At present 100-300 μm are recommended, but the use of smaller beads (M1 70-150 μm) - commonly used at treating liver metastasis- is being evaluated in clinical trials. Many working groups have introduced them in their protocols, particularly with small size HCCs and they are extremely promising thanks to their bigger penetrability[183]; and (7) - Repeat TACE in 2-4 wk, if needed, to get initial complete response, which is being related to survival benefit[184].
Ablative therapies and chemoembolization form the interventional treatments recommended by BCLC staging and treatment strategy, with simplicity as one of its known advantages. Other classifications as Japanese guidelines[185] stands for suggest other treatment options together with first line therapies in different stages or subgroups of them.
The huge variability of patients with HCC makes necessary to create a tailored approach that nowadays it is an undeniable clinical tendency[186]. We should adjust to each patient the most suitable treatment for its particular case, after a multidisciplinary assessment. The combination of locoregional therapies sometimes offers this maximal flexibility. This approach seems to be particularly valuable in patients with multifocal disease and nodules > 3 cm.
Among combine therapies, there are more experience with the combination of TACE and RFA (TACE first). Therefore, perfusion tissue is reduced and heat loss by perfusion mediated tissue cooling is minimized making possible larger ablation zone with wider safety margin[187]. Thus, sometimes downstaging is possible, above all with HCC 3-5 cm.
In the recent years, several groups perform RFA followed by TACE (RFA first). This way, TACE acts over a transitional zone with sub lethal hyperthermia and increase vascular permeability. This forms an increase delivery, uptake and susceptibility to chemotherapeutics ideal to treat microsatellites outside RFA zone[188].
Radioembolization is an alternative to TACE with less evidence and minor applicability. It needs to join interventional radiology and nuclear medicine units, which is restricted to only a few hospitals. Besides, technically is more complex than TACE and require an anatomical previous vascular map, because many times is necessary to embolize the arteries that communicate the target liver places with other adjacent organs as gallbladder or stomach that could be damaged.
Although is not included in the BCLC recommended treatments, it would be indicated in stage B HCC as an alternative to TACE and some stage C HCC with portal thrombosis that is not a contraindication of this technique. Some working groups consider it a first option in tumour > 5 cm or when > 4 nodules are present[174]. Ongoing RCTs are needed to unequivocally confirm the survival benefit provided by transarterial radioembolization in many cohort studies.
Sorafenib is a small molecule that inhibits tumour-cell proliferation, tumour angiogenesis and it is a multi-tyrosine kinase inhibitor and nowadays is the only drug that have demonstrated survival benefits in patients with advanced HCC. The initial phase II and phase III studies showed positive results with better survival in patients treated with sorafenib. The benefit of sorafenib was to increase the median survival from 7.9 mo in the placebo group to 10.7 mo in the sorafenib group. In addition, sorafenib showed a significant benefit in terms of time to progression, but objective responses rates were low[189]. These results were corroborated in other phase III study conducted in Asia[190]. This drug is only indicated in patients with preserved liver function and advanced disease not susceptible of other therapies and in this group of patients have an acceptable safety profile with manageable adverse events. The initial results were very promising because it was the first time that a systemic therapy demonstrated benefits effects in patients with HCC. Two subsequent trials, the Space (Sorafenib or placebo in combination with TACE for intermediate-stage HCC)[191] and the Storm (Sorafenib or placebo after resection or ablation to prevent recurrence of HCC)[192] have failed to demonstrated efficacy of sorafenib as adjuvant in combination with locally therapies. In the next years, new novel drugs, with a slightly different profile in terms of targets and intensity, have been tried both in first-line and second-line therapy. Until now, none of these drugs (sunitinib, brivanib, linifanib and combination of erlotinib and sorafenib) have proven to be better than sorafenib in first-line trials, in terms of survival. Second-line trails with brivanib, everolimus and ramucirumab have also failed to show benefits compared with placebo.
The EASL and AASLD recommend the use of sorafenib in patients with HCC advanced stage and preserved liver function.
