Published online Dec 8, 2016. doi: 10.4254/wjh.v8.i34.1497
Peer-review started: July 4, 2016
First decision: August 10, 2016
Revised: September 2, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 8, 2016
Processing time: 157 Days and 9.3 Hours
To determine the accuracy of fractional excretion of sodium (FeNa) in the diagnosis of hepatorenal syndrome (HRS).
Eighty-eight liver transplantation candidates with renal dysfunction and/or proteinuria were included in the study sample. The baseline characteristics of the patients were obtained. All the 88 patients underwent iothalamate glomerular filtration rate testing, 24-h urine collection for urinary sodium and protein excretions, random urine for sodium and creatinine testing, and percutaneous kidney biopsy. FeNa was calculated using the equation [(urine sodium × serum creatinine)/(serum sodium × urine creatinine)] × 100%. Diuretic use was recorded among the participants. Patients on renal replacement therapy were not included in the original sample.
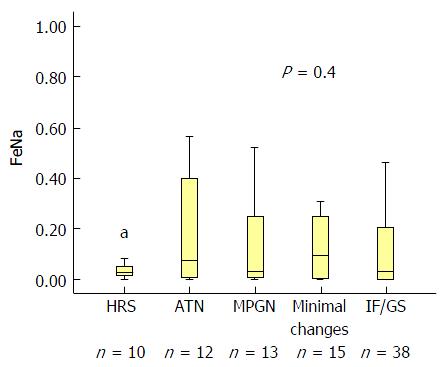
Seventy-seven (87%) of the 88 patients had FeNa < 1%. FeNa < 1% was present in 10/10, 10/12, 11/13, 12/15 and 34/38 in patients with HRS, acute tubular necrosis, membranoproliferative glomerulonephritis, minimal histological findings (≤ 30%) and advanced (≥ 30%-40%) interstitial fibrosis and/or glomerulosclerosis, respectively (P = 0.4). FeNa < 1% was 100% sensitive and 14% specific in diagnosing HRS. Receiver operating characteristic curve confirmed the poor accuracy of FeNa < 1% in diagnosing HRS (area under the curve = 0.58, P = 0.47). Calculated positive predictive value and negative predictive value for FeNa < 1% in HRS diagnosis were 46% and 100%, respectively. When used as a continuous variable, FeNa did not correlate with kidney biopsy findings (P = 0.41).
FeNa < 1% was common in cirrhotic patients with renal dysfunction and it did not differentiate between HRS and other causes of renal pathologies. HRS diagnosis should be avoided in patients with FeNa > 1%.
Core tip: In this retrospective analysis of patients with advanced end-stage liver disease, we describe three main concepts. First, our data indicates that fractional excretion of sodium (FeNa) < 1% is a common finding in this group of patients irrespective of the etiology of their renal dysfunction. Second, our study suggests that FeNa < 1% cannot differentiate hepatorenal syndrome (HRS) from other causes of renal pathology. And third, we statistically measured the performance of FeNa < 1% in patients with HRS using kidney biopsy findings as golden diagnostic standard.
- Citation: Alsaad AA, Wadei HM. Fractional excretion of sodium in hepatorenal syndrome: Clinical and pathological correlation. World J Hepatol 2016; 8(34): 1497-1501
- URL: https://www.wjgnet.com/1948-5182/full/v8/i34/1497.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i34.1497
Kidney dysfunction is common in patients with end-stage liver disease (ESLD). It is estimated that nearly 20% to 25% of patients with ESLD will have some type of kidney dysfunction during their course of disease[1]. The spectrum of kidney disease can range from reversible kidney injury like acute kidney injury [whether prerenal azotemia or acute tubular necrosis (ATN)] to permanent and chronic kidney damage (CKD) and fibrosis. CKD could be either from causes unrelated to the liver disease such as diabetes, or related to the liver disease such as hepatitis C virus infection.
