Published online Dec 28, 2016. doi: 10.4254/wjh.v8.i36.1617
Peer-review started: June 30, 2016
First decision: August 5, 2016
Revised: October 25, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: December 28, 2016
Processing time: 181 Days and 22.6 Hours
To investigate the incidence of spontaneous bacterial peritonitis (SBP) in pre-transplant patients and its effect on post transplant mortality and graft failure.
We conducted a retrospective cohort study of patient records from the organ procurement and transplant network data set. Patients were identified by the presence of SBP pre-transplant. Univariate post-transplant survival models were constructed using the Kaplan-Meier technique and multivariate models were constructed using the Cox proportional hazards model. Variables that affected post-transplant graft survival were identified in the SBP population.
Forty-seven thousand eight hundred and eighty patient records were included in the analysis for both groups, and 1966 (4.11%) patients were identified in the data set as having pre-transplant SBP. Patients that had pre-transplant SBP had higher rates of graft loss from recurrent hepatitis C virus (HCV) (3.6% vs 2.0%, P < 0.0001), infections leading to graft loss (1.9% vs 1.3%, P = 0.02), primary non-function (4.3% vs 3.0%, P < 0.0001) and chronic rejection (1.1% vs 0.7%, P = 0.04). Kaplan-Meier survival analysis showed a statistically significant difference in all-cause survival in patients with a history of SBP vs those without (P < 0.0001). Pre-transplant history of SBP was independently predictive of mortality due to recurrent HCV (HR = 1.11, 95%CI: 1.02-1.21, P < 0.017) after liver transplantation.
HCV patients prior to the advent of directing acting anti-viral agents had a higher incidence of pre-transplant SBP than other patients on the liver transplant wait list. SBP history pre-transplant resulted in a higher rate of graft loss due to recurrent HCV infection and chronic rejection.
Core tip: Prevention of spontaneous bacterial peritonitis (SBP) pre-transplant may affect graft outcomes and ultimately patient survival post-transplant. Patients with hepatitis C virus (HCV) in whom therapy is deferred until the time of transplant due to hepatic decompensation, may benefit from expedited treatment if they possess a history of SBP to avoid complications related to HCV recurrence.
- Citation: Shah NL, Intagliata NM, Henry ZH, Argo CK, Northup PG. Spontaneous bacterial peritonitis prevalence in pre-transplant patients and its effect on survival and graft loss post-transplant. World J Hepatol 2016; 8(36): 1617-1622
- URL: https://www.wjgnet.com/1948-5182/full/v8/i36/1617.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i36.1617
According to the Scientific Registry of Transplant Recipients database, since 2005 over 6000 liver transplants have been performed on an annual basis. Hepatitis C virus (HCV) has consistently been the most common indication for liver transplantation and challenging due to the possibility of viral recurrence post-transplant. Recurrent HCV had been a major problem for patients post liver transplantation, but with the new direct acting anti-viral (DAA) therapies, this problem is steadily declining. Even with resolution of this challenge, identifying factors that may accelerate graft loss or damage is still essential.
Originally established for mortality post transjugular intrahepatic portosystemic shunts, the laboratory based Model for End Stage Liver Disease (MELD and MELD-Na) is now the primary measure for liver organ allocation in the United States[1]. Since the use of MELD began in 2002, wait list mortality has significantly decreased as patients are prioritized effectively, however patients on the wait list still suffer from complications of end stage liver disease and portal hypertension such as gastrointestinal bleeding, encephalopathy, ascites, renal failure, and infection[2].
Liver disease patients, with decreased levels of complement proteins and decreased opsonization, live in a relatively immunocompromised state and are at high risk of developing bacterial, viral, or fungal infections[3]. Ascites reportedly occurs in 7% to 23% of hospitalized end stage liver disease patients[4]. An initial episode of spontaneous bacterial peritonitis (SBP) occurs in about 10% of these patients[5]. Infection related to SBP can have severe ramifications in the development of renal failure and mortality of patients while on the transplant wait list.
The utility of MELD in organ allocation is well established, but its extrapolation to post-transplant survival or graft outcomes is still unclear. Further, in the post MELD era, limited studies have investigated the role of SBP on post-transplant outcomes[6]. Therefore, in order to understand the role of infections on post-transplant outcomes, we aimed to investigate the incidence of SBP in pre-transplant patients and its effect on post transplant mortality and graft failure in the era prior to DAA therapy.
We investigated the United States organ procurement and transplant network (OPTN) dataset for liver transplants from February 2002 until November 2009 for liver graft recipients with a reported history of pre-transplant SBP. All patients who eventually underwent liver transplantation were included in the analysis. If the patient did not have a history of SBP or if the question was left blank (or “unknown” was selected) on the Adult Liver Transplant Recipient Registration Worksheet submitted to the United Network for Organ Sharing, the recipient was assumed to not have pretransplant SBP. The population with a history of SBP was compared to those without the history of SBP for multiple pre- and post-transplant characteristics. Recipient etiology of disease was categorized as HCV, hepatitis B, non-alcoholic steatohepatitis or cryptogenic, alcohol alone, cholestatic liver disease, autoimmune, liver malignancy, or other. Etiologies of graft failure included biliary, de novo autoimmune hepatitis, recurrent (non-viral) disease, infection, primary non-function, recurrent viral hepatitis, acute rejection, chronic rejection, and vascular thrombosis. Laboratory (non-exception) MELD scores were used for all recipients.
Demographics, recipient, donor, and surgical characteristics were compared between groups using the χ2 test for categorical variables and the Student t-test for continuous variables. Univariate post-transplant survival models were constructed using the Kaplan-Meier technique and multivariate models were constructed using the Cox proportional hazards model. Variables known to influence post-transplant survival from previous studies or those variables found to be significant in the univariate analysis to a level of less than 0.20 were included in multivariate models using a whole model (non-stepwise) analysis. Because of the finding of a relationship between SBP and graft failure due to recurrent HCV, a multivariate logistic regression model was developed to determine those variables independently predictive of recurrent HCV. No data imputation was used. All statistical testing was two sided and the level of type one error deemed to be statistically significant was assumed to be less than or equal to 0.05. All dataset manipulation and analysis was performed using SAS (version 9.2, Cary, NC, United States). Local institutional review board approval was not required for analysis of this de-identified dataset.
The OPTN data set contained information on 47880 patients transplanted during the study period. The charactersitics of the study population are outlined in Table 1. Of this population, 1966 (4.11%) patients were reported to have a history of pre-transplant SBP. Patients with a history of SBP tended to be older (50.5 mean years in the SBP population vs 48.0 in the non-SBP population, P < 0.0001), male (72.7% vs 65.2%, P < 0.0001), and have a higher MELD score at the time of liver transplantation (25.3 vs 20.3, P < 0.0001). The etiology of liver disease was sigificantly different between those recipients who had pretransplant SBP compared to those that did not. HCV was signficantly more prevelant in the SBP population (41.1% vs 29.5%, P < 0.0001).
| Population characteristic | History of pre-transplant SBP (n = 1966) | No history of SBP (n = 45914) | P-value |
| Recipient age, yr | 50.51 (49.96-51.07) | 47.97 (47.81-48.13) | < 0.0001 |
| Donor age, yr | 38.08 (38.84-39.61) | 38.29 (38.12-38.46) | 0.165 |
| MELD score at transplant | 25.28 (24.83-25.74) | 20.34 (20.25-20.44) | < 0.0001 |
| Male | 1429 (72.69) | 29950 (65.23) | < 0.0001 |
| Ethnicity African American | 149 (7.58) | 4591 (10.00) | 0.027 |
| Etiology of recipient liver disease | < 0.0001 | ||
| Alcohol alone | 318 (16.17) | 4621 (10.06) | |
| Autoimmune | 64 (3.26) | 1149 (2.50) | |
| Cholestatic disease | 113 (5.75) | 3337 (7.27) | |
| Hepatitis B | 69 (3.51) | 1014 (2.21) | |
| Hepatitis C | 809 (41.15) | 13557 (29.53) | |
| Liver malignancy | 126 (6.41) | 6435 (14.02) | |
| NASH/cryptogenic | 175 (8.90) | 4214 (9.18) | |
| Other | 292 (14.85) | 11587 (25.24) | |
| Liver retransplantation | 145 (7.38) | 3711 (8.08) | 0.259 |
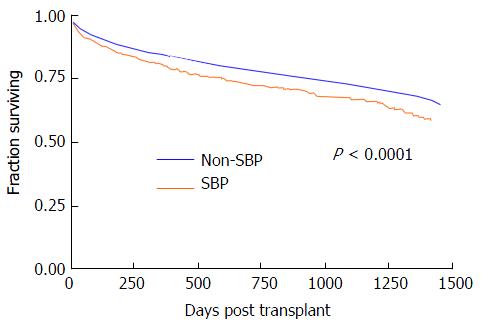
Table 2 shows the distribution and causes of graft failure in the post-transplant time period. While overall graft loss was uncommon, compared to those without a history of SBP, patients that had pre-transplant SBP had higher rates of graft loss from recurrent HCV (3.6% vs 2.0%, P < 0.0001), infections leading to graft loss (1.9% vs 1.3%, P = 0.02), primary non-function (4.3% vs 3.0%, P < 0.0001) and chronic rejection (1.1% vs 0.7%, P = 0.04). In regards to all-cause survival, patients having pre-transplant SBP had worse unadjusted one year post-transplant survival (82.8% vs 86.5%, P < 0.0001) and this difference widened at two years (76.5% vs 81.6%, P < 0.0001). Figure 1 shows the Kaplan-Meier survival analysis. There was a statistically significant difference in all-cause survival in patients with a history of SBP vs those without (P < 0.0001).
| Cause of graft failure | History of pre-transplant SBP (n = 1966) | No history of SBP (n = 45914) | P-value |
| Biliary | 24 (1.22) | 423 (0.92) | 0.176 |
| De novo autoimmune hepatitis | 2 (0.10) | 17 (0.04) | 0.182 |
| Recurrent viral hepatitis | 71 (3.61) | 936 (2.04) | < 0.0001 |
| Infection | 37 (1.88) | 585 (1.27) | 0.020 |
| Primary non-function | 84 (4.27) | 1352 (2.94) | < 0.0001 |
| Recurrent non-viral disease | 26 (1.32) | 547 (1.19) | 0.600 |
| Acute rejection | 18 (0.92) | 289 (0.63) | 0.120 |
| Chronic rejection | 21 (1.07) | 312 (0.68) | 0.042 |
| Vascular thrombosis | 44 (2.24) | 811 (1.77) | 0.122 |
In order to account for multiple factors influencing graft loss in this population, we developed a multivariable logistic regression model including factors known to affect survival rates: Age of recipient, age of donor, MELD score, history of previous transplant, and history of HCV. Table 3 shows the results of this analysis. A pre-transplant history of SBP was found to be an independent risk factor for post-transplant graft failure imparting a 57% increased risk of graft failure (OR = 1.57, 95%CI: 1.22-2.02, P < 0.001). Table 4 shows the results of a mutivariate proprortional hazards survival model predicting death due to recurrent HCV. Once again, a pre-transplant history of SBP was independently predictive of mortality due to recurrent HCV (HR = 1.11, 95%CI: 1.02-1.21, P < 0.017) after liver transplantation.
| Odds ratio | 95%CI | P-value | |
| History of pre-transplant SBP | 1.567 | 1.218-2.017 | < 0.001 |
| Hepatitis C (vs hepatitis B) | 6.777 | 5.864-7.832 | < 0.0001 |
| Previous transplant | 2.349 | 1.923-2.868 | < 0.0001 |
| MELD at transplant | 0.989 | 0.982-0.996 | 0.0014 |
| Age of recipient | 0.992 | 0.986-0.997 | 0.0041 |
| Age of donor | 1.023 | 1.019-1.027 | < 0.0001 |
| Hazard ratio | 95%CI | P-value | |
| History of pre-transplant SBP | 1.112 | 1.019-1.212 | 0.017 |
| Hepatitis C | 1.176 | 1.126-1.228 | < 0.0001 |
| Previous transplant | 1.979 | 1.860-2.107 | < 0.0001 |
| Gender male | 0.983 | 0.941-1.027 | 0.443 |
| Ethnicity non-African American | 0.921 | 0.866-0.980 | 0.010 |
| MELD at transplant | 1.016 | 1.014-1.018 | < 0.0001 |
| Age of recipient | 1.009 | 1.007-1.010 | < 0.0001 |
| Age of donor | 1.009 | 1.008-1.010 | < 0.0001 |
We have shown that HCV patients prior to the advent of DAA agents had a higher incidence of pre-transplant SBP than other patients on the liver transplant wait list. Further, SBP history resulted in a higher rate of graft loss due to recurrent HCV infection and chronic rejection. This group also had an 11.2% greater risk of post-transplant mortality. In a multivariate Cox regression model, SBP was found to be an independent risk factor for post-transplant mortality.
Patients with a diagnosis of active HCV infection at the time of transplant have a known predisposition to graft failure due to HCV recurrence. However, the rate of progression to cirrhosis and graft failure is unpredictable. Certain known risk factors such as the donor’s age, post-transplant CMV infection, HIV co-infection, the use of T cell depleting therapies, pulsed steroids, and other donor risk factors have all been associated with poorer outcomes[7]. SBP has not been studied in this regard. Our results show that a history of pre-transplant SBP may contribute to worse survival and an increased risk of graft failure. As our study shows, 40% of pre-transplant SBP patients in this cohort suffered from HCV, whereas only 30% of a control, non-SBP pre-transplant group suffered from HCV.
Prior treatment regimens, which included interferon, to decrease HCV levels in pre-transplant candidates possessed their own risks and had shown an increase the rate of bacterial infections in these patients[8]. The rate of infection, especially the incidence of SBP in patients not receiving prophylaxis therapy, in Child Pugh B/C patients was higher than matched controls[8]. Several etiologies had been proposed, including the neutropenic and granulo-toxic effect of interferon causing an increase susceptibility to bacterial infections. These studies show an increased risk of infection, including SBP, in HCV patients with poor liver function. With our data showing the deleterious effect of SBP on transplant outcomes, it supports the careful choice of HCV patients that were selected in the past to undergo interferon based therapies pre-transplant and the importance of SBP prophylaxis in this group with proper indications[8,9].
Limited studies from the pre-MELD era did not show that pre-transplant SBP influenced post-transplant outcomes. A study following 100 patients, showed that 32% of patients developed a pre-transplant infection. The infections ranged from SBP (35.6%), blood stream infections (28.9%), cellulitis (13.3%), pneumonia (8.9%), urinary tract infections (6.7%), and other infections (6.7%)[10]. After following this group, the study found that patients with pre-transplant infections were less likely to be transplanted from home and required longer hospital stays, but the mortality at 90 and 180 d post-transplant did not differ significantly compared to individuals without pre-transplant infections. As shown by our Kaplan Meier survival curves in Figure 1, the survival of the two groups from our analysis of the OPTN database seems to be similar, but then diverges significantly starting at day 250. Therefore, even though the previously mentioned study did not find differences in survival, we feel that with longer follow-up this difference could have been statistically significant.
Another single center study, reported that patients with a history of SBP had more severe liver disease as measured by MELD and CTP score, but had similar post-transplant mortality to those without a history of SBP[11]. The patients who developed SBP most commonly suffered from liver disease related to HCV or alcohol. The mean follow-up time period for this study was 3 years. While mortality or infection rates were not affected, these patients were more likely to require abdominal surgery 1 year post transplant for hernia repairs, bleeding, and vascular complications[11]. This study reinforces our findings of the large burden of SBP on pre-transplant cirrhosis patients and the higher rate of SBP in HCV patients. However, the relatively small sample size and inclusion of patients transplanted in the pre-MELD era may not be as applicable to the general population. Our cohort studies the transplants occurring after 2002, and includes a multi-center analysis with almost 48000 patient records.
There are several limitations to our study. One of the major limitations is the incomplete reporting of SBP in the OPTN database. We believe it is safe to assume that reported instances of SBP are accurate and have a clear documented episode recognized by the listing health care provider, which prompted this designation. However, the low rates of patients with SBP pre-transplant as compared to other studies in the literature, raises the speculation of recall bias from reporting centers on patient’s history of SBP. The exact determination of SBP may not be uniform across all centers, and without full accessibility to numbers of SBP episodes, exact cell counts, or ascites fluid analysis we are dependent solely on information from the database. Further, those patients with severe infection due to SBP were likely excluded from transplant listing. Finally, while we may assume that survival statistics and graft loss are accurate in the OPTN database, other post-transplant variables are often incomplete including immunosuppression data and other details regarding HCV recurrence[12]. By using a worst case scenario analysis, if we assume that all patients in the OPTN database without data entered for SBP are included in the control group. Any mortality registered in these patients would only reduce the differences between our groups.
It has been shown that HCV patients being treated with interferon based therapies were at an increased risk of developing SBP[8]. It is unclear if this association is due to the underlying viral hepatitis disease process or related to the treatment regimen that were used. Regardless, stronger surveillance measures and antibiotic prophylaxis pre-transplant SBP may be necessary to improve post-transplant outcomes. Also, the new issue as we transition fully to DAA regimens is the decision to treat cirrhosis patients pre vs post-transplant[13,14]. If patients live in a region with a relatively high average MELD at time of transplant, treating HCV infection pre-transplant may put these patients into MELD “purgatory” - stable MELD that will not increase transplant prioritization, but continued low quality of life[15]. Due to the low rates of drug interactions between the DAAs and immunosuppression, some patients at higher MELD are deferred for therapy until after transplant. From these results and our experience prior to the advent of DAAs, in those HCV patients we deem necessary to treat post-transplant, we should consider prioritizing therapy in those with a history of SBP. Further, we should strive to reduce donor risk factors, minimize pulsed steroids or T cell depleting immunosuppression to prevent post-transplant HCV recurrence and graft failure[16,17]. Over time, we predict the number of transplants performed in the United States stemming from HCV infection to decrease, but the decision to treat these patients around the time of transplant and avoiding SBP will continue to be a challenge.
This study examines the effect of spontaneous bacterial peritonitis in cirrhosis patients on the wait list for liver transplantation. Using the patients identified in the organ procurement and transplant network (OPTN)/United Network for Organ Sharing (UNOS) database, in the era prior to direct acting anti-viral agents, we investigated the effect of spontaneous bacterial peritonitis (SBP) on liver transplant graft loss and post-transplant mortality.
Future studies focused on the prevention of pre-transplant infections, like SBP, and the effect of direct acting anti-viral (DAA) therapies on graft loss and mortality is a potential area to study. Further, as the treatment paradigm for pre vs post-transplant hepatitis C virus (HCV) therapy changes, it may be worthwhile to examine the change it produces in graft loss from HCV complications.
From our knowledge, this is the first article to assess the effect of SBP on post transplant outcomes from the OPTN dataset. Further it provides a collective experience of our outcomes from the pre-DAA therapy era which can be used as a basis for future comparison.
This article has shown the importance of avoiding SBP infections in the pre-transplant population. Aggressive prophylaxis and treatment of this infection may have long term implications with regards to graft survival and mortality.
SBP: Spontaneous bacterial peritonitis - infection found in the ascites fluid in the peritoneum; DAA: Direct acting anti-viral therapies - the new class of HCV medications which directly inhibits replication; OPTN/UNOS: The national organization responsible for managing organ allocation, obtaining individual center’s data on transplantation, and reporting collective outcomes.
This is a good paper.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: El-Shabrawi MHH, Stanciu C S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2067] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 2. | Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1225] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 3. | Ono Y, Watanabe T, Matsumoto K, Ito T, Kunii O, Goldstein E. Opsonophagocytic dysfunction in patients with liver cirrhosis and low responses to tumor necrosis factor-alpha and lipopolysaccharide in patients’ blood. J Infect Chemother. 2004;10:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343-1346. [PubMed] |
| 5. | Cheruvattath R, Balan V. Infections in Patients With End-stage Liver Disease. J Clin Gastroenterol. 2007;41:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Leong J, Huprikar S, Schiano T. Outcomes of spontaneous bacterial peritonitis in liver transplant recipients with allograft failure. Transpl Infect Dis. 2016;18:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009;9:1707-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Carrión JA, Martínez-Bauer E, Crespo G, Ramírez S, Pérez-del-Pulgar S, García-Valdecasas JC, Navasa M, Forns X. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: A retrospective study. J Hepatol. 2009;50:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142-153. [PubMed] |
| 10. | Sun HY, Cacciarelli TV, Singh N. Impact of pretransplant infections on clinical outcomes of liver transplant recipients. Liver Transpl. 2010;16:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Mounzer R, Malik SM, Nasr J, Madani B, Devera ME, Ahmad J. Spontaneous bacterial peritonitis before liver transplantation does not affect patient survival. Clin Gastroenterol Hepatol. 2010;8:623-628.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Gillespie BW, Merion RM, Ortiz-Rios E, Tong L, Shaked A, Brown RS, Ojo AO, Hayashi PH, Berg CL, Abecassis MM. Database comparison of the adult-to-adult living donor liver transplantation cohort study (A2ALL) and the SRTR U.S. Transplant Registry. Am J Transplant. 2010;10:1621-1633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Russo FP, Zanetto A, Burra P. Timing for treatment of HCV recurrence after liver transplantation: the earlier the better. Transpl Int. 2016;29:694-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Suraweera D, Saab EG, Tong MJ, Saab S. Timing of Hepatitis C Antiviral Therapy in Liver Transplant Recipients With Direct-acting Agents. Exp Clin Transplant. 2016;14:243-251. [PubMed] |
| 15. | Carrion AF, Khaderi SA, Sussman NL. Model for end-stage liver disease limbo, model for end-stage liver disease purgatory, and the dilemma of treating hepatitis C in patients awaiting liver transplantation. Liver Transpl. 2016;22:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, Ray C. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005;42:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Bhat I, Mukherjee S. Hepatitis C recurrence after liver transplantation. Panminerva Med. 2009;51:235-247. [PubMed] |