HCC is a tumour with high incidence in patients with liver cirrhosis and is currently the leading cause of death in this group of patients. It is expected a decreases in incidence in the coming decades due to better management of patients infected with HBV and HCV. The vaccination against hepatitis B, the extended use of antiviral drugs with a high genetic barrier, which remain at undetectable viral load levels and the higher rate of sustained viral response in patients with chronic HCV with the new generation of antiviral drugs will reduce the incidence of this tumour in the future. On the other hand, increasingly numbers of studies have identified protective factors such as treatment with beta-blockers or statins, and perhaps in the future the use of some of these drugs will be recommended in selected cirrhotic patients. On the other hand, the improvement in the quality of imaging techniques allows establishing a diagnosis without histological confirmation in a high percentage of patients. New radiologic classifications, although promising, need more studies to be accepted universally. Once confirmed the diagnosis, the staging of the tumour allows us to decide the best therapeutic approach. Although several prognostic classifications have been described, the BCLC classification has been supported by American and European clinical practice guidelines. In addition, it allows deciding the best therapy according to the stage. The mainstays of treatment of HCC are surgery, radiological approach and systemic drugs. Since it is the treatment of choice to better outcomes in terms of survival, the indications of liver transplantation are in constant review. The expanded criteria and the downstaging have helped to expand the number of patients who are eligible for this option, with acceptable survival and recurrence after the transplant. On the other hand, the percutaneous ablative techniques have obtained good results in terms of response and survival, similar to surgical resection, in selected cases. In patients at intermediate stages, chemoembolization with particles has improved the results against the conventional chemoembolization with a similar rate of adverse effects. Sorafenib is the only systemic drug that has demonstrated survival benefits in advanced-stage patients and therefore remains the standard of care in this group. So far, any drug has shown survival benefits in second-line therapy after progression with sorafenib.
P- Reviewer: Lau WY, Morris DLL, Zhu ZH S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 3. | Available from: http://www-dep.iarc.fr/. |
| 4. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2140] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 5. | Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 795] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 6. | Prates MD, Torres FO. A cancer survey in Lourenço Marques, Portuguese East Africa. J Natl Cancer Inst. 1965;35:729-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 8. | Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Lett. 2009;286:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 10. | Bralet MP, Régimbeau JM, Pineau P, Dubois S, Loas G, Degos F, Valla D, Belghiti J, Degott C, Terris B. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology. 2000;32:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 12. | Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938-945, 945.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 240] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 13. | Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD; HALT-C Trial Group. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 16. | Jung KS, Kim SU, Ahn SH, Park YN, Kim do Y, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 17. | Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303-S309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | London WT, McGlynn KA. Liver cancer. Cancer epidemiology and prevention. 3rd ed. New York: Oxford University Press 2006; 763e86. [DOI] [Full Text] |
| 19. | Stuver S, Trichopolous D. Cancer of the liver and biliary tract. Textbook of cancer epidemiology. 2rd ed. New york: Oxford University Press 2008; 308e32. [DOI] [Full Text] |
| 20. | Boffetta P, Boccia S, La Vecchia C. Cancer of the liver and biliary tract. In: Boffetta P, Boccia S, La Vecchia C, editors. A quick guide to cancer epidemiology. Springer 2014; [DOI 10.1007/978-3-319-05068-3]. |
| 21. | Boffetta P; IARC. IARC monographs on the evaluation of carcinogenic risks to humans. 1994;59. |
| 22. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1973] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 23. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1838] [Cited by in RCA: 1758] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 24. | Yu MW, Chen CJ. Hepatitis B and C viruses in the development of hepatocellular carcinoma. Crit Rev Oncol Hematol. 1994;17:71-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432-438. [PubMed] |
| 26. | Villeneuve JP, Desrochers M, Infante-Rivard C, Willems B, Raymond G, Bourcier M, Côté J, Richer G. A long-term follow-up study of asymptomatic hepatitis B surface antigen-positive carriers in Montreal. Gastroenterology. 1994;106:1000-1005. [PubMed] |
| 27. | Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956. [PubMed] |
| 29. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ; Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 915] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 30. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 31. | Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, Shih WL, Kao JH, Chen DS, Chen CJ. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 422] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 32. | Muñoz N, Lingao A, Lao J, Estève J, Viterbo G, Domingo EO, Lansang MA. Patterns of familial transmission of HBV and the risk of developing liver cancer: a case-control study in the Philippines. Int J Cancer. 1989;44:981-984. [PubMed] |
| 33. | Kuper H, Hsieh C, Stuver SO, Mucci LA, Tzonou A, Zavitsanos X, Lagiou P, Trichopoulos D. Birth order, as a proxy for age at infection, in the etiology of hepatocellular carcinoma. Epidemiology. 2000;11:680-683. [PubMed] |
| 34. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1816] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 35. | Dragosics B, Ferenci P, Hitchman E, Denk H. Long-term follow-up study of asymptomatic HBsAg-positive voluntary blood donors in Austria: a clinical and histologic evaluation of 242 cases. Hepatology. 1987;7:302-306. [PubMed] |
| 36. | Chen CJ, Yang HI, Iloeje UH, Su J, Jen CL, You SL, Liaw YF. Time-dependent relative risk of hepatocellular carcinoma for markers of chronic hepatitis B. The REVEAL HBV study (abstract). Hepatology. 2005;42 Suppl 1:722A. |
| 37. | Tong MJ, Blatt LM, Kao JH, Cheng JT, Corey WG. Basal core promoter T1762/A1764 and precore A1896 gene mutations in hepatitis B surface antigen-positive hepatocellular carcinoma: a comparison with chronic carriers. Liver Int. 2007;27:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Simonetti J, Bulkow L, McMahon BJ, Homan C, Snowball M, Negus S, Williams J, Livingston SE. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 39. | Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, Lau K, Yuen JC, Lai CL. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 40. | Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 41. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 42. | Shen YC, Hsu C, Cheng CC, Hu FC, Cheng AL. A critical evaluation of the preventive effect of antiviral therapy on the development of hepatocellular carcinoma in patients with chronic hepatitis C or B: a novel approach by using meta-regression. Oncology. 2012;82:275-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2013;38:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 44. | Yu MW, You SL, Chang AS, Lu SN, Liaw YF, Chen CJ. Association between hepatitis C virus antibodies and hepatocellular carcinoma in Taiwan. Cancer Res. 1991;51:5621-5625. [PubMed] |
| 45. | Huang YT, Yang HI, Jen CL, Iloeje UH, Su J, You SL, Wang LY, Sun CA, Chen CJ. Suppression of hepatitis B virus replication by hepatitis C virus: combined effects on risk of hepatocellular carcinoma (abstract). Hepatology. 2005;42:230A. |
| 46. | Benvegnù L, Fattovich G, Noventa F, Tremolada F, Chemello L, Cecchetto A, Alberti A. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer. 1994;74:2442-2448. [PubMed] |
| 47. | Bruix J, Barrera JM, Calvet X, Ercilla G, Costa J, Sanchez-Tapias JM, Ventura M, Vall M, Bruguera M, Bru C. Prevalence of antibodies to hepatitis C virus in Spanish patients with hepatocellular carcinoma and hepatic cirrhosis. Lancet. 1989;2:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 508] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 48. | Colombo M, Kuo G, Choo QL, Donato MF, Del Ninno E, Tommasini MA, Dioguardi N, Houghton M. Prevalence of antibodies to hepatitis C virus in Italian patients with hepatocellular carcinoma. Lancet. 1989;2:1006-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Omland LH, Jepsen P, Krarup H, Christensen PB, Weis N, Nielsen L, Obel N, Sørensen HT, Stuver SO; DANVIR cohort study. Liver cancer and non-Hodgkin lymphoma in hepatitis C virus-infected patients: results from the DANVIR cohort study. Int J Cancer. 2012;130:2310-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Lewis S, Roayaie S, Ward SC, Shyknevsky I, Jibara G, Taouli B. Hepatocellular carcinoma in chronic hepatitis C in the absence of advanced fibrosis or cirrhosis. AJR Am J Roentgenol. 2013;200:W610-W616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, Fujii H, Rusyn I. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1182-1190. [PubMed] |
| 53. | Suruki RY, Mueller N, Hayashi K, Harn D, DeGruttola V, Raker CA, Tsubouchi H, Stuver SO. Host immune status and incidence of hepatocellular carcinoma among subjects infected with hepatitis C virus: a nested case-control study in Japan. Cancer Epidemiol Biomarkers Prev. 2006;15:2521-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 55. | Liang TJ, Ghany MG. Therapy of hepatitis C--back to the future. N Engl J Med. 2014;370:2043-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 56. | Ikeda K, Marusawa H, Osaki Y, Nakamura T, Kitajima N, Yamashita Y, Kudo M, Sato T, Chiba T. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med. 2007;146:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Marcellin P, Pequignot F, Delarocque-Astagneau E, Zarski JP, Ganne N, Hillon P, Antona D, Bovet M, Mechain M, Asselah T. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol. 2008;48:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 58. | Chou YH, Chiou HJ, Tiu CM, Chiou SY, Lee SD, Hung GS, Wu SC, Kuo BI, Lee RC, Chiang JH. Duplex Doppler ultrasound of hepatic Schistosomiasis japonica: a study of 47 patients. Am J Trop Med Hyg. 2003;68:18-23. [PubMed] |
| 59. | Ezzat S, Abdel-Hamid M, Eissa SA, Mokhtar N, Labib NA, El-Ghorory L, Mikhail NN, Abdel-Hamid A, Hifnawy T, Strickland GT. Associations of pesticides, HCV, HBV, and hepatocellular carcinoma in Egypt. Int J Hyg Environ Health. 2005;208:329-339. [PubMed] |
| 60. | Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 969] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 61. | Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 872] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 62. | Yu SZ. Primary prevention of hepatocellular carcinoma. J Gastroenterol Hepatol. 1995;10:674-682. [PubMed] |
| 63. | Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, Chen GC, Chen G, Yu SZ. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis. 1996;17:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 442] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 64. | Tsai JF, Chuang LY, Jeng JE, Ho MS, Hsieh MY, Lin ZY, Wang LY. Betel quid chewing as a risk factor for hepatocellular carcinoma: a case-control study. Br J Cancer. 2001;84:709-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Tsai JF, Jeng JE, Chuang LY, Ho MS, Ko YC, Lin ZY, Hsieh MY, Chen SC, Chuang WL, Wang LY. Habitual betel quid chewing as a risk factor for cirrhosis: a case-control study. Medicine (Baltimore). 2003;82:365-372. [PubMed] |
| 66. | Trichopoulos D, Bamia C, Lagiou P, Fedirko V, Trepo E, Jenab M, Pischon T, Nöthlings U, Overved K, Tjønneland A. Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case-control study. J Natl Cancer Inst. 2011;103:1686-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 67. | Mayans MV, Calvet X, Bruix J, Bruguera M, Costa J, Estève J, Bosch FX, Bru C, Rodés J. Risk factors for hepatocellular carcinoma in Catalonia, Spain. Int J Cancer. 1990;46:378-381. [PubMed] |
| 68. | Tanaka K, Hirohata T, Takeshita S, Hirohata I, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, Ishibashi H. Hepatitis B virus, cigarette smoking and alcohol consumption in the development of hepatocellular carcinoma: a case-control study in Fukuoka, Japan. Int J Cancer. 1992;51:509-514. [PubMed] |
| 69. | Mohamed AE, Kew MC, Groeneveld HT. Alcohol consumption as a risk factor for hepatocellular carcinoma in urban southern African blacks. Int J Cancer. 1992;51:537-541. [PubMed] |
| 70. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 71. | Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology. 1994;106:1085-1105. [PubMed] |
| 72. | Chiesa R, Donato F, Tagger A, Favret M, Ribero ML, Nardi G, Gelatti U, Bucella E, Tomasi E, Portolani N. Etiology of hepatocellular carcinoma in Italian patients with and without cirrhosis. Cancer Epidemiol Biomarkers Prev. 2000;9:213-216. [PubMed] |
| 73. | Munaka M, Kohshi K, Kawamoto T, Takasawa S, Nagata N, Itoh H, Oda S, Katoh T. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2003;129:355-360. [PubMed] |
| 74. | Covolo L, Gelatti U, Talamini R, Garte S, Trevisi P, Franceschi S, Franceschini M, Barbone F, Tagger A, Ribero ML. Alcohol dehydrogenase 3, glutathione S-transferase M1 and T1 polymorphisms, alcohol consumption and hepatocellular carcinoma (Italy). Cancer Causes Control. 2005;16:831-838. [PubMed] |