Hepatorenal syndrome (HRS) is a form of prerenal azotemia that is unique to patients with liver cirrhosis and ascites that is diagnosed after excluding other causes of renal impairment[2,3]. HRS occurs in nearly 8% of patients with ascites and 75% of patients with HRS will require dialysis[2].
Early studies have demonstrated that kidneys procured from HRS patients had normal renal histology and exhibited immediate allograft function after transplantation[4]. Pathophysiologically, patients with HRS experience severe vasoconstriction that involves various vascular beds including the kidneys with subsequent reduction in renal blood flow, glomerular filtration rate, and reciprocal increase in proximal tubular sodium re-absorption[5]. Indeed, urinary sodium excretion is low in patients with HRS[6,7]. Classic teaching has utilized FeNa cut-off of < 1% or > 1% to differentiate between prerenal (including HRS) and intrinsic renal dysfunctions especially those caused by ATN. While this FeNa cut-off has been used as discriminatory tool discriminatory tool in cirrhotic patients presenting with elevated serum creatinine, its accuracy has not been tested in diagnosing HRS against a gold standard such as kidney biopsy. Our program utilizes kidney biopsy in evaluating selected liver transplant candidates with renal dysfunction.
In this study, we sought to determine the accuracy of FeNa < 1% in HRS diagnosis using histological data as a gold standard for comparison.
After obtaining Mayo Clinic Institutional Review Board approval, we retrospectively reviewed the electronic medical record of 88 patients with ESLD who were undergoing LT evaluation at Mayo Clinic in Jacksonville, Florida. All 88 patients had renal dysfunction defined as an iothalamate glomerular filtration rate (GFR) of less than 40 mL/min per 1.73 m2 or the presence of proteinuria or hematuria. Patients with fulminant hepatic failure were not included.
All study patients had undergone renal biopsy after a stabilization of platelets count of less than 50000 × 106/L with platelets transfusion, and an international normalized ratio of less than 1.5 by fresh frozen plasma transfusion.
Data for GFR and 24-h urine collection for urinary sodium excretion, protein excretion, random urine sodium, and random creatinine were collected. Patients who underwent renal replacement therapy within the last 6 wk prior to evaluation were not included in the original sample, as their serum and urine electrolyte values will be modulated by dialysis. Diuretic use was recorded.
Kidney biopsy specimens were assessed using light microscopy, immunofluorescence and electron microscopy and were interpreted by an experienced nephro-pathologist as previously described[8]. Patients were grouped according to the primary kidney biopsy diagnosis into five main groups: HRS (normal kidney pathology), ATN, membranoproliferative glomerulonephritis (MPGN), minimal histological changes [defined as 10%-30% interstitial fibrosis (IF) and/or glomerulosclerosis (GS)] and advanced (> 30%) IF and/or GS.
FeNa was calculated using the equation: [(urine sodium x serum creatinine)/(serum sodium x urine creatinine)] × 100.
Patients with fulminant hepatic failure were not included in this analysis.
The primary outcome was to calculate the sensitivity, specificity, positive predictive value (PPV) and (NPV) of FeNa < 1% in diagnosing HRS. Secondary outcome was to measure the correlation between FeNa as a continuous variable and the kidney biopsy diagnosis.
Using SPSS software version 22, (Cary, NC), we analyzed the data of the 88 LT patients. Continuous variables were presented as mean ± SD; categorical variables were presented as number (%). The sensitivity, specificity, PPV and NPV in HRS diagnosis were calculated. A receiver operating characteristic (ROC) curve was constructed to assess the diagnostic accuracy of FeNa < 1% in diagnosing HRS.
Table 1 summarizes the baseline characteristics of the 88 liver transplantation patients. The mean ± SD age of the cohort was 60 ± 7 years and the majority were of male gender (65%). Seventy two percent of the patients were using at least one diuretic at time of FeNa calculation. Out of the 88 LT candidates, 77 (87%) had FeNa < 1%. As demonstrated in Table 1, FeNa < 1% was present in 10/10, 10/12, 11/13, 12/15 and 34/38 in patients with HRS, ATN, MPGN, minimal histological changes (10%-30% fibrosis) and advanced (≥ 30%) IF and/or glomerulosclerosis (GS), respectively (P = 0.4).
| Variable | |
| Age | 60 ± 7 |
| Male gender | 57 (65) |
| Cause of ESLD | |
| HCV infection | 40 (45) |
| NASH | 13 (15) |
| Alcoholic cirrhosis | 12 (14) |
| Cryptogenic cirrhosis | 10 (11) |
| Other | 13 (15) |
| MELD score | 17.5 ± 5.8 |
| History of diabetes | 35 (40) |
| History of hypertension | 40 (45) |
| Iothalamate GFR mL/min per 1.73 m2 | 28 ± 14 |
| Serum creatinine (mg/dL) | 1.9 ± 0.9 |
| Serum Na (mEq/dL) | 137 ± 5 |
| 24-h urinary protein excretion (mg/d) | 87 (0-13625) |
| 24-h urinary Na excretion (mEq/d) | 56 (0-238) |
| 24-urine protein > 150 mg/d | 35 (40) |
| Hematuria | 40 (45) |
| Diuretic use | 64 (72) |
| FeNa < 1 | 77 (87) |
| Kidney Biopsy | |
| HRS | 10 (11) |
| ATN | 12 (14) |
| MPGN | 13 (15) |
| Minimal histology | 15 (17) |
| ≥ 30%-40% IF/GS | 38 (43) |
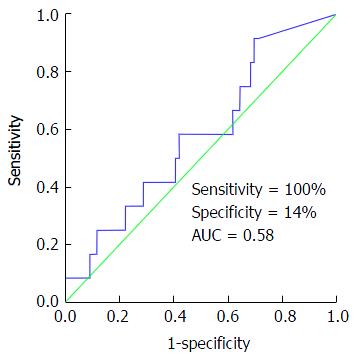
FeNa < 1% was 100% sensitive and 14% specific in diagnosing HRS. Calculated PPV and NPV for FeNa < 1% in the setting of HRS were 46% and 100%, respectively. Figure 1 represents the result of the ROC curve assessing the performance of FeNa < 1% in HRS diagnosis. As demonstrated in Figure 1, FeNa < 1% showed poor accuracy in diagnosing HRS with an area under the curve (AUC) of 0.58, P = 0.47. To assess the effect of diuretic use on the performance of FeNa < 1% in HRS diagnosis, we compared urinary sodium indices between patients on diuretics (n = 64) and those not on diuretic (n = 24) treatment. There was no observed difference in the 24-h urinary sodium excretion, FeNa (as a continuous variable) and number of patients with FeNa < 1% between those using and not using diuretics at time of FeNa calculation (P > 0.3 for all). Also, the sensitivity (100%), specificity (12.5%), PPV (14%) and NPV (100%) of FeNa < 1% in diagnosing HRS did not differ when patients not using diuretics were excluded from the analysis.
We then correlated FeNa as continuous variable with the kidney biopsy finding. Although FeNa was lowest in HRS patients it did not differentiate between HRS and other renal pathologies (Figure 2).
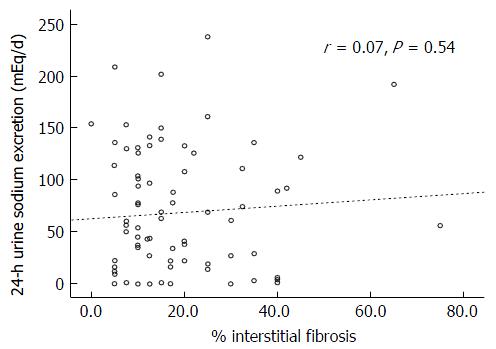
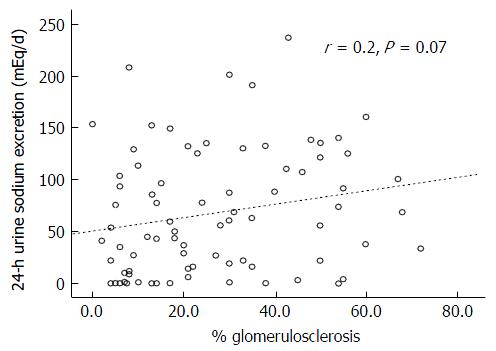
There was also no correlation between the degrees of the IF (r = 0.07, P = 0.54) or GS (r = 0.2, P = 0.07) and the 24-h urinary sodium excretion (Figures 3 and 4, respectively). This lack of correlation was not affected by diuretic use (data not shown).
Our study indicates that the majority of ESLD patients with renal dysfunction of unknown etiology or duration have a low 24-h urinary sodium excretion and a FeNa < 1% irrespective of renal pathology on kidney biopsy. Using kidney biopsy findings as a gold standard, we were able to determine the accuracy of FeNa < 1% in diagnosing HRS. Our results defined the sensitivity, specificity, PPV and NPV of FeNa < 1% in HRS as 100%, 14%, 46% and 100%, respectively. These results suggest that a FeNa > 1% excludes HRS diagnosis but a FeNa < 1% is not useful in diagnosing HRS.
Previous studies confirm that cirrhotic patients without renal dysfunction have low urinary sodium excretion rates and increased renal tubular reabsorption due to the activation of various neuro-hormonal mechanism and subsequent increase in renal tubular sodium re-absorption[5,9]. In patient with renal dysfunction however, low urinary sodium excretion implies maintained tubular integrity and favors either prerenal azotemia or HRS diagnosis[5]. Results of this study challenge this understanding. Our findings indicated that urinary sodium excretion was similarly low in cirrhotic patients with renal dysfunction due to tubular injury (ATN), glomerular disease (MPGN), irreversible renal damage (advanced IF/GS) or normal renal histology (HRS). There was also no correlation between the degree of IF or GS and the 24-h urinary sodium excretion. These results indicate that a low urinary sodium excretion is present in the majority of cirrhotic patients with renal dysfunction and does not reflect an intact renal tubular integrity but rather reflects the avid renal sodium retention state in these patients irrespective of the cause of renal dysfunction. It is also important to mention that diuretic use did not affect urinary sodium indices or the performance of FeNa < 1% in HRS diagnosis which support the avid sodium retention state in these patients with advanced cirrhosis.
FeNa is more sensitive than urinary sodium concentration in detecting prerenal causes of renal dysfunction as the serum sodium level is factored into the equation. Previously published reports, however, indicated that although FeNa was lowest in patients with HRS, FeNa did not differentiate HRS and other causes of renal dysfunction including ATN[6,10]. These studies did not include kidney biopsy evidence of HRS (normal renal histology) and the diagnosis of HRS was made solely on clinical grounds[10,11].
The main strength of the current study is that we used kidney biopsy findings to diagnose HRS, ATN and other renal pathologies. By using histological data, we were able to confirm that FeNa < 1% is present in almost 90% of cirrhotic patients with renal dysfunction. Although FeNa was lowest in HRS and FeNa < 1% was present in 100% of patients with HRS, FeNa did not differentiate between HRS and other causes of acute or chronic renal dysfunction. Of note a previous prospective study demonstrated that FeNa < 1% was present in only 0% to 4% of patients with ATN and no history of liver disease[12]. In contrast, in the current study 10 of 12 patients (83%) with biopsy evidence of ATN had a FeNa < 1%. Previous studies also demonstrated that FeNa < 1% had a sensitivity and specificity of 58%-78% and 75%-81% in diagnosing prerenal azotemia, respectively, in subjects without liver disease and varied according to diuretic use[13]. The results of the current study indicate that the sensitivity and specificity of FeNa < 1% in the diagnosis of HRS is much different than in non-cirrhotic patients with prerenal azotemia and that they are not affected by diuretic use. This difference is likely due to the intense renal vasoconstriction manifesting in cirrhotic patients with subsequent increase in renal sodium reabsorption and the diuretic resistant state these patients develop with worsening liver disease.
The performance of FeNa < 1% in diagnosing HRS was overall poor but the test had high sensitivity and high NPV (both 100%), indicating that in patients with negative test results (i.e., FeNa > 1%) HRS diagnosis should be excluded.
The current study is limited by the small number of patients particularly those with normal biopsy findings and HRS diagnosis which could have affected the results. Another important limitation of the study is the lack of detailed information on dietary sodium intake and doses and class of the diuretic medications used. We also measured FeNa at a single time point prior to the kidney biopsy to minimize the selection bias. Future studies should address if serial measurements of FeNa in a given patient will confer similar results.
In conclusion, the current study indicates that FeNa < 1% is common finding in patients with ESLD and renal dysfunction and has a poor accuracy in diagnosing HRS. Our results also indicate that HRS diagnosis should be avoided in patients with FeNa > 1%. Further studies with large number of patients are needed to confirm the findings of this study.
Kidney dysfunction is common in patients with end-stage liver disease (ESLD). It is estimated that nearly 20% to 25% of patients with ESLD will have some type of kidney dysfunction during their course of disease.
Classic teaching has utilized fractional excretion of sodium (FeNa) cut-off of < 1% or > 1% to differentiate between prerenal [including hepatorenal syndrome (HRS)] and intrinsic renal dysfunctions especially those caused by acute tubular necrosis. While this FeNa cut-off has been a useful discriminatory tool in cirrhotic patients presenting with elevated serum creatinine, its accuracy has not been tested in diagnosing HRS against a gold standard such as kidney biopsy.
The authors program utilizes kidney biopsy in evaluating selected liver transplant candidates with renal dysfunction.
The authors measured FeNa at a single time point prior to the kidney biopsy to minimize the selection bias. Future studies should address if serial measurements of FeNa in a given patient will confer similar results.
In patients with end stage cirrhosis and renal dysfunction (glomerular filtration rate < 40) the estimated FeNa < 1 could not discriminate between HRS and intrarenal kidney injury. The findings are quite relevant, since FeNa is used most often to exclude intrarenal disease in cirrhotic patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chok KSH, Lenz K S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Huschak G, Kaisers UX, Laudi S. [Hepatorenal syndrome]. Anaesthesist. 2013;62:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Mohanty A, Garcia-Tsao G. Hyponatremia and Hepatorenal Syndrome. Gastroenterol Hepatol (NY). 2015;11:220-229. [PubMed] |
| 4. | Iwatsuki S, Popovtzer MM, Corman JL, Ishikawa M, Putnam CW, Katz FH, Starzl TE. Recovery from “hepatorenal syndrome” after orthotopic liver transplantation. N Engl J Med. 1973;289:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 152] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Cárdenas A, Arroyo V. Mechanisms of water and sodium retention in cirrhosis and the pathogenesis of ascites. Best Pract Res Clin Endocrinol Metab. 2003;17:607-622. [PubMed] |
| 6. | Bataller R, Ginès P, Guevara M, Arroyo V. Hepatorenal syndrome. Semin Liver Dis. 1997;17:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Wadei HM, Geiger XJ, Cortese C, Mai ML, Kramer DJ, Rosser BG, Keaveny AP, Willingham DL, Ahsan N, Gonwa TA. Kidney allocation to liver transplant candidates with renal failure of undetermined etiology: role of percutaneous renal biopsy. Am J Transplant. 2008;8:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Bichet DG, Van Putten VJ, Schrier RW. Potential role of increased sympathetic activity in impaired sodium and water excretion in cirrhosis. N Engl J Med. 1982;307:1552-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 235] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89:47-50. [PubMed] |
| 13. | Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |