Published online Dec 18, 2016. doi: 10.4254/wjh.v8.i35.1576
Peer-review started: June 28, 2016
First decision: September 7, 2016
Revised: September 26, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: December 18, 2016
Processing time: 173 Days and 4.6 Hours
To investigate and summarize the literature regarding the diagnosis and management of intrahepatic pancreatic pseudocysts (IHPP).
A literature search was performed using PubMed (MEDLINE) and Google Scholar databases, followed by a manual review of reference lists to ensure that no articles were missed. All articles, case reports, systematic reviews, letters to editors, and abstracts were analyzed and tabulated. Bivariate analyses were performed, with significance accepted at P < 0.05. Articles included were primarily in the English language, and articles in other languages were reviewed with native speakers or, if none available, were translated with electronic software when possible.
We found 41 published articles describing 54 cases since the 1970s, with a fairly steady rate of publication. Patients were predominantly male, with a mean age of 49 years. In 42% of published cases, the IHPP was the only reported pseudocyst, but 58% also had concurrent pseudocysts in other extrapancreatic locations. Average IHPP size was 9.5 cm and they occurred most commonly (48%) in the left hemiliver. Nearly every reported case was managed with an intervention, most with a single intervention, but some required up to three interventions. Percutaneous treatment with either simple aspiration or with an indwelling drain were the most common interventions, frequently performed along with stenting of the pancreatic duct. The size of the IHPP correlated significantly with both the duration of treatment (P = 0.006) and with the number of interventions required (P = 0.031). The duration of therapy also correlated with the initial white blood cell (WBC) count (P = 0.048).
Diagnosis of IHPP is difficult and often missed. Initial size and WBC are predictive of the treatment required. With appropriate intervention, most patients achieve resolution.
Core tip: Intrahepatic pancreatic pseudocysts (IHPPs) are rare and the pathophysiology is not entirely clear, but they likely result from proteolytic pancreatic fluid tracking from the pancreas into the surrounding tissue. This fluid may then migrate along planes such as the hepatogastric or hepatoduodenal ligaments, to penetrate the hepatic parenchyma. The initial size of the IHPP and the initial white blood cell are predictive of the number of treatments required and the overall duration of treatment required. Percutaneous approaches have been successful and result in good clinical outcomes.
- Citation: Demeusy A, Hosseini M, Sill AM, Cunningham SC. Intrahepatic pancreatic pseudocyst: A review of the world literature. World J Hepatol 2016; 8(35): 1576-1583
- URL: https://www.wjgnet.com/1948-5182/full/v8/i35/1576.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i35.1576
A pancreatic pseudocyst is an abnormal collection of pancreatic fluid generally due to pancreatitis, exists for at least 4 wk, have a well-defined wall, and contain essentially no solid material[1,2]. They are more commonly seen in patients with alcohol-associated pancreatitis (20%) than with gallstone pancreatitis (6.6%)[3]. Although most commonly immediately peripancreatic or intrapancreatic, they can occur in truly extrapancreatic locations throughout the peritoneal cavity as well as the mediastinum[4,5].
Extrapancreatic pseudocysts are relatively uncommon, estimated to occur in up to 22% of patients with pancreatic pseudocysts[5]. The location depends on where the pancreatic enzymes are released and the path they travel. One of the least common locations for truly extrapancreatic pseudocysts is within the liver[4,5]. Here we describe such a case of an intrahepatic pancreatic pseudocyst (IHPP), and exhaustively review, and analyze, the world literature on IHPP.
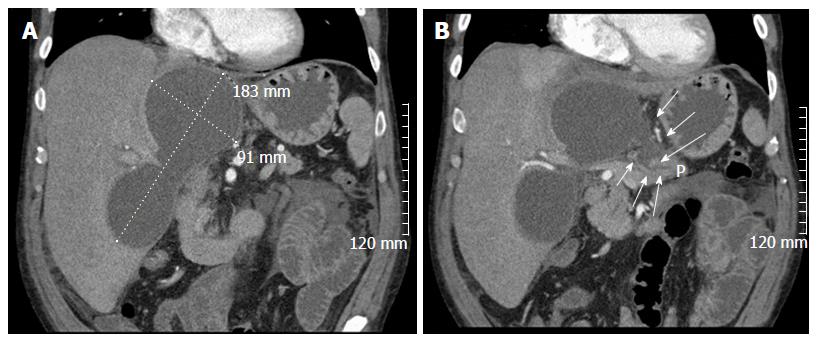
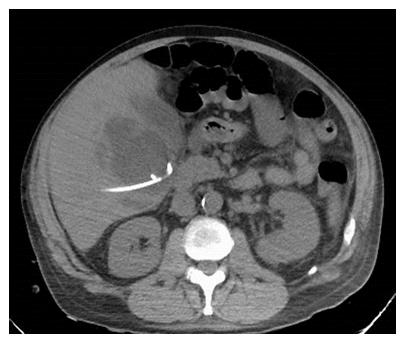
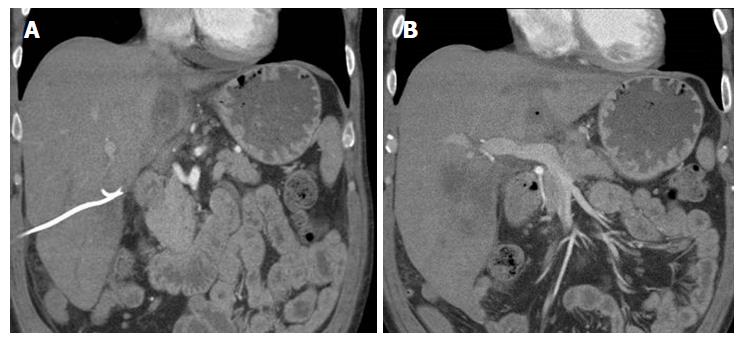
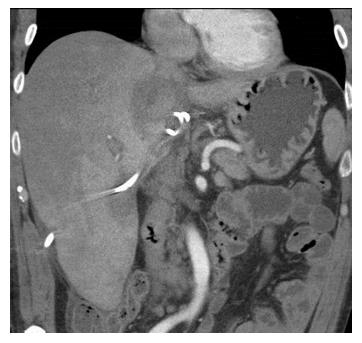
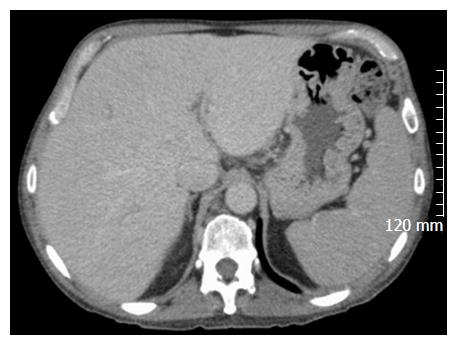
A 56-year-old male with a history of acute alcoholic pancreatitis presented with intermittent chronic abdominal pain. Magnetic resonance imaging revealed a 1.3-cm lesion in the body of the pancreas consistent with a small pancreatic pseudocyst. Computed tomography (CT) 4 mo later revealed a new, 18-cm-long, bilobed fluid collection, wrapped about the hepatoduodenal ligament, not only communicating with the original fluid collection but also insinuating itself deeply into the hepatic parenchyma (Figures 1A), with evidence of communication to the erstwhile intrapancreatic pseudocyst (Figure 1B). Given worsening right upper quadrant abdominal pain, fever, chills, anorexia and significant weight loss, and an unknown age of the new IHPP, percutaneous transhepatic drainage was performed of the more superficial, inferior lobe (Figure 2, fluid was high-amylase and culture-negative), as well as endoscopic pancreatic sphincterotomy, and pancreatic-duct stenting. Follow-up CT one week later revealed a significant reduction in the size of both lobes of the pseudocyst. Three weeks later, however, he developed worsening abdominal fullness, pain and fevers. Repeat CT showed the superficial, inferior lobe to be well drained with the pigtail in place (Figure 3A), but the deeper superior collection was found to be larger containing a small bubble of gas (Figure 3B), with the connecting bridge collapsed. The drain was therefore repositioned into this deeper lobe (Figure 4, culture-positive). Following this procedure, the patient improved clinically and was discharged on 4 more weeks of IV antibiotics. Two weeks later he required aspiration of a small liver abscess (low-amylase, culture-positive), although his pseudocysts remained collapsed. At this point the drain was removed. Interval imaging one month and three months (Figure 5) later revealed no residual fluid collections and he remains drain-free, off antibiotics, gaining weight, and productive at work.
A PubMed and Google Scholar search using key words “pseudocyst”, “pancreatic”, and “intrahepatic” followed by extensive cross-reference review revealed 41 published articles on patients with IHPP. All articles, case reports, systematic reviews which also added a case, letters to editors, and abstracts were analyzed and the data tabulated for comprehensive review and statistical analysis. Bivariate analyses were performed in Statistical Package for the Social Sciences (IBM Corporation, New York, NY, United States). Statistical review of the study was performed by a biomedical statistician.
Articles included were primarily in the English language, but also included French, German, Portuguese, Czech, Korean, and Japanese. Foreign-language articles were reviewed with native speakers or, if native speakers were not available, then the articles were translated with electronic software when possible.
We identified 41 articles containing 54 cases of IHPP in the literature, the earliest identified case being published in 1974 (Table 1). These are primarily single case reports and mini case series but included two relatively thorough review articles which reviewed 26 cases[6] and 23 cases[7]. Two of the cases were notable in that the IHPP formation was thought to be secondary to ectopic pancreatic tissue and not an inflammatory pancreatic process[8,9]. In many of the cases (42%), the IHPP was the only reported pseudocyst, but a significant number also had other concurrent pseudocysts, the most common of which were intra- or peripancreatic pseudocysts (71%).
| Ref. | Year | Language | Clinical features |
| Gautier-Benoit et al[12] | 1974 | French | Abdominal pain, weight loss |
| Cécile et al[10] | 1974 | French | Same patient as published by Gautier |
| Quevedo et al[16] | 1975 | Portuguese | Unknown location, died prior to intervention |
| Siegelman et al[4] | 1980 | English | Edematous pancreas, IHPP aspirated |
| Epstein et al[21] | 1982 | English | 2 patients. Abdominal pain, distension, vomiting, diarrhea, chest pain, ascites |
| Hospitel et al[18] | 1983 | French | Abdominal pain, jaundice, palpable liver |
| Atienza et al[38] | 1987 | French | Weight loss, hepatomegaly, splenomegaly |
| Roche et al[11] | 1987 | French | Abdominal pain, febrile |
| Shimayama et al[39] | 1988 | Japanese | Abdominal pain |
| Lantink et al[22] | 1989 | English | Abdominal pain |
| Schaefer et al[8] | 1989 | German | Abdominal pain, anorexia, DVT/PE |
| Okuda et al[34] | 1991 | English | 2 patients, abdominal pain, anorexia, guarding; 1 resolved spontaneously |
| Slim et al[40] | 1992 | French | Right epigastric pain |
| Aiza et al[37] | 1993 | English | Abd pain, fever, weight loss |
| Hamm et al[5] | 1993 | German | 8 patients |
| Králík et al[9] | 1993 | Czech | Abdominal pain, pruritis, dark urine, light stools |
| Wang et al[27] | 1993 | English | Abdominal pain, weight loss |
| Scappaticci et al[35] | 1995 | English | Abdominal pain, blood per rectum |
| Bayo Poleo et al[41] | 1997 | Spanish | Epigstric pain and tenderness, perotinitis |
| Lederman et al[23] | 1997 | French | Abdominal pain, palpable liver |
| Mehler et al[30] | 1998 | French | 3 patients, abdominal pain, vomiting, diarrhea, jaundice, guarding |
| Mofredj et al[6] | 2000 | English | Abdominal pain, tenderness, guarding, diarrhea |
| Sugiyama et al[42] | 2000 | Japanese | Abdominal pain |
| Shibaski et al[33] | 2002 | English | Abdominal pain, nausea, vomiting, epigastric tenderness |
| Bong et al[43] | 2003 | Korean | Abdominal pain |
| Ancel et al[44] | 2005 | French | Abdominal pain |
| Balzan et al[29] | 2005 | English | Abdominal pain, cystic dystrophy of duodenal wall |
| Bhasin et al[25] | 2005 | English | Abdominal pain |
| Gamanagatti et al[20] | 2006 | English | Abdominal pain, rigid abdomen |
| Les et al[17] | 2006 | English | Vomiting, melena, tachycardia |
| Casado et al[26] | 2007 | English | Abdominal pain, nausea |
| Yi et al[45] | 2008 | Korean | Abdominal pain |
| Al-Ani et al[19] | 2009 | English | Epigastric pain, fever, diaphoresis, guarding, palpable abdominal mass |
| Atia et al[36] | 2009 | English | Abdominal pain, nausea, vomiting, hepatomegaly |
| Chahal et al[13] | 2009 | English | Abdominal pain |
| Guesmi et al[7] | 2009 | English | Abdominal pain, vomiting, weight loss |
| Bhasin et al[24] | 2010 | English | 2 patients, abdominal pain |
| Kibria et al[14] | 2010 | English | Abdominal pain |
| Baydar et al[15] | 2013 | English | Abdominal pain, nausea, vomiting, jaundice |
| Devangan et al[28] | 2015 | English | Abdominal pain, weight loss, anorexia, palpable epigastric mass |
| Martínez-Sanz et al[46] | 2015 | English | Abdominal pain |
| Current case | 2016 | English | Abdominal pain |
Diagnosis of an IHPP can be difficult as it is uncommon and it is not often part of the initial differential of a patient presenting with abdominal pain. Furthermore, if the presentation is delayed, imaging may reveal the IHPP but without inflammatory changes of the pancreas. Abdominal pain was the primary complaint in 91% of cases, but physical exam was generally nonspecific. Only 17% (n = 9) of patients were noted to have a palpable abdominal mass or hepatomegaly, and 15% (n = 8) had peritoneal signs. Initial diagnosis was often via CT (53%) or ultrasound (US) (33%) but nearly every patient in our database (91% of cases where imaging is mentioned) did get a CT scan at some point in the diagnostic or therapeutic process, and CT is generally considered to be the imaging modality of choice for these patients currently. Prior to the widespread availability of the CT scan, however, a significant workup was often done to identify the etiology of a patient’s presentation and in some cases would include a gastrointestinal transit studies, endoscopy, venogram, arteriogram, or exploratory laparotomy where the lesions were finally identified[10-12]. Endoscopy has been used effectively in several cases, not only including initial diagnosis[13], but also with therapeutic intervention[14,15], as discussed further below.
The diagnosis of an IHPP was often delayed with the lesions often initially being mistaken for intrahepatic biliary dilatation, hemangioma, hepatic cyst, pyogenic liver abscess, amebic abscess, biloma, malignancy, echinococcal cyst, or even peritoneal tuberculosis[10,13,16-19]. Although IHPP lesions may be clinically suspected in a patient based on the presentation and radiological imaging, definitive diagnosis was rarely made until analysis of the cystic fluid was performed demonstrating a high amylase content[6,7,17,20,21].
Despite advancements in, and the increasing availability of, imaging modalities, especially the CT scan, the number of reported cases and the type of management techniques have not evolved significantly. There are no widely accepted management guidelines for IHPPs and therefore clinicians have tailored the treatment to the individual patient based on judgment, taking into account many factors, such as underlying etiology, location of the pseudocyst, concomitant lesions, and other patient comorbidities.
Most patients reviewed were symptomatic (91% of reported cases) and required either transcutaneous or surgical intervention. Prior to the development of advanced radiological imaging, more patients underwent a laparotomy and open drainage[10,12,22].
In recent years, however, several less invasive methods have been used to manage IHPPs. Unlike the more commonplace peripancreatic or intrapancreatic pseudocysts, for IHPPs the most common method was percutaneous aspiration or drainage (Table 2) which provided a definitive diagnosis, and was usually well tolerated with minimal complications in these patients[6,7,23]. Simple needle aspiration alone with either US or CT guidance was performed as often as drainage (Table 2). While it aided in the definitive diagnosis by providing an amylase value of the fluid, it was often not completely therapeutic, with 38% of the aspiration-only cases in the literature requiring additional interventions.
| Mean age (range) | Gender (%) | No. of IHPP (% of cases) | Size (range) | Location (%, n) | No. of interventions (%, n)1 | Intervention(%, n)2 | Infection(%, n)3 |
| 49 (15-76) yr | Male (80%) | 1 (67) | 9.5 (3-18) cm | Right lobe (11%, 6) | 0 (9%, 4) | Operative (25%, 15) | Culture positive (16%, 5) |
| 2 (15) | Left lobe (48%, 26) | 1 (60%, 27) | Simple aspiration (28%, 17) | ||||
| Female (20%) | 3 (13) | Right and left lobes (17%, 9) | 2 (24%, 11) | Percutaneous drainage (28%, 17) | Culture negative (84%, 27) | ||
| 4 (4) | Unavailable (24%, 13) | 3 (7%, 3) | Endoscopic (8%, 5) |
In addition to either percutaneous drainage or aspiration, there were several other approaches or adjunctive procedures which have been utilized to manage an IHPP. Although most cases are managed percutaneously or operatively, there is an increasing experience with endoscopic approaches. These have included endoscopic retrograde pancreatography (ERCP) with pancreatic duct stenting, endoscopic transpapillary nasopancreatic drainage, pancreatic duct balloon dilatation, and ERCP-guided aspiration (Table 2)[13-15,24,25]. Bhasin et al[24,25] for example, reviewed 11 patients with atypically located pseudocysts, treated with ERCP and transpapillary nasopancreatic drainage. Placement of a nasopancreatic drain across the disruption was successful in 10 of the 11 patients (90.9%), with resolution of the extrapancreatic pseudocysts in 4-8 wk, with a follow-up period of 3-70 mo.
Operative interventions on patients with IHPPs have been generally reserved for those refractory to, or inappropriate for, nonoperative treatment, such as cases of diagnostic uncertainty[26], rupture[22], or severe infection[27]. All 15 operative interventions (Table 2) to manage these IHPPs were open operations and included partial resection with drainage of the cavity into a Roux limb[8,9,22] and complete resection/excision of the lesion[26,28]. In 10 reports the operation was the first intervention, in 4 reports it was the second intervention, and in one report it was the third intervention (likely, see below). The four second-intervention operations followed percutaneous aspiration in two cases[8,22] and percutaneous drainage in two cases[12,29]. The one third-intervention report[5], however, included 19 extrapancreatic pseudcysts, eight of which were intrahepatic, but it is not clearly reported which if any of those eight IHPP patients underwent which operation. We found no report of postoperative pancreatic fistula development complicating operation.
Although spontaneous resolution of pseudocysts with conservative (noninterventional) management has been reported, complications in these cases included persistent nausea and vomiting, rupture, fistula tract formation, abscess formation if not sterile, or obstruction of the venous or biliary system due to mass effect.
Outcomes were generally very good for patients presenting with these IHPP, with 45% of patients achieved complete resolution of both the cyst and symptoms. In addition, 21% of patients experienced partial resolution of the cyst but total resolution of their symptoms by the time of the follow-up. In our analysis, we noted a statistically significant correlation between the size of the IHPP and both the duration of treatment (P = 0.006) and the number of interventions required (P = 0.031).
Infection of these pseudocysts was reported in 16% of the cases (Table 2), but an organism is not always reported and it is usually unknown whether organisms were part of the original process, or later infected the pseudocyst. Many cases were associated with leukocytosis [mean reported white blood cell (WBC) count of 15000] but without correlation to positive cultures on pseudocyst aspiration. Although there is no correlation between infection and final outcome, we did note a statistically significant positive correlation between the initial WBC count and the duration of treatment (P = 0.048).
There are four reported deaths in the IHPP literature, three of which had undergone a percutaneous drainage procedure[7,16,20,30]. Of note, two of these cases had an infectious component either of the intrahepatic pseudocyst or another concomitant pseudocyst[20,30].
IHPPs frequently present with abdominal pain and are diagnosed with either US or CT imaging. Although the mechanism by which IHPPs develop is not entirely clear, the time to presentation varies tremendously with reports ranging from 6 d to 2 mo[26,29]. It is understood, however, that although a collection of pancreatic fluid is not called a “pseudocyst” until it has been present for at least 4 wk, according to the 2012 revision of the Atlanta classification and definitions by international consensus[1], many of the IHPP reports reviewed here predate that nomenclature. Therefore, we have retained the term “pseudocyst” in these cases.
The process of IHPP formation begins of course with an inflammatory or traumatic episode during which pancreatic duct disruption occurs, resulting in the leakage of pancreatic fluid into the surrounding tissue. Then, once the pancreatic proteolytic enzymes are found outside the pancreatic parenchyma, they may migrate along planes (e.g., hepatogastric, hepatoduodenal) or, by digesting tissue, across planes into the hepatic parenchyma. The end result of this is often observable by imaging and on anatomical-pathological findings, evidencing rupture of the main pancreatic duct and active communication with the intrahepatic collection, as shown in several reported cases[5,21,31-34], and in our case (Figure 1). However, communication does not always persist and in these select cases, may actually be more amenable to conservative management or observation.
The most common extrapancreatic location for pancreatic pseudocyst development is within the lesser sac and may be seen alone or along with an IHPP[4]. An IHPP may be either subcapsular or intraparynchymal with CT imaging of the former characterized by peripheral location and a biconvex appearance[20,29]. They are further characterized by their spatial location in ether the right lobe, left lobe, or involving both lobes. It has been hypothesized that the location of the pancreatic inflammation (e.g., head vs tail) is correlated with the tract the fluid takes and eventual location in the liver of the IHPP with several different paths described[4,5,13,15,16,19,33-37]. However, we did not find this to be a statistically significant correlation. The left lobe was by far the most common location for an IHPP (Table 2) with fluid that likely traveled through the hepatogastric ligament.
Although IHPPs may resolve spontaneously, this is uncommon. As in our case, symptoms, or occasionally diagnostic uncertainly, generally require intervention to prevent complications such as infection, fistula, rupture, and mass-effect obstruction of the biliary or portal systems. Our experience certainly echoes that in the literature, viz., that percutaneous or surgical drainage is usually well tolerated and results in resolution of the pseudocyst and improvement in associated symptoms. Treatment of course depends on the location, size, and effects of the pseudocyst, patient stability, and whether or not the lesion remains in persistent communication with the pancreas. In addition to the primary drainage methods to address the IHPP, several adjunctive procedures have been done, some of which were reportedly novel for this indication. Examples include placement of pancreatic duct stent, endoscopic placement of a nasopancreatic drain, or FNA during endoscopy[13,24,25]. Recurrence of these pseudocysts has not been described in the literature although is certainly possible, and indeed likely, that there were recurrences, the absence of which may be due to lack of longitudinal follow-up, lack of publication, or the rarity of the condition.
Our case was particularly interesting in that the pseudocyst was very large and bilobed, originating around the hepatoduodenal ligament and extending into the liver. The interval presentation between his pancreatitis flair and initial presentation allowed the pancreas to return to fairly normal appearance. This supports the idea that the hepatoduodenal ligament may be a critical structure in the formation of IHPPs.
In conclusion, although IHPPs are often not included in the differential diagnosis of a patient presenting with an intrahepatic lesion, in the right setting and population of patients, it should be considered as an important differential diagnosis. Analysis of this sparse literature has been instructive in revealing a significant correlation between the size of the IHPP and both the duration of treatment and the number of interventions required. The duration of therapy was also correlated with the initial WBC count. These observations may help with prediction of the clinical course in future cases.
The authors have summarized and analyzed the literature on intrahepatic pancreatic pseudocysts (IHPP), to facilitate an appreciation for this study’s relevance and to help understand its significance for the field as a whole.
Current important areas in the research field as related this study include the establishment of a registry.
The key advances in the current study is the recognition that size of the IHPP correlates with both the duration of treatment and the number of interventions required. The duration of therapy was also correlated with the initial white blood cell count.
These observations may help with prediction of the clinical course in future cases.
This is an interesting paper on intrahepatic pseudocyst. Conclusions are interesting. Please elaborate if possible more on the role of endoscopic treatment in such cases.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Furihata M, Somani P S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (45)] |
| 2. | Sabo A, Goussous N, Sardana N, Patel S, Cunningham SC. Necrotizing pancreatitis: a review of multidisciplinary management. JOP. 2015;16:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Cho JH, Kim TN, Kim SB. Comparison of clinical course and outcome of acute pancreatitis according to the two main etiologies: alcohol and gallstone. BMC Gastroenterol. 2015;15:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Siegelman SS, Copeland BE, Saba GP, Cameron JL, Sanders RC, Zerhouni EA. CT of fluid collections associated with pancreatitis. AJR Am J Roentgenol. 1980;134:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Hamm B, Franzen N. [Atypically located pancreatic pseudocysts in the liver, spleen, stomach wall and mediastinum: their CT diagnosis]. Rofo. 1993;159:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Mofredj A, Cadranel JF, Dautreaux M, Kazerouni F, Hadj-Nacer K, Deplaix P, Francois G, Danon O, Lukumbo S, Collot G. Pancreatic pseudocyst located in the liver: a case report and literature review. J Clin Gastroenterol. 2000;30:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Guesmi F, Zoghlami A, Saidi Y, Najeh N, Dziri C. Pancreatic pseudocysts located in the liver: a systematic review of the literature. Tunis Med. 2009;87:801-804. [PubMed] |
| 8. | Schaefer B, Meyer G, Arnholdt H, Hohlbach G. [Heterotopic pancreatic pseudocyst of the liver]. Chirurg. 1989;60:556-558. [PubMed] |
| 9. | Králík J, Pesula E. [A pancreatic pseudocyst in the liver]. Rozhl Chir. 1993;72:91-93. [PubMed] |
| 10. | Cécile JP, Gautier-Benoit G, Luez J, Gaquière A. [False cyst of the pancreas with intrahepatic development]. J Radiol Electrol Med Nucl. 1974;55:51-54. [PubMed] |
| 11. | Roche J, Frairot A, Volle L, Bory R. [Intrahepatic localization of pancreatic pseudocyst. Treatment by simple puncture under ultrasonography]. Presse Med. 1987;16:2230. [PubMed] |
| 12. | Gautier-Benoit C, Luez J, Cécile JP. [Pseudocyst of the pancreas with intra-hepatic development]. Sem Hop. 1974;50:1235-1237. [PubMed] |
| 13. | Chahal P, Baron TH, Topazian MD, Levy MJ. EUS-guided diagnosis and successful endoscopic transpapillary management of an intrahepatic pancreatic pseudocyst masquerading as a metastatic pancreatic adenocarcinoma (with videos). Gastrointest Endosc. 2009;70:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kibria R, Akram S, Ali SA. Successful endoscopic transpapillary management of intrahepatic pancreatic pseudocyst. JOP. 2010;11:41-44. [PubMed] |
| 15. | Baydar B, Cantürk F, Alper E, Aslan F, Akpınar Z, Cengız O, Kandemır A, Ünsal B. Intrahepatic localization of pancreatic pseudocyst: a case report. Turk J Gastroenterol. 2013;24:447-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Quevedo FC, Achilles P, Franco MF. [Pancreatic psuedocysts involving the liver and the spleen. Report of 2 cases]. Rev Hosp Clin Fac Med Sao Paulo. 1975;30:371-374. [PubMed] |
| 17. | Les I, Córdoba J, Vargas V, Guarner L, Boyé R, Pineda V. Pancreatic pseudocyst located in the liver. Rev Esp Enferm Dig. 2006;98:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Hospitel S, Guinot B, Teyssou H, Meyblum J, Tessier JP. [Intrahepatic localization of a pancreatic pseudocyst]. J Radiol. 1983;64:355-358. [PubMed] |
| 19. | Al-Ani R, Ramadan K, Abu-Zidan FM. Intrahepatic pancreatic pseudocyst. N Z Med J. 2009;122:75-77. [PubMed] |
| 20. | Gamanagatti S, Kandpal H, Mishra V. Acute pancreatitis complicated by intrasplenic and intrahepatic pseudocysts. Eur J Radiol Extra. 2006;60: 29-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Epstein BM, Conidaris C. Pseudocysts involving the left lobe of the liver. CT demonstration. Br J Radiol. 1982;55:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Lantink JA, Heggelman BG, Geerdink RA. Intrahepatic rupture of a pancreatic pseudocyst: sonographic and CT demonstration. AJR Am J Roentgenol. 1989;152:1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Lederman E, Cajot O, Canva-Delcambre V, Ernst O, Notteghem B, Paris JC. [Pseudocysts of the left liver: uncommon complication of acute pancreatitis]. Gastroenterol Clin Biol. 1997;21:340-341. [PubMed] |
| 24. | Bhasin DK, Rana SS, Nanda M, Chandail VS, Masoodi I, Kang M, Kalra N, Sinha SK, Nagi B, Singh K. Endoscopic management of pancreatic pseudocysts at atypical locations. Surg Endosc. 2010;24:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Bhasin DK, Rana SS, Chandail VS, Nanda M, Nadkarni N, Sinha SK, Nagi B. An intra-hepatic pancreatic pseudocyst successfully treated endoscopic transpapillary drainage alone. JOP. 2005;6:593-597. [PubMed] |
| 26. | Casado D, Sabater L, Calvete J, Mayordomo E, Aparisi L, Sastre J, Lledo S. Multiple intrahepatic pseudocysts in acute pancreatitis. World J Gastroenterol. 2007;13:4655-4657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Wang SJ, Chen JJ, Changchien CS, Chiou SS, Tai DI, Lee CM, Kuo CH, Chiu KW, Chuah SK. Sequential invasions of pancreatic pseudocysts in pancreatic tail, hepatic left lobe, caudate lobe, and spleen. Pancreas. 1993;8:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Devangan M, Sonkar SK, Sharma S. A rare case of pancreatic pseudocyst involving liver and spleen. Int J Med Sci Res Pract. 2015;2:150-152. |
| 29. | Balzan S, Kianmanesh R, Farges O, Sauvanet A, O’toole D, Levy P, Ruszniewski P, Ogata S, Belghiti J. Right intrahepatic pseudocyst following acute pancreatitis: an unusual location after acute pancreatitis. J Hepatobiliary Pancreat Surg. 2005;12:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Mehler CI, Soyer P, Kardache M, Pelage JP, Boudiaf M, Panis Y, Abitbol M, Hamzi L, Rymer R. [Computed tomography of intrahepatic pancreatic pseudocysts]. J Radiol. 1998;79:751-755. [PubMed] |
| 31. | Goyal S, Raju R, Yadav S. Pancreatic pseudocyst of gastrohepatic ligament: a case report and review of management. JOP. 2012;13:439-442. [PubMed] |
| 32. | Gould L, Khademi M, Guarnaccia M, Patel NK. Pancreatic pseudocyst simulating an intrahepatic mass. Am J Gastroenterol. 1979;72:75-78. [PubMed] |
| 33. | Shibasaki M, Bandai Y, Ukai T. Pancreatic pseudocyst extending into the liver via the hepatoduodenal ligament: a case report. Hepatogastroenterology. 2002;49:1719-1721. [PubMed] |
| 34. | Okuda K, Sugita S, Tsukada E, Sakuma Y, Ohkubo K. Pancreatic pseudocyst in the left hepatic lobe: a report of two cases. Hepatology. 1991;13:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Scappaticci F, Markowitz SK. Intrahepatic pseudocyst complicating acute pancreatitis: imaging findings. AJR Am J Roentgenol. 1995;165:873-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Atia A, Kalra S, Rogers M, Murthy R, Borthwick TR, Smalligan RD. A wayward cyst. JOP. 2009;10:421-424. [PubMed] |
| 37. | Aiza I, Barkin JS, Casillas VJ, Molina EG. Pancreatic pseudocysts involving both hepatic lobes. Am J Gastroenterol. 1993;88:1450-1452. [PubMed] |
| 38. | Atienza P, Couturier D, Grandjouan S, Guerre J, Bettan L, Chapuis Y, Vasile N. [Intrahepatic collections of fluid of pancreatic origin. A case]. Presse Med. 1987;16:1195-1198. [PubMed] |
| 39. | Shimayama T, Katsuki T, Kosai S, Yogi Y. [A case of pancreatic pseudocyst intruded into the right lobe of the liver]. Nihon Shokakibyo Gakkai Zasshi. 1988;85:1708-1711. [PubMed] |
| 40. | Slim K, Hendaoui L, Larabi B. [Multiple intrahepatic pseudocysts in acute pancreatitis]. Gastroenterol Clin Biol. 1992;16:902. [PubMed] |
| 41. | Bayo Poleo R, Zaheri M, Córdoba López A. [Bleeding intrahepatic cyst in a patient with chronic pancreatitis]. Gastroenterol Hepatol. 1997;20:46-47. [PubMed] |
| 42. | Sugiyama H, Sasaki M, Asano T, Kawai H, Kato T, Moriwaki H, Kuroiwa M. [A case of pancreatic pseudocyst intruded into the left lobe of the liver]. Nihon Shokakibyo Gakkai Zasshi. 2000;97:605-611. [PubMed] |
| 43. | Bong HK, Kim JK, Lee SY, Lee JS, Lee MS, Kim JH, Cho SW, Shim CS. A case of chronic pancreatitis complicated by intrahepatic pseudocyst. Korean J Gastroenterol. 1993;25: 1375-1380. |
| 44. | Ancel D, Lefebvre M, Peyrin-Biroulet L, Chone L, Sido A, Regent D, Bigard MA. [Pancreatic pseudocysts of the right hepatic lobe during acute biliary pancreatitis]. Gastroenterol Clin Biol. 2005;29:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Yi CY, Na GJ, Baek HC, Kim JH, Bae SH, Kim DH, Je IS, Kwon BP. [A case of intrahepatic pseudocyst complicating acute pancreatitis]. Korean J Gastroenterol. 2008;51:56-59. [PubMed] |
| 46. | Martínez-Sanz N, González-Valverde FM, Vicente-Ruiz M, Pastor-Pérez P, Ruiz-Marín M, Albarracín-Marín-Blázquez A. Intrahepatic pancreatic pseudocyst: a case report. Rev Esp Enferm Dig. 2015;107:249-250. [PubMed] |