| 75. | Yu MC, Tong MJ, Govindarajan S, Henderson BE. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J Natl Cancer Inst. 1991;83:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498-502. [PubMed] |
| 77. | El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 350] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 79. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1018] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 80. | Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 81. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 82. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, Kawata S, Uto H, Takami S, Sumida Y, Takamura T, Kawanaka M, Okanoue T; Japan NASH Study Group, Ministry of Health, Labour, and Welfare of Japan. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433; quiz e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 83. | Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 84. | Deugnier YM, Guyader D, Crantock L, Lopez JM, Turlin B, Yaouanq J, Jouanolle H, Campion JP, Launois B, Halliday JW. Primary liver cancer in genetic hemochromatosis: a clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology. 1993;104:228-234. [PubMed] |
| 85. | Perlmutter DH. Pathogenesis of chronic liver injury and hepatocellular carcinoma in alpha-1-antitrypsin deficiency. Pediatr Res. 2006;60:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Polio J, Enriquez RE, Chow A, Wood WM, Atterbury CE. Hepatocellular carcinoma in Wilson’s disease. Case report and review of the literature. J Clin Gastroenterol. 1989;11:220-224. [PubMed] |
| 87. | Dawn BM, Todd S, Kim SI, Glucksman M. Biochemistry and molecular biology. Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins 2007; . |
| 88. | Liang Y, Yang Z, Zhong R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology. 2012;56:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 89. | Stewart MF. Review of hepatocellular cancer, hypertension and renal impairment as late complications of acute porphyria and recommendations for patient follow-up. J Clin Pathol. 2012;65:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 90. | Andant C, Puy H, Bogard C, Faivre J, Soulé JC, Nordmann Y, Deybach JC. Hepatocellular carcinoma in patients with acute hepatic porphyria: frequency of occurrence and related factors. J Hepatol. 2000;32:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 91. | Andant C, Puy H, Faivre J, Deybach JC. Acute hepatic porphyrias and primary liver cancer. N Engl J Med. 1998;338:1853-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Andersson C, Bjersing L, Lithner F. The epidemiology of hepatocellular carcinoma in patients with acute intermittent porphyria. J Intern Med. 1996;240:195-201. [PubMed] |
| 93. | Bengtsson NO, Hardell L. Porphyrias, porphyrins and hepatocellular cancer. Br J Cancer. 1986;54:115-117. [PubMed] |
| 94. | Gubler JG, Bargetzi MJ, Meyer UA. Primary liver carcinoma in two sisters with acute intermittent porphyria. Am J Med. 1990;89:540-541. [PubMed] |
| 95. | Hardell L, Bengtsson NO, Jonsson U, Eriksson S, Larsson LG. Aetiological aspects on primary liver cancer with special regard to alcohol, organic solvents and acute intermittent porphyria--an epidemiological investigation. Br J Cancer. 1984;50:389-397. [PubMed] |
| 96. | Kauppinen R, Mustajoki P. Acute hepatic porphyria and hepatocellular carcinoma. Br J Cancer. 1988;57:117-120. [PubMed] |
| 97. | Lithner F, Wetterberg L. Hepatocellular carcinoma in patients with acute intermittent porphyria. Acta Med Scand. 1984;215:271-274. [PubMed] |
| 98. | Liu Y, He Y, Li T, Xie L, Wang J, Qin X, Li S. Risk of primary liver cancer associated with gallstones and cholecystectomy: a meta-analysis. PLoS One. 2014;9:e109733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Tajada M, Nerín J, Ruiz MM, Sánchez-Dehesa M, Fabre E. Liver adenoma and focal nodular hyperplasia associated with oral contraceptives. Eur J Contracept Reprod Health Care. 2001;6:227-230. [PubMed] |
| 100. | Korula J, Yellin A, Kanel G, Campofiori G, Nichols P. Hepatocellular carcinoma coexisting with hepatic adenoma. Incidental discovery after long-term oral contraceptive use. West J Med. 1991;155:416-418. [PubMed] |
| 101. | Gordon SC, Reddy KR, Livingstone AS, Jeffers LJ, Schiff ER. Resolution of a contraceptive-steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Intern Med. 1986;105:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Maheshwari S, Sarraj A, Kramer J, El-Serag HB. Oral contraception and the risk of hepatocellular carcinoma. J Hepatol. 2007;47:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Freedman ND, Cross AJ, McGlynn KA, Abnet CC, Park Y, Hollenbeck AR, Schatzkin A, Everhart JE, Sinha R. Association of meat and fat intake with liver disease and hepatocellular carcinoma in the NIH-AARP cohort. J Natl Cancer Inst. 2010;102:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 104. | Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 105. | Luo J, Yang Y, Liu J, Lu K, Tang Z, Liu P, Liu L, Zhu Y. Systematic review with meta-analysis: meat consumption and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2014;39:913-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 106. | Agency for Toxic Substances and Disease Registry. Toxicological profile for N nitrosodimethylamine. Atlanta, GA: US Department of Health and Human Services 1989; . |
| 107. | Turati F, Edefonti V, Talamini R, Ferraroni M, Malvezzi M, Bravi F, Franceschi S, Montella M, Polesel J, Zucchetto A. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55:1416-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 108. | Tanabe KK, Lemoine A, Finkelstein DM, Kawasaki H, Fujii T, Chung RT, Lauwers GY, Kulu Y, Muzikansky A, Kuruppu D. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. JAMA. 2008;299:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 109. | De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 110. | Iavarone M, Lampertico P, Iannuzzi F, Manenti E, Donato MF, Arosio E, Bertolini F, Primignani M, Sangiovanni A, Colombo M. Increased expression of vascular endothelial growth factor in small hepatocellular carcinoma. J Viral Hepat. 2007;14:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [PubMed] |
| 112. | Suzuki K, Hayashi N, Miyamoto Y, Yamamoto M, Ohkawa K, Ito Y, Sasaki Y, Yamaguchi Y, Nakase H, Noda K. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res. 1996;56:3004-3009. [PubMed] |
| 113. | Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, Imaoka S, Tsujimoto M, Higashiyama S, Monden M. Expression and clinical significance of the erbB family in intrahepatic cholangiocellular carcinoma. Pathol Res Pract. 2001;197:95-100. [PubMed] |
| 114. | Zhong JH, You XM, Gong WF, Ma L, Zhang Y, Mo QG, Wu LC, Xiao J, Li LQ. Epidermal growth factor gene polymorphism and risk of hepatocellular carcinoma: a meta-analysis. PLoS One. 2012;7:e32159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Clifford RJ, Zhang J, Meerzaman DM, Lyu MS, Hu Y, Cultraro CM, Finney RP, Kelley JM, Efroni S, Greenblum SI. Genetic variations at loci involved in the immune response are risk factors for hepatocellular carcinoma. Hepatology. 2010;52:2034-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 116. | Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 117. | Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 118. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 119. | Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando-Lemaire V, Ganne-Carrie N, Trinchet JC, Vicaut E, Beaugrand M. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev Res (Phila). 2012;5:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Sawada N, Inoue M, Iwasaki M, Sasazuki S, Shimazu T, Yamaji T, Takachi R, Tanaka Y, Mizokami M, Tsugane S; Japan Public Health Center-Based Prospective Study Group. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142:1468-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 121. | Huang RX, Duan YY, Hu JA. Fish intake and risk of liver cancer: a meta-analysis. PLoS One. 2015;10:e0096102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 122. | Zhang W, Shu XO, Li H, Yang G, Cai H, Ji BT, Gao J, Gao YT, Zheng W, Xiang YB. Vitamin intake and liver cancer risk: a report from two cohort studies in China. J Natl Cancer Inst. 2012;104:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, Franceschi S, Montella M, Trichopoulou A, La Vecchia C. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 124. | Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 125. | Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1413-1421.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 126. | Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148:118-125; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 127. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [PubMed] |
| 128. | Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 129. | Trevisani F, De Notariis S, Rapaccini G, Farinati F, Benvegnù L, Zoli M, Grazi GL, Del PP, Di N, Bernardi M; Italian Liver Cancer Group. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol. 2002;97:734-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 130. | Pascual S, Irurzun J, Zapater P, Such J, Sempere L, Carnicer F, Palazón JM, de la Iglesia P, Gil S, de España F. Usefulness of surveillance programmes for early diagnosis of hepatocellular carcinoma in clinical practice. Liver Int. 2008;28:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 131. | Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 318] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 132. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 133. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 134. | Jha RC, Mitchell DG, Weinreb JC, Santillan CS, Yeh BM, Francois R, Sirlin CB. LI-RADS categorization of benign and likely benign findings in patients at risk of hepatocellular carcinoma: a pictorial atlas. AJR Am J Roentgenol. 2014;203:W48-W69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 135. | Darnell A, Forner A, Rimola J, Reig M, García-Criado Á, Ayuso C, Bruix J. Liver Imaging Reporting and Data System with MR Imaging: Evaluation in Nodules 20 mm or Smaller Detected in Cirrhosis at Screening US. Radiology. 2015;275:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 136. | Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 137. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 138. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 139. | Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 140. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 141. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 142. | Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, Makuuchi M, Kojiro M, Ichida T, Arii S. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:884-892. [PubMed] |
| 143. | Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol. 2010;53:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 144. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3302] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 145. | Reig M, Darnell A, Forner A, Rimola J, Ayuso C, Bruix J. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin Liver Dis. 2014;34:444-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 146. | Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022. [PubMed] |
| 147. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 148. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5310] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 149. | Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 150. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 151. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 152. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 153. | Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003;9:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 154. | Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 155. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 156. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 157. | Kneteman NM, Oberholzer J, Al Saghier M, Meeberg GA, Blitz M, Ma MM, Wong WW, Gutfreund K, Mason AL, Jewell LD. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl. 2004;10:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 158. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 159. | Silva M, Moya A, Berenguer M, Sanjuan F, López-Andujar R, Pareja E, Torres-Quevedo R, Aguilera V, Montalva E, De Juan M. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14:1449-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 160. | Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, Qian JM, Zhou J, Xu Y, Qiu SJ. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol. 2009;135:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 161. | Guiteau JJ, Cotton RT, Washburn WK, Harper A, O’Mahony CA, Sebastian A, Cheng S, Klintmalm G, Ghobrial M, Halff G. An early regional experience with expansion of Milan Criteria for liver transplant recipients. Am J Transplant. 2010;10:2092-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 162. | Raj A, McCall J, Gane E. Validation of the “Metroticket” predictor in a cohort of patients transplanted for predominantly HBV-related hepatocellular carcinoma. J Hepatol. 2011;55:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 163. | Lei JY, Wang WT, Yan LN. “Metroticket” predictor for assessing liver transplantation to treat hepatocellular carcinoma: a single-center analysis in mainland China. World J Gastroenterol. 2013;19:8093-8098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 164. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 165. | Gordon-Weeks AN, Snaith A, Petrinic T, Friend PJ, Burls A, Silva MA. Systematic review of outcome of downstaging hepatocellular cancer before liver transplantation in patients outside the Milan criteria. Br J Surg. 2011;98:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 166. | Toso C, Mentha G, Kneteman NM, Majno P. The place of downstaging for hepatocellular carcinoma. J Hepatol. 2010;52:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 167. | Shiina S. Image-guided percutaneous ablation therapies for hepatocellular carcinoma. J Gastroenterol. 2009;44 Suppl 19:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 168. | Lencioni R, Crocetti L, Cioni D, Pina CD, Oliveri F, De Simone P, Brunetto M, Filipponi F. Single-session percutaneous ethanol ablation of early-stage hepatocellular carcinoma with a multipronged injection needle: results of a pilot clinical study. J Vasc Interv Radiol. 2010;21:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 169. | Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 170. | Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 171. | Ikeda K, Seki T, Umehara H, Inokuchi R, Tamai T, Sakaida N, Uemura Y, Kamiyama Y, Okazaki K. Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol. 2007;31:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 172. | Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 329] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 173. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 174. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 175. | Takayasu K, Arii S, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 176. | Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57:1258-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 177. | Leoni S, Piscaglia F, Serio I, Terzi E, Pettinari I, Croci L, Marinelli S, Benevento F, Golfieri R, Bolondi L. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: experience of the Bologna Liver Oncology Group. Dig Liver Dis. 2014;46:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 178. | Matsui O, Miyayama S, Sanada J, Kobayashi S, Khoda W, Minami T, Kozaka K, Gabata T. Interventional oncology: new options for interstitial treatments and intravascular approaches: superselective TACE using iodized oil for HCC: rationale, technique and outcome. J Hepatobiliary Pancreat Sci. 2010;17:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 179. | Miyayama S, Matsui O, Yamashiro M, Ryu Y, Takata H, Takeda T, Aburano H, Shigenari N. Visualization of hepatic lymphatic vessels during transcatheter arterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:1111-1117. [PubMed] |
| 180. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 181. | Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, Martin RC, O’Grady E, Real MI, Vogl TJ. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 182. | Basile A, Carrafiello G, Ierardi AM, Tsetis D, Brountzos E. Quality-improvement guidelines for hepatic transarterial chemoembolization. Cardiovasc Intervent Radiol. 2012;35:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 183. | Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, Civelli EM, Vaiani M, Bhoori S, Pellegrinelli A. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015;38:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 184. | Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E, Spyridopoulos T, Chatziioannou A, Sotirchos V, Karampelas T. Chemoembolization of hepatocellular carcinoma with HepaSphere 30-60 μm. Safety and efficacy study. Cardiovasc Intervent Radiol. 2014;37:165-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 185. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 186. | Bolondi L, Cillo U, Colombo M, Craxì A, Farinati F, Giannini EG, Golfieri R, Levrero M, Pinna AD, Piscaglia F. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 187. | Sugimori K, Morimoto M, Shirato K, Kokawa A, Tomita N, Saito T, Nozawa A, Hara M, Sekihara H, Tanaka K. Radiofrequency ablation in a pig liver model: effect of transcatheter arterial embolization on coagulation diameter and histologic characteristics. Hepatol Res. 2002;24:164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 188. | Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259-2267. [PubMed] |
| 189. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 190. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4649] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 191. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. SPACE: Sorafenib or placebo in combination with transarterial chemoembolization with doxorubicin-eluting beads for intermediate-stage hepatocellular carcinoma: Phase II, randomized, double-blind SPACE trial. J Clin Oncol. 2012;30:A154. |
| 192. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 193. | Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 194. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 195. | Carr BI, Kondragunta V, Buch SC, Branch RA. Therapheutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatment in unresectable hepatocellular carcinoma: a two cohort study. Cancer. 2010;116:1305-1314. [RCA] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 196. | Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |