Published online Mar 8, 2016. doi: 10.4254/wjh.v8.i7.368
Peer-review started: August 3, 2015
First decision: September 14, 2015
Revised: October 24, 2015
Accepted: December 3, 2015
Article in press: December 4, 2015
Published online: March 8, 2016
Processing time: 215 Days and 0.4 Hours
AIM: To assess whether reasons for hepatitis C virus (HCV) therapy non-initiation differentially affect racial and ethnic minorities with human immunodeficiency virus (HIV)/HCV co-infection.
METHODS: Analysis included co-infected HCV treatment-naïve patients in the University of North Carolina CFAR HIV Clinical Cohort (January 1, 2004 and December 31, 2011). Medical records were abstracted to document non-modifiable medical (e.g., hepatic decompensation, advanced immunosuppression), potentially modifiable medical (e.g., substance abuse, severe depression, psychiatric illness), and non-medical (e.g., personal, social, and economic factors) reasons for non-initiation. Statistical differences in the prevalence of reasons for non-treatment between racial/ethnic groups were assessed using the two-tailed Fisher’s exact test. Three separate regression models were fit for each reason category. Odds ratios and their 95%CIs (Wald’s) were computed.
RESULTS: One hundred and seventy-one patients with HIV/HCV co-infection within the cohort met study inclusion. The study sample was racially and ethnically diverse; most patients were African-American (74%), followed by Caucasian (19%), and Hispanic/other (7%). The median age was 46 years (interquartile range = 39-50) and most patients were male (74%). Among the 171 patients, reasons for non-treatment were common among all patients, regardless of race/ethnicity (50% with ≥ 1 non-modifiable medical reason, 66% with ≥ 1 potentially modifiable medical reason, and 66% with ≥ 1 non-medical reason). There were no significant differences by race/ethnicity. Compared to Caucasians, African-Americans did not have increased odds of non-modifiable [adjusted odds ratio (aOR) = 1.47, 95%CI: 0.57-3.80], potentially modifiable (aOR = 0.72, 95%CI: 0.25-2.09) or non-medical (aOR = 0.90, 95%CI: 0.32-2.52) reasons for non-initiation.
CONCLUSION: Race/ethnicity alone is not predictive of reasons for HCV therapy non-initiation. Targeted interventions are needed to improve access to therapy for all co-infected patients, including minorities.
Core tip: Historically, hepatitis C virus (HCV) treatment rates have been low in patients with human immunodeficiency virus (HIV) co-infection, especially for African-American patients. Identifying the reasons for treatment non-initiation may help improve treatment rates among racially and ethnic minorities. In our study of patients with HIV/HCV coinfection, non-modifiable medical reasons, potentially modifiable medical reasons, and non-medical reasons for non-treatment were common among all patients, regardless of their race/ethnicity. There is a need to recognize and overcome potential treatment barriers in order to improve HCV treatment uptake in this patient population.
- Citation: Oramasionwu CU, Kashuba ADM, Napravnik S, Wohl DA, Mao L, Adimora AA. Non-initiation of hepatitis C virus antiviral therapy in patients with human immunodeficiency virus/hepatitis C virus co-infection. World J Hepatol 2016; 8(7): 368-375
- URL: https://www.wjgnet.com/1948-5182/full/v8/i7/368.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i7.368
Hepatitis C virus (HCV) treatment rates have been low in patients who are co-infected with human immunodeficiency virus (HIV). Up until 2011, when the first direct-acting antivirals (DAAs) became available, only one-third of co-infected patients were deemed eligible to receive HCV therapy, of whom less than one-third initiated HCV treatment[1-4]. Of great concern is the proportion of racial and ethnic minorities with co-infection that have not received HCV therapy. Nearly half of United States patients with HIV/HCV co-infection are African-American[5-7]. Previous studies involving older HCV regimens [pegylated interferon plus ribavirin (pegIFN-RBV)] reported that African-Americans were less likely than Caucasians to initiate HCV therapy[6,8,9]. Of co-infected patients in the HIV Outpatient Study during 1999-2007, African-Americans had a lower likelihood of HCV treatment than Caucasians (HR = 0.3, 95%CI: 0.2-0.6)[6]. African-American patients have been shown to not initiate therapy due to presence of IFN-related contraindications or to defer therapy due to lack of symptoms[10,11].
Non-initiation of HCV therapy in co-infected patients is attributed to diverse factors such as patient- and provider-level barriers, perceived risks and benefits of therapy, and patient ineligibility to receive therapy due to medical contraindications[12]. Examples of medical conditions that sometimes precluded treatment with older regimens include hepatic decompensation, active injection drug use (IDU), alcohol abuse, severe depression, and advanced HIV-associated immunosuppression[13-16].
Although these treatment-related barriers have been identified in the general co-infected population, scant research has documented their prevalence in co-infected minorities. Some reasons for non-treatment, such as substance abuse, are potentially modifiable. Addressing them could help improve access to HCV therapy in minorities. Despite the clinical promise of the DAAs, it is possible that some of the historical challenges to treating patients with HIV/HCV co-infection are still obstacles to treatment, particularly for minority patients[1,10,17]. The objectives of this study were to document reasons for non-treatment with HCV antiviral therapy and to assess how they differentially affect racial and ethnic minorities with HIV/HCV co-infection.
This was a retrospective study of patients with HIV/HCV co-infection enrolled in the University of North Carolina (UNC) Center for AIDS Research HIV Clinical Cohort. This prospective cohort began enrolling patients in 1996 and includes over 4000 HIV-infected patients ≥ 18 years of age who receive HIV care at UNC. The cohort, approved by the UNC Institutional Review Board, has ongoing enrollment and participants provide written informed consent. Data for the cohort are retrieved from two sources. Patient demographic characteristics and laboratory values are retrieved electronically, whereas patient medication histories and comorbid conditions are obtained by standardized and comprehensive electronic medical record reviews.
This study examined patients with HIV and HCV infection who had never received treatment for HCV and who had at least one outpatient clinic visit between January 1, 2004 and December 31, 2011. Patients were included in the study if they had the following: (1) a concomitant diagnosis of HCV based on positive HCV serostatus (as determined by HCV antibody test enzyme-linked immunosorbant assay/enzyme immunoassay); and (2) a positive HCV recombinant immunoblot assay (RIBA) test, detectable HCV RNA or HCV genotype test results. Patients with a history of HCV antiviral therapy were excluded. Anti-HCV therapy was defined as interferon, pegIFN, RBV, telaprevir, or boceprevir. The study period (2004-2011) was selected to best capture the timeframe when combination therapy with pegIFN-RBV was the standard of treatment for most patients with co-infection.
Baseline variables were retrieved from the cohort database and included patient demographics and clinical characteristics at time of HCV diagnosis. Baseline clinical characteristics were measurements taken proximal (allowing a 30-d window) to the date of the first positive HCV test. Demographic variables included age, gender, race/ethnicity (African-American, Caucasian, or Hispanic/other), and insurance coverage (private, public, none, or other). Clinical characteristics included CD4, HIV-1 RNA, HCV RNA, HCV genotype, HIV risk category (risk categories were not mutually exclusive), prior AIDS-defining clinical conditions, and use of highly active antiretroviral therapy (HAART), defined as a combination of three or more antiretroviral drugs. Prior to May 1, 2007, HCV RNA assays were measured in copies/mL, whereas subsequent HCV RNA assays were measured in IU/mL. Results for both assays are presented, where applicable, within the study period.
We reviewed individual medical records to identify reasons cited in the clinic notes by providers for not initiating HCV therapy. Reasons for treatment non-initiation were then categorized as non-modifiable medical reasons, potentially modifiable medical reasons, or non-medical reasons. Non-modifiable medical reasons included death (patients with a poor life expectancy or patients that died before treatment was ever initiated), hepatic decompensation, advanced immunosuppression (CD4 < 200) not controlled by antiretroviral therapy, renal insufficiency, uncontrolled autoimmune conditions, or hematological disease. Potentially modifiable medical reasons included active or recent (within the past six months) IDU/cocaine use, alcohol use, severe depression (defined as depression with suicidal ideation), psychiatric illness, or pregnancy/unwillingness to use contraception. Lastly, non-medical reasons included personal factors (e.g., refusal of available therapies, poor adherence to care), social factors (e.g., social instability, homelessness/lack of housing, lack of transportation), and economic factors (e.g., lack of health insurance, prohibitive cost).
Descriptive analyses were conducted on baseline variables, including demographic and clinical characteristics. For each type of reason for non-treatment, the prevalence of the sub-categories by racial/ethnic groups was computed. Statistical differences in the prevalence of reasons for non-treatment between racial/ethnic groups were assessed using the two-tailed Fisher’s exact test. For each reason type (non-modifiable medical, potentially modifiable medical, and non-medical), risk factors such as age, gender, race/ethnicity, insurance status, and select HIV clinical characteristics were analyzed using multivariate logistic regression. Three separate regression models were fit for each reason type; the three reason types were the dependent variables in the respective models. Odds ratios and their 95%CIs (Wald’s) were computed. All data analyses were conducted using SAS software (version 9.2; SAS Institute Inc., Cary, North Carolina, United States). All statistical analyses were performed by Lu Mao, a trained biostatistician with the UNC CFAR Biostatistics Core.
Within the cohort, 246 patients had a positive HCV serostatus and either a positive HCV RIBA test or detectable HCV RNA at baseline. Of these, 75 patients (30%) were excluded during the chart review process due to lack of HCV genotype results or due to reported history of antiviral therapy. We present results for the 171 patients (70%) that met criteria for this study. Baseline demographic and clinical characteristics are summarized in Table 1. The median age was 46 years [interquartile range (IQR) = 39-50] and most patients were male (74%). The study sample was racially and ethnically diverse; most patients were African-American (74%), followed by Caucasian (19%), and Hispanic/other (7%). This largely reflects the racial/ethnic makeup of the clinical cohort. More than one-third of patients lacked any insurance coverage (37%).
| Variable | Patients (n = 171) |
| Patient demographics | |
| Age (median, IQR) | 46 (39, 50) |
| Male gender, n (%) | 126 (73.7) |
| Race/ethnicity, n (%) | - |
| Caucasian | 32 (18.7) |
| African-American | 126 (73.7) |
| Hispanic/other | 13 (7.6) |
| Insurance, n (%) | - |
| Private | 23 (13.5) |
| Public | 67 (39.2) |
| None | 64 (37.4) |
| Other | 17 (9.9) |
| HIV clinical characteristics | |
| HIV-1 RNA (log10 copies/mL) (median, IQR) | 4.3 (2.7, 5) |
| CD4 (cells/μL) (median, IQR) | 299 (91, 517) |
| HAART, n (%) | 125 (73.1) |
| Prior AIDS-defining clinical condition, n (%) | 37 (21.6) |
| HIV risk category, n (%)1 | - |
| MSM | 41 (24) |
| Injection drug use | 97 (56.7) |
| HCV clinical characteristics | |
| HCV RNA log10 copies/mL (median, IQR)2 | 5.8 (5.7, 5.8) |
| HCV RNA log10 (IU/mL) (median, IQR)3 | 6.5 (6.2, 6.7) |
| HCV genotype, n (%)4 | - |
| Genotype 1 | 157 (91.8) |
| Genotype 2 | 8 (4.7) |
| Genotype 3 | 6 (3.5) |
| Genotype 4 | 1 (0.6) |
At baseline, patients had a median (IQR) HIV-1 RNA of 4.3 (2.7-5) log10 copies/mL, a median (IQR) CD4 299 (91-517) cells/μL, and 73% of patients were treated with HAART. Twenty-five patients (15%) had a baseline median (IQR) HCV RNA of 5.8 (5.7, 5.8) log10 copies/mL (values reported prior to May 1, 2007) and 10 patients (6%) had a baseline median (IQR) HCV RNA of 6.5 (6.2, 6.7) log10 IU/mL (values reported after May 1, 2007). The most predominant HCV genotype was genotype 1 (92%), followed by genotype 2 (5%), genotype 3 (3%), and genotype 4 (< 1%).
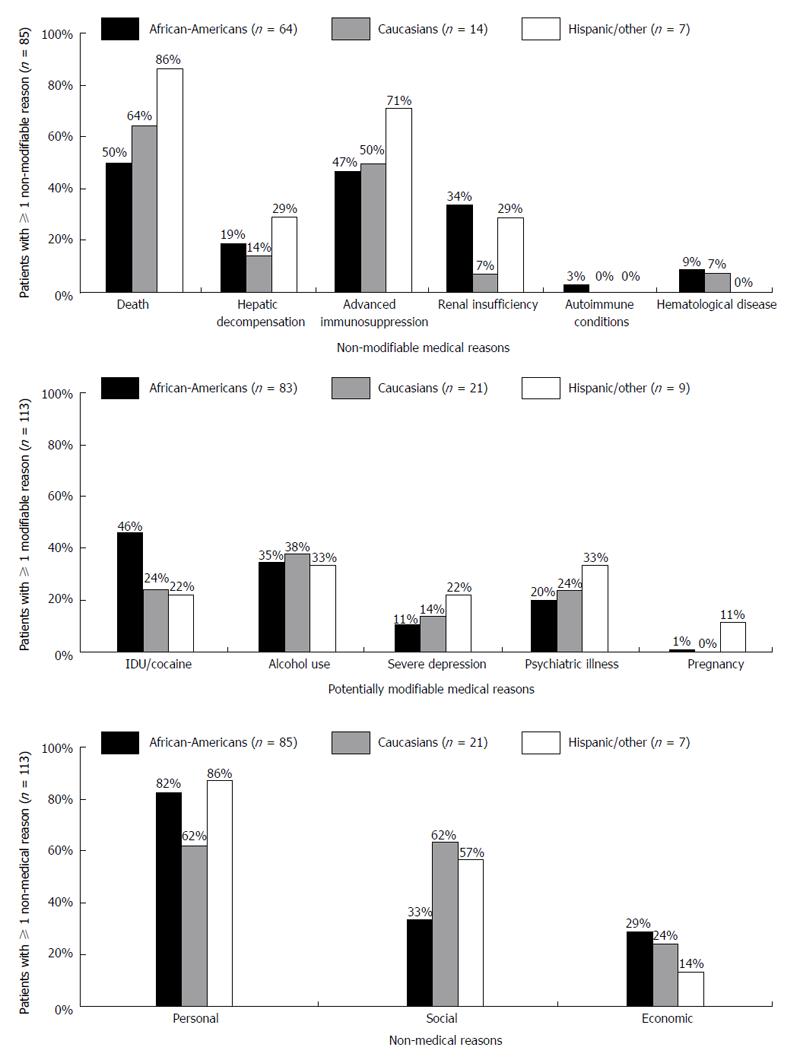
Reasons for HCV non-treatment did not vary significantly by race/ethnicity (Table 2). Subcategories for each reason type are illustrated in Figure 1. At least one non-modifiable medical reason was documented in approximately half of all patients. Patient death was the most common non-modifiable medical reason in all three racial/ethnic groups, followed by advanced immunosuppression. Two-thirds of patients in each racial/ethnic group had at least one potentially modifiable reason for not initiating therapy (range 66%-69% across racial/ethnic groups); of these, IDU/cocaine use and psychiatric illness was the most common, followed by alcohol use and severe depression. Non-medical reasons were also common in each racial/ethnic group; these were most often due to personal and social reasons, and least commonly due to economic reasons.
| Total | Race/ethnicity | ||||
| (n = 171) | African-American(n = 126) | Caucasian(n = 32) | Hispanic/other(n = 13) | P value1 | |
| ≥ 1 non-modifiable medical reason, n (%) | 85 (49.7) | 64 (50.8) | 14 (43.8) | 7 (53.8) | 0.806 |
| ≥ 1 potentially modifiable medical reason, n (%) | 113 (66.1) | 83 (65.9) | 21 (65.6) | 9 (69.2) | 1.000 |
| ≥ 1 non-medical reason, n (%) | 113 (66.1) | 85 (67.5) | 21 (65.6) | 7 (53.8) | 0.597 |
We evaluated age, gender, race/ethnicity, insurance, HIV-1 RNA, CD4, and prior AIDS-defining clinical conditions as factors independently associated with reasons for not initiating therapy (Table 3). Compared to Caucasian race/ethnicity, African-American race/ethnicity was not associated with having at least one non-modifiable medical reason [adjusted odds ratio (aOR) = 1.47, 95%CI: 0.57-3.80], potentially modifiable medical reason (aOR = 0.72, 95%CI: 0.25-2.09), or non-medical reason (aOR = 0.90, 95%CI: 0.32-2.52).
| Variable | Non-modifiable medical reason | Potentially modifiable medical reason | Non-medical reason |
| aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | |
| Age (yr) | 1.00 (0.96-1.05) | 0.93 (0.87-0.98) | 0.99 (0.94-1.04) |
| Gender | |||
| Female | Ref | Ref | Ref |
| Male | 1.07 (0.47-2.43) | 0.77 (0.30-1.95) | 1.57 (0.66-3.71) |
| Race/ethnicity | |||
| Caucasian | Ref | Ref | Ref |
| African-American | 1.47 (0.57-3.80) | 0.72 (0.25-2.09) | 0.90 (0.32-2.52) |
| Hispanic/other | 1.94 (0.34-11.18) | 1.65 (0.14-18.83) | 0.46 (0.08-2.88) |
| Insurance | |||
| Private | Ref | Ref | Ref |
| Public | 1.30 (0.39-4.27) | 2.27 (0.65-7.93) | 0.69 (0.15-3.10) |
| None | 0.64 (0.19-2.05) | 1.13 (0.34-3.76) | 0.44 (0.13-1.56) |
| Other | 0.82 (0.32-3.13) | 3.03 (0.59-15.6) | 0.50 (0.09-2.68) |
| HIV-1 RNA log10 | 1.08 (0.79-1.49) | 0.95 (0.67-1.34) | 1.11 (0.80-1.56) |
| CD4 | 0.99 (0.99-1.00) | 1.00 (0.99-1.00) | 0.99 (0.99-1.00) |
| Prior AIDS-defining clinical condition | 0.46 (0.09-2.37) | 0.58 (0.10-3.28) | 1.49 (0.15-14.65) |
While low uptake of older HCV antiviral regimens in HIV/HCV co-infected patients, particularly in racial and ethnic minorities, is well-documented in the literature, the reasons for low uptake are less clear[6,8,16]. This study evaluated reasons cited by the provider for non-initiation of HCV therapy in a cohort of untreated patients. Patients in our study were predominantly African-American and largely had genotype 1, which is comparable to other studies[1,18-21]. Our findings suggest that race/ethnicity alone is not predictive of having at least one reason for not initiating therapy. Rather, a key finding of this study was the high prevalence of multiple reasons for non-treatment, regardless of racial/ethnic group.
Nearly one-third of all patients in this study died without ever receiving HCV therapy. Of note, it cannot be assumed that patients who died would have ever initiated therapy while alive. Advanced immunosuppression (CD4 < 200) was also a common reason for non-treatment in our study. The majority (73%) of patients were on HAART at baseline, yet more than half of all patients had advanced immunosuppression documented as a reason for non-treatment, a finding that has been noted previously. In a study of patients with HIV/HCV co-infection at one of three Los Angeles HIV clinics, HAART use was common (> 90%), yet CD4 ≤ 200 was independently associated with decreased HCV treatment acceptance (OR = 0.08, 95%CI: 0.01-0.40)[13]. Treatment guidelines suggest postponing HCV antiviral therapy in HIV/HCV co-infected patients with CD4 < 200 and recommend HAART initiation to preserve and restore immune function[22]. Treatment-related factors such as adherence and regimen appropriateness can influence immune response. CD4 and HIV-1 RNA are indirect, objective measures of these treatment-related factors. However, we only evaluated baseline CD4 and HIV-1 RNA values, which precluded us from drawing inferences about the effects of adherence and regimen appropriateness on immunosuppression and resultant non-initiation of HCV therapy.
As expected, IDU/cocaine use was reported as a potentially modifiable reason for not initiating therapy. Past studies have classified substance abuse as an absolute contraindication to HCV therapy[23]. In a recent systematic review evaluating barriers to HCV therapy in HIV/HCV co-infected patients, substance abuse was the most frequently cited barrier[23]. However, substance users should not be routinely excluded from treatment, as substance use can fluctuate with time[24]. Recent guidelines recommend deferral of treatment if active or ongoing substance abuse is expected to interfere with regimen adherence, and frequent re-evaluation of each patient’s adherence to routine medical care, other comorbidities, and potential for reinfection[15,24].
Personal and social factors were commonly reported in all racial/ethnic groups as reasons for not initiating therapy. Engagement in care and adherence are often perceived by the provider as an indicator of treatment readiness and are based on characteristics such as mental health, clinic attendance, substance use, and the patient’s attitudes and beliefs about therapy[25]. These factors can decrease the likelihood of patient referral for HCV care.
Historically, patients with HIV/HCV, most often African-Americans, have had poor virologic response to HCV therapy[26-29]. African-Americans have been shown to be less likely to accept pegIFN-RBV antiviral therapy when it was recommended by their providers[13]. It has been suggested that patient awareness of low sustained virologic response (SVR) among African-Americans with genotype 1 may contribute to decisions to refuse therapy[11]. The high proportion of personal factors for non-treatment in our study could have also been attributed to patient refusal of pegIFN-RBV in anticipation of more effective, and ultimately more convenient, therapies. DAA-based therapy demonstrates excellent efficacy in clinical trials; 87% SVR has been attained in African-Americans[30]. Nevertheless, some of the reasons for non-treatment identified in the present study are still likely barriers to the DAAs.
Current treatment guidelines acknowledge substance abuse, psychiatric disorders, and lack of access (e.g., cost, insurance, distance to provider) as barriers to current HCV treatment regimens that include DAAs[31]. Several strategies are proposed to increase HCV treatment, particularly DAA therapy, in patients with HIV/HCV. At the patient level, pre-treatment education, management of comorbidities and mental health conditions, and harm reduction counseling in individuals with continued substance abuse can be provided through patient referral for specialty services, such as substance abuse treatment and psychiatric therapy[24]. Strategies at the provider level include collaborative care models with primary care providers and HCV specialists[32], and co-localization models that combine HCV treatment and care with other primary medical care or substance abuse treatment and social services[31]. Systems-level strategies are needed, such as medication patient assistance programs, removal of Medicaid state restrictions regarding substance abuse and HCV therapy, decrease prescription prior authorization requirements, and ultimately, to lower DAA drug prices in order to increase treatment access for patients[33].
Our study is subject to limitations. The UNC clinic is a large academic center and may not be representative of patients receiving care in other clinic settings. All baseline variables were retrieved from the clinical cohort database; however, individual patient records were reviewed to ascertain reasons for non-treatment in the medical record. Some limitations of medical record data collection include variability in documentation across clinic providers, missing data due to errors that occur during clinic visit narrative dictation and transcription, and lack of specificity for patient information. Listed reasons were based on providers’ cited reasons for not initiating HCV therapy. These may differ from patient-reported barriers to care that are specific to racial and ethnic groups, such as medical mistrust[34]. As our study was designed to focus on the untreated, we were unable to make any causal associations between documented reasons and why patients were not treated. We did not assess continuity of HAART. Given that advanced immunosuppression greatly contributed to having a non-modifiable medical reason, it is possible that patients who had advanced immunosuppression documented as a reason for non-treatment were maintained on HAART, but did not experience the full clinical benefits of HAART due to regimen adherence, regimen appropriateness, and/or due to inability for some patients to achieve immune reconstitution[35]. We did not measure these factors in our study. Lastly, we did not evaluate differences in HCV treatment by race/ethnicity, and were therefore, unable to determine if any treatment disparities exist among patients in the UNC clinic.
In summary, reasons for non-treatment did not differentially affect racial and ethnic minorities co-infected with HIV/HCV. Rather, there was a high prevalence of multiple reasons for non-treatment in patients, regardless of racial/ethnic group. The advent of DAAs has undoubtedly revolutionized HCV care, however, there is still a need to recognize and overcome potential treatment barriers in order to improve treatment uptake and eradicate HCV in this patient population.
The authors would like to thank Oksana Zakharova, Sam Stinnette, Christine Sun, Dan-Thanh Nguyen, Heather Moore, and Joshua Toliver for their assistance with data extraction and data collection for this study.
Historically, hepatitis C virus (HCV) treatment rates have been low in patients with human immunodeficiency virus (HIV) co-infection, especially for African-American patients. Identifying the reasons for treatment non-initiation may help improve treatment rates among racially and ethnic minorities.
The authors’ findings suggest that race/ethnicity alone is not predictive of having at least one reason for not initiating therapy. Rather, a key finding of this study was the high prevalence of multiple reasons for non-treatment, regardless of racial/ethnic group.
While low uptake of older HCV antiviral regimens in HIV/HCV co-infected patients, particularly in racial and ethnic minorities, is well documented in the literature, the reasons for low uptake are less clear. This study evaluated reasons cited by the provider for non-initiation of HCV therapy in a cohort of untreated patients.
This study demonstrates that there is a need to recognize and overcome potential treatment barriers in order to improve HCV treatment uptake in this patient population.
This article is of interest to clinicians that manage patients with HIV/HCV coinfection.
P- Reviewer: Larrubia JR, Li ZF, Wang K S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, Thomas DL, Sulkowski MS. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Restrepo A, Johnson TC, Widjaja D, Yarmus L, Meyer K, Clain DJ, Bodenheimer HC, Min AD. The rate of treatment of chronic hepatitis C in patients co-infected with HIV in an urban medical centre. J Viral Hepat. 2005;12:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Hooshyar D, Napravnik S, Miller WC, Eron JJ. Effect of hepatitis C coinfection on discontinuation and modification of initial HAART in primary HIV care. AIDS. 2006;20:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ananthakrishnan AN, McGinley EL, Fangman J, Saeian K. Hepatitis C/HIV co-infection is associated with higher mortality in hospitalized patients with hepatitis C or HIV. J Viral Hepat. 2010;17:720-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Vellozzi C, Buchacz K, Baker R, Spradling PR, Richardson J, Moorman A, Tedaldi E, Durham M, Ward J, Brooks JT. Treatment of hepatitis C virus (HCV) infection in patients coinfected with HIV in the HIV Outpatient Study (HOPS), 1999-2007. J Viral Hepat. 2011;18:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Johnson TL, Toliver JC, Mao L, Oramasionwu CU. Differences in outpatient care and treatment utilization for patients with HIV/HCV coinfection, HIV, and HCV monoinfection, a cross-sectional study. BMC Infect Dis. 2014;14:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Backus LI, Boothroyd DB, Phillips BR, Mole LA. Pretreatment assessment and predictors of hepatitis C virus treatment in US veterans coinfected with HIV and hepatitis C virus. J Viral Hepat. 2006;13:799-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Butt AA, Justice AC, Skanderson M, Good C, Kwoh CK. Rates and predictors of hepatitis C virus treatment in HCV-HIV-coinfected subjects. Aliment Pharmacol Ther. 2006;24:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Schaeffer S, Khalili M. Reasons for HCV non-treatment in underserved African Americans: implications for treatment with new therapeutics. Ann Hepatol. 2015;14:234-242. [PubMed] |
| 11. | Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci. 2007;52:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Mehta SH, Thomas DL, Sulkowski MS, Safaein M, Vlahov D, Strathdee SA. A framework for understanding factors that affect access and utilization of treatment for hepatitis C virus infection among HCV-mono-infected and HIV/HCV-co-infected injection drug users. AIDS. 2005;19 Suppl 3:S179-S189. [PubMed] |
| 13. | Osilla KC, Wagner G, Garnett J, Ghosh-Dastidar B, Witt M, Bhatti L, Goetz MB. Patient and provider characteristics associated with the decision of HIV coinfected patients to start hepatitis C treatment. AIDS Patient Care STDS. 2011;25:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Osilla KC, Ryan G, Bhatti L, Goetz M, Witt M, Wagner G. Factors that influence an HIV coinfected patient’s decision to start hepatitis C treatment. AIDS Patient Care STDS. 2009;23:993-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medical Association of the Infectious Diseases Society of America. [Accessed 2015 Mar 12]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. |
| 16. | Butt AA, Tsevat J, Leonard AC, Shaikh OS, McMahon D, Khan UA, Dorey-Stein Z, Lo Re V 3rd. Effect of race and HIV co-infection upon treatment prescription for hepatitis C virus. Int J Infect Dis. 2009;13:449-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Walley AY, White MC, Kushel MB, Song YS, Tulsky JP. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat. 2005;28:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Adeyemi OM, Jensen D, Attar B, Ghaoui R, Gallagher M, Wolen D, Cotler SJ. Hepatitis C treatment eligibility in an urban population with and without HIV coinfection. AIDS Patient Care STDS. 2004;18:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Backus LI, Boothroyd D, Deyton LR. HIV, hepatitis C and HIV/hepatitis C virus co-infection in vulnerable populations. AIDS. 2005;19 Suppl 3:S13-S19. [PubMed] |
| 20. | Butt AA, Khan UA, Shaikh OS, McMahon D, Dorey-Stein Z, Tsevat J, Lo Re V 3rd. Rates of HCV treatment eligibility among HCV-monoinfected and HCV/HIV-coinfected patients in tertiary care referral centers. HIV Clin Trials. 2009;10:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Pearlman BL. Hepatitis C virus infection in African Americans. Clin Infect Dis. 2006;42:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. [Accessed March 12, 2015]. Available from: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. |
| 23. | Oramasionwu CU, Moore HN, Toliver JC. Barriers to hepatitis C antiviral therapy in HIV/HCV co-infected patients in the United States: a review. AIDS Patient Care STDS. 2014;28:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Robaeys G, Grebely J, Mauss S, Bruggmann P, Moussalli J, De Gottardi A, Swan T, Arain A, Kautz A, Stöver H. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2013;57 Suppl 2:S129-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Wagner GJ, Ryan GW. Hepatitis C virus treatment decision-making in the context of HIV co-infection: the role of medical, behavioral and mental health factors in assessing treatment readiness. AIDS. 2005;19 Suppl 3:S190-S198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Sterling RK, Stravitz RT, Luketic VA, Sanyal AJ, Contos MJ, Mills AS, Shiffman ML. A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans. Clin Gastroenterol Hepatol. 2004;2:469-473. [PubMed] |
| 27. | Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, Belle SH, Hoofnagle JH, Kleiner DE. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | Ioannou GN, Scott JD, Yang Y, Green PK, Beste LA. Rates and predictors of response to anti-viral treatment for hepatitis C virus in HIV/HCV co-infection in a nationwide study of 619 patients. Aliment Pharmacol Ther. 2013;38:1373-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Martel-Laferrière V, Brinkley S, Bichoupan K, Posner S, Stivala A, Perumalswami P, Schiano T, Sulkowski M, Dieterich D, Branch A. Virological response rates for telaprevir-based hepatitis C triple therapy in patients with and without HIV coinfection. HIV Med. 2014;15:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 31. | American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. [Accessed 2015 Jan 15]. Available from: http://www.hcvguidelines.org/full-report-view. |
| 32. | Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, Parish B, Burke T, Pak W, Dunkelberg J. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 807] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 33. | Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207 Suppl 1:S19-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Jordan AE, Masson CL, Mateu-Gelabert P, McKnight C, Pepper N, Bouche K, Guzman L, Kletter E, Seewald RM, Des-Jarlais DC. Perceptions of drug users regarding hepatitis C screening and care: a qualitative study. Harm Reduct J. 2013;10:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Geng EH, Deeks SG. CD4+ T cell recovery with antiretroviral therapy: more than the sum of the parts. Clin Infect Dis. 2009;48:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |