Published online Nov 18, 2016. doi: 10.4254/wjh.v8.i32.1402
Peer-review started: March 18, 2016
First decision: April 19, 2016
Revised: May 6, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: November 18, 2016
Processing time: 245 Days and 7.7 Hours
To make efficacy and safety comparison of telbivudine-raodmap and tenofovir-roadmap in hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) patients.
This was the first prospective, randomised, two-arm, open-label, non-inferiority study in HBeAg-negative CHB patients that compared telbivudine and tenofovir administered as per roadmap concept. Patients were treated up to 24 wk and, depending on virologic response, continued the same therapy or received add-on therapy up to 104 wk. Eligible patients received an additional 52 wk of treatment in the extension period (i.e., up to 156 wk). Patients who developed virologic breakthrough (VB) while on monotherapy also received add-on therapy. The primary efficacy endpoint was the rate of patients achieving hepatitis B virus (HBV) DNA < 300 copies/mL at week 52. Secondary efficacy endpoints included the rates of HBV DNA < 300 and < 169 copies/mL, HBV DNA change from baseline, alanine aminotransferase normalisation, hepatitis B surface antigen (HBsAg) loss, HBsAg seroconversion, VB, and emergence of resistance at various timepoints throughout the study. Safety and estimated glomerular filtration rate (eGFR) were also analysed.
A total of 241 patients were randomised. Non-inferiority of telbivudine arm to tenofovir arm was demonstrated at week 52 (± 7 d window), with over 91% of patients in each treatment arm achieving HBV DNA level < 300 copies/mL. Both arms were similar in terms of key secondary efficacy variables at weeks 104 and 156. The percentage of patients achieving HBV DNA < 300 copies/mL remained high and was similar in the telbivudine and tenofovir arms at both weeks 104 and 156. Over 82% of patients in both arms achieved alanine aminotransferase normalisation at week 52, and this percentage remained high at weeks 104 and 156. Telbivudine treatment progressively reduced serum HBsAg levels from baseline while no change was reported in quantitative HBsAg during therapy with tenofovir. Both treaments showed acceptable safety profiles. The telbivudine arm showed eGFR improvement unlike the tenofovir arm.
Efficacy was shown for both telbivudine-roadmap and tenofovir-roadmap regimens in HBeAg-negative CHB patients over 156 wk. Telbivudine arm was associated with renal improvement.
Core tip: This was the first prospective, randomised, non-inferiority study in hepatitis B e antigen-negative chronic hepatitis B patients that compared telbivudine and tenofovir administered as per roadmap concept. Both treatments based on the roadmap approach were effective over a 156 wk treatment period. Non-inferiority of telbivudine arm to tenofovir arm was demonstrated at week 52, with over 91% of patients in each treatment arm achieving hepatitis B virus DNA level < 300 copies/mL. Both treaments showed acceptable safety profiles. Moreover, telbivudine showed an improvement in estimated glomerular filtration rate from baseline.
- Citation: Krastev Z, Petrova D, Kotzev I, Celen MK, Mendelson M, Chandra R, Pandey P, Hamed K. Telbivudine vs tenofovir in hepatitis B e antigen-negative chronic hepatitis B patients: OPTIMA roadmap study. World J Hepatol 2016; 8(32): 1402-1413
- URL: https://www.wjgnet.com/1948-5182/full/v8/i32/1402.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i32.1402
Approximately 240-400 million people worldwide are chronically infected with hepatitis B virus (HBV), with a wide variation of prevalence among countries, and more than 780000 people die every year due to acute or chronic hepatitis B (CHB)[1-3]. Although CHB may be treated with interferon or nucleos(t)ide analogue (NA) antivirals, emergence of resistance due to prolonged NA therapy or incomplete suppression of HBV still remains an important concern[4]. Several studies have suggested that the use of response-guided add-on therapy is associated with a higher rate of virologic response and reduced antiviral resistance as compared to sequential monotherapy[5,6].
Early virologic response has been used as a guide to predict better outcomes and to reduce the risk of antiviral resistance[7,8]. As previously reported[9,10], the roadmap concept uses early virologic response at week 24 to individualize ongoing management of CHB patients. Patients with a complete response at week 24 can remain on their initial therapy, whereas treatment modification that may include the addition of a second drug is done for those with an inadequate virologic response. This strategy is relevant mainly in patients receiving NA with a low genetic barrier to resistance (clevudine, emtricitabine, lamivudine, telbivudine)[10]. In hepatitis B e antigen (HBeAg)-positive CHB patients treated with telbivudine, a response-guided treatment optimization strategy with telbivudine based on the roadmap concept has been demonstrated to improve the clinical outcomes of patients with a suboptimal antiviral response[11,12].
The aim of this study, OPTIMA, was to assess the efficacy and safety of telbivudine and tenofovir regimens, when administered using the roadmap concept, in HBeAg-negative patients with CHB. This was the first study that compared efficacy of the 2 regimens in a prospective manner. The safety of the combination of telbivudine and tenofovir, for which limited data are currently available, was also evaluated.
OPTIMA was a prospective, randomised, 2-arm, open-label study (ClinicalTrials.gov ID: NCT01379508) that enrolled patients between February 2011 and October 2012 in 8 countries (Austria, Bulgaria, Germany, Greece, Italy, Russia, Spain and Turkey). This study was approved by the Institutional Review Board at each participating centre, and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from each patient before enrolment.
Eligible patients were randomised via an interactive voice response system in a 1:1 ratio to either telbivudine arm (600 mg/d) or tenofovir arm (300 mg/d) (Figure 1). Randomisation was stratified by the screening HBV DNA level (< 7 log10 copies/mL or ≥ 7 log10 copies/mL) and alanine aminotransferase (ALT) level [< 3 × upper limit of normal (ULN) or ≥ 3 × ULN].
This study used the response-guided add-on strategy (roadmap concept). For patients with HBV DNA ≥ 300 copies/mL (≥ 51 IU/mL) at week 24, tenofovir was added to telbivudine by week 26 in the telbivudine arm, and telbivudine was added to tenofovir by week 26 in the tenofovir arm. For patients with HBV DNA < 300 copies/mL at week 24, telbivudine and tenofovir monotherapies in the respective arms were continued. Patients who developed virologic breakthrough (VB) while on monotherapy received add-on therapy. However, patients who developed VB after week 24 while on combination therapy were discontinued from the study.
Eligible patients were male or female ≥ 18 years of age, with detectable hepatitis B surface antigen (HBsAg) for ≥ 6 mo, HBeAg-negative with positive hepatitis B e antibody, available liver histology report within 12 mo before screening compatible with CHB (patients without evaluable liver histology were eligible if they had clinical evidence of compensated liver cirrhosis or non-invasive methods that support the diagnosis of moderate to severe liver inflammation and/or fibrosis), serum HBV DNA > 2000 IU/mL, and serum ALT level > 1 × ULN and < 10 × ULN at the screening visit. Patients with ALT ≤ 1 × ULN at screening were eligible if they had at least moderate liver inflammation or fibrosis, clinical evidence of compensated cirrhosis, or ALT level > 1 × ULN within the last 6 mo.
Main exclusion criteria included co-infection with hepatitis C virus, hepatitis D virus or human immunodeficiency virus; hepatic decompensation; liver disease other than CHB; any nucleos(t)ide or interferon/immunomodulator treatment in the previous 6 mo; chronic renal insufficiency or serum creatinine clearance < 50 mL/min; history of myopathy, myositis, or persistent muscle weakness; pregnant or nursing (lactating) women; or history of malignancy of any organ system (other than localized basal cell carcinoma of the skin).
Patients were allowed to recieve an additional 52 wk of treatment in the extension period (i.e., up to 156 wk) if they had HBV DNA < 300 copies/mL at both weeks 92 and 104, and serum creatinine clearance ≥ 50 mL/min at two consecutive visits including week 104.
The primary efficacy endpoint was the rate of patients achieving HBV DNA < 300 copies/mL (51 IU/mL) at week 52. Secondary efficacy endpoints included the rates of patients with HBV DNA < 300 copies/mL at weeks 104 and 156, and HBV DNA < 169 copies/mL (29 IU/mL) (lower limit of detection) at weeks 24, 52, 104 and 156; change from baseline in HBV DNA; ALT normalisation at weeks 52, 104 and 156; HBsAg loss and HBsAg seroconversion; VB; and emergence of resistance. In addition, subgroup analyses were performed for secondary efficacy endpoints by baseline HBV DNA (i.e., < 7 log10 copies/mL or ≥ 7 log10 copies/mL).
VB was defined as an increase of HBV DNA by at least 1 log10 copies/mL (or 1 log10 IU/mL) above nadir on 2 consecutive visits, or at the last on-treatment visit in patients who did not have a primary non-response. Emergence of resistance was assessed as the rate of confirmed treatment-emergent genotypic resistance and was assessed at the time of confirmed VB and at week 24 in patients with viral load ≥ 300 copies/mL, it was calculated cumulatively at weeks 52, 104 and 156.
HBV DNA detection and quantification were performed at a central laboratory using the COBAS TaqMan real-time polymerase chain reaction assay (Roche Molecular Systems, Branchburg, NJ, United States).
Safety assessments included monitoring of adverse events (AEs), vital signs, and graded laboratory abnormalities. Estimated glomerular filtration rate (eGFR), calculated by the modification of diet in renal disease formula was recorded. AEs of special interest (muscle and renal function related events) were also reported.
For the primary efficacy analysis, study treatments were compared for non-inferiority.
Based on the assumptions of 96% and 97% HBV DNA < 300 copies/mL at week 52 in the telbivudine arm and the tenofovir arm, respectively, and an approximately 10% dropout rate, it was estimated that 120 randomised patients per arm would provide 87% power for the non-inferiority testing on the primary analysis. Non-inferiority in efficacy of telbivudine arm to tenofovir arm was to be claimed if the lower limit of the 2-sided confidence interval (CI) for the difference was above the pre-determined non-inferiority margin (-10%).
A weighted Cochran-Mantel-Haenszel method, adjusting for randomisation strata [HBV DNA (< or ≥ 7 log10 copies/mL) and ALT (< or ≥ 3 × ULN) levels], was used to assess comparative therapeutic response rates.
For continuous variables, summary statistics of absolute value and of change from baseline, including mean, standard deviation (SD), median, minimum, and maximum were used. For dichotomous endpoints, statistical summaries included count and percentage of patients with a positive response (response rate) and also 95%CI for the response rate.
The intent-to-treat (ITT) population consisted of all patients who received at least one dose of study drug and had at least one post-baseline assessment of serum HBV DNA. The roadmap ITT (rITT) population consisted of all patients who did not discontinue before week 24 and did not deviate from the protocol defined rules of receiving add-on at week 24 (i.e., patients who received the add-on therapy at week 24 if they had HBV DNA ≥ 300 copies/mL, or did not receive the add-on at week 24 if they had HBV DNA < 300 copies/mL). The modified ITT (mITT) population consisted of all patients in the ITT population who were eligible and enrolled in the extension period beyond week 104. The per-protocol population consisted of all patients in the ITT population who had no major protocol deviations.
All efficacy observations on or after censoring date were treated as missing. A patient’s censoring date was the date of the first occurrence of: One day after the last dose of the study drug, the start of first prohibited CHB-related medication, pregnancy, or a specific major protocol deviation. To assess the robustness of the results due to missing data, the analysis of primary and all secondary efficacy endpoints were performed based on the rITT and ITT analysis populations. The mITT population was used only for the week 156 analysis.
The primary efficacy endpoint (week 52) analysis was performed on the rITT population. The analyses presented include: (1) assessments within the ± 7 d protocol-prespecified visit window around the scheduled week 52 date; (2) missing data at week 52 treated as failure; (3) missing data imputed using the earliest available assessment within the 28 d window starting from the scheduled week 52 date; and (4) missing data imputed using the last observation carried forward (LOCF).
Secondary efficacy parameters including HBV DNA, ALT normalisation, HBsAg loss, and HBsAg seroconversion were analysed using two imputation methods for missing data: (1) missing data treated as failure; and (2) missing data imputed using the earliest available assessment within the 28 d window starting from the scheduled visit for weeks 52 (except HBV DNA < 300 copies/mL), 104 and 156. VB and eGFR were analysed using the LOCF imputation method for missing data. Treatment-emergent genotypic resistance was analysed using cumulative imputation method for missing data. Missing eGFR assessments were imputed using the LOCF method.
Analyses of endpoints using LOCF imputation at weeks 104 and 156 are presented for the rITT and mITT populations, respectively.
A total of 241 patients (121 in the telbivudine arm and 120 in the tenofovir arm) were randomised in this study. A total of 22 (18.2%) patients in the telbivudine arm and 13 (10.8%) patients in the tenofovir arm discontinued prematurely from the study. The most common reasons for discontinuation in the telbivudine arm were consent withdrawal (n = 7), lost to follow-up (n = 5), and administrative reasons (n = 4). In the tenofovir arm, the most common reasons for discontinuation were AEs (n = 5), consent withdrawal (n = 4), and lost to follow-up (n = 3).
Major protocol deviations were reported in 11 (9.1%) patients in the telbivudine arm and 8 (6.7%) patients in the tenofovir arm. The most commonly reported major deviations were patients on monotherapy with confirmed VB not starting add-on therapy within 2 wk of laboratory confirmation of VB (n = 9), patients with a positive HBeAg result (n = 6), and patients not completing 3 wk of treatment before the third visit (n = 4).
The safety population comprised 120 patients in each of the 2 treatment arms. One patient in the telbivudine arm was excluded from the safety population as this patient did not receive any study treatment. Of the 241 randomized patients, 235 patients were included in the ITT population, with 117 (96.7%) in the telbivudine arm and 118 (98.3%) in the tenofovir arm. Six patients were excluded from the ITT population (4 patients in the telbivudine arm due to no post-baseline HBV DNA assessments, non-compliance with the study conduct, or no study treatment received; and 2 patients in the tenofovir arm because of no post-baseline HBV DNA assessments and viral resistance at baseline). A total of 113 (93.4%) patients in the telbivudine arm and 117 (97.5%) patients in the tenofovir arm comprised the rITT population. Five patients (4 in the telbivudine arm and 1 in the tenofovir arm) that were included in the ITT population were excluded from the rITT population because they discontinued before week 24 and were not eligible for or enrolled into the roadmap concept period (weeks 24 to 104).
The per-protocol population consisted of 107 (88.4%) patients in the telbivudine arm and 111 (92.5%) patients in the tenofovir arm. A total of 17 patients (10 in the telbivudine arm and 7 in the tenofovir arm) were included in the ITT and rITT populations but were excluded from the per-protocol population because of major protocol deviations. The mITT population consisted of 79 (65.3%) patients in the telbivudine arm and 89 (74.2%) patients in the tenofovir arm.
Treatment arms were balanced with respect to demographics and baseline characteristics, with no clinically meaningful differences between the telbivudine and tenofovir arms (Table 1). Most (86.0% telbivudine, 91.7% tenofovir) patients were infected with HBV genotype D, and the mean HBV DNA at baseline was 6.2 log10 copies/mL in the telbivudine arm and 6.0 log10 copies/mL in the tenofovir arm, with 70.2% and 71.7% of patients, respectively, having a baseline HBV DNA < 7 log10 copies/mL.
| Patients characteristics | Telbivudine (n = 121) | Tenofovir (n = 120) |
| Age, mean (SD), yr | 42.1 (11.5) | 43.3 (12.6) |
| Median (min-max) | 42.0 (19-70) | 44.0 (18-73) |
| Male gender, n (%) | 86 (71.1) | 82 (68.3) |
| Race, Caucasian, n (%) | 117 (96.7) | 118 (98.3) |
| Body mass index, mean (SD), kg/m2 | 25.8 (4.1) | 25.7 (4.0) |
| Median (min-max) | 25.6 (16.5-40.4) | 25.2 (18.4-39.8) |
| Genotype, n (%) | ||
| A | 6 (5.0) | 2 (1.7) |
| B | 1 (0.8) | 0 (0.0) |
| C | 0 (0.0) | 1 (0.8) |
| D | 104 (86.0) | 110 (91.7) |
| G | 1 (0.8) | 0 (0.0) |
| Other | 1 (0.8) | 0 (0.0) |
| Unknown | 8 (6.6) | 7 (5.8) |
| HBV DNA, mean (SD), log10 copies/mL | 6.2 (1.5) | 6.0 (1.4) |
| Median (min-max) | 6.1 (3.2-9.5) | 5.9 (2.5-9.9) |
| < 7 log10, n (%) | 85 (70.2) | 86 (71.7) |
| ≥ 7 log10, n (%) | 36 (29.8) | 34 (28.3) |
| Serum alanine aminotransferase, mean (SD), IU/L | 79.8 (84.1) | 78.2 (86.1) |
| Median (min-max) | 53.0 (13-494) | 49.0 (5-568) |
| Serum aspartate aminotransferase, mean (SD), IU/L | 54.0 (52.8) | 52.5 (47.1) |
| Median (min-max) | 35.0 (13-347) | 35.0 (13-322) |
| Creatine phosphokinase, mean (SD), IU/L | 118.6 (64.4) | 160.1 (299.3) |
| Median (min-max) | 104.0 (35-430) | 111.0 (36-2976) |
| eGFR1, mean (SD), (mL/min per 1.73 m2) | 97.4 (17.9) | 95.8 (16.4) |
| Median (min-max) | 96.6 (60.9-147.1) | 94.2 (60.5-138.4) |
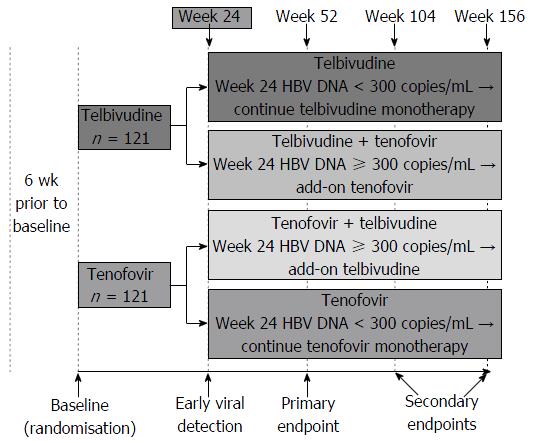
Virologic response (HBV DNA < 300 copies/mL) at week 52 was achieved in more than 91% of patients in each treatment arm (Figure 2A). The primary endpoint analysis showed that the antiviral efficacy of telbivudine-roadmap was non-inferior to that of tenofovir-roadmap application at week 52 in the rITT population; the lower bound of the 95%CI for the difference between the 2 treatment arms was above the non-inferiority margin of 10%: -9.4% (utilizing assessments within the ±7 d protocol-prespecified visit window); 8.3% for the 28 d window imputation; and -7.9% for the LOCF imputation. Using missing data as treatment failure, non-inferiority was not demonstrated (lower bound of the 95%CI: -10.5%, just below the protocol defined non-inferiority margin) (Table 2). In this analysis, HBV DNA samples from 6 patients (4 in the telbivudine arm and 2 in the tenofovir arm), although resulted in < 300 copies/mL, were considered as missing because they were not obtained at the week 52 visit date itself (i.e., patients were counted as treatment failures).
| Parameters | Telbivudine (n = 113) | Tenofovir (n = 117) | Difference between arms and 95%CI |
| Patients achieving HBV DNA < 300 copies/mL (51 IU/mL) at week 52, n (%) | |||
| ± 7 d protocol-prespecified visit window | 104 (91.9) | 111 (95.0) | -3.1% (-9.4%, 3.1%)1 |
| Treating missing as failure | 103 (91.0) | 111 (95.0) | -4.0% (-10.5%, 2.5%)1 |
| 28 d imputation | 105 (92.7) | 111 (95.0) | -2.3% (-8.3%, 3.8%)1 |
| Last observation carried forward | 108 (95.4) | 116 (99.2) | -3.8% (-7.9%, 0.4%)1 |
| Change from baseline in HBV DNA levels (log10 copies/mL) by visit, mean (SD) | P-value | ||
| Week 24 | -4.001 (1.256) | -4.122 (1.165) | P < 0.00012 |
| Week 52 | -4.356 (1.473) | -4.305 (1.343) | P < 0.00012 |
| Week 104 | -4.281 (1.753) | -4.349 (1.382) | P < 0.00012 |
The primary endpoint analysis at week 52 in the per-protocol population supported the non-inferiority of the telbivudine arm to the tenofovir arm (98.0% in the telbivudine arm and 99.0% in the tenofovir arm, lower bound of the 95%CI: 4.3%).
Virologic responses: Percentage of patients achieving HBV DNA < 300 copies/mL (51 IU/mL) at weeks 24 and 104, and by baseline viral load at weeks 24, 52 and 104 in the rITT population: The percentage of patients achieving HBV DNA < 300 copies/mL in the telbivudine and tenofovir arms at week 24 was 80.5% and 89.7%, and at week 104, 93.8% and 99.1%, respectively (Figure 2A).
In patients with lower baseline viral load (HBV DNA level < 7 log10 copies/mL) at week 24, telbivudine and tenofovir regimens were similar in terms of viral load reduction with 93.8% and 95.2% of patients achieving HBV DNA levels < 300 copies/mL in the telbivudine and tenofovir arms, respectively. At weeks 52 and 104, telbivudine and tenofovir regimens seemed to be similar in terms of viral load reduction, with over 92% of patients achieving HBV DNA levels < 300 copies/mL at weeks 52 and 104 (Figure 2A). The proportion of patients in each arm with higher baseline viral load (≥ 7 log10 copies/mL) was relatively small to make any meaningful interpretation.
Change from baseline in HBV DNA levels from week 24 to week 104 in the rITT population: A statistically significant (P < 0.0001) reduction in HBV DNA levels vs baseline was achieved in both treatment arms at week 24 and was sustained through week 104 (Table 2).
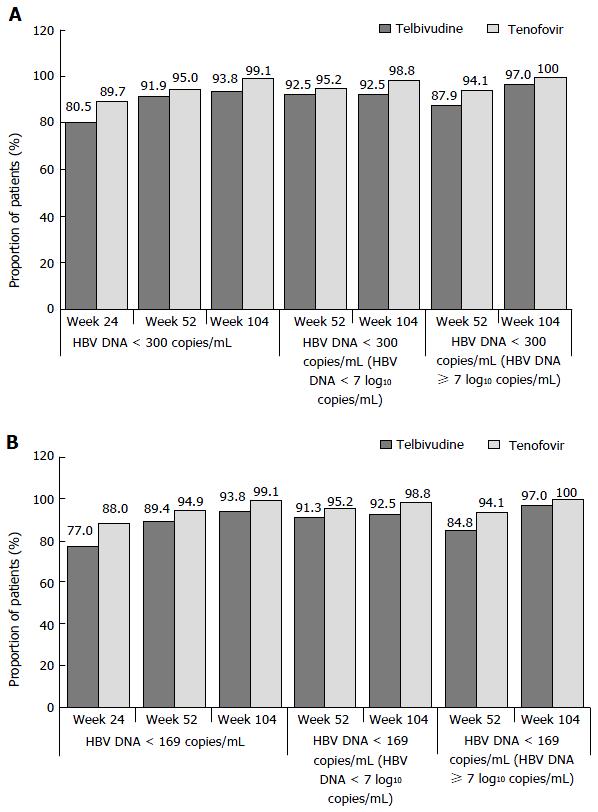
Intensification with tenofovir or telbivudine for HBV DNA ≥ 300 copies/mL at week 24 or for VB post week 24 through week 104 in the rITT population; response at week 104 (HBV DNA < 300 copies/mL) according to the requirement for add-on therapy at week 24: A greater number of patients in the telbivudine arm required add-on therapy compared with the tenofovir arm (35 patients in the telbivudine arm including 22 patients requiring add-on therapy at week 24 and 13 requiring add-on therapy post week 24 vs 11 patients in the tenofovir arm, all requiring add-on therapy at week 24).
The proportion of patients in the telbivudine arm achieving HBV DNA < 300 copies/mL at week 104 was greater in those who required tenofovir add-on therapy at week 24 (100%, 21/21 patients) than patients who were in the telbivudine monotherapy group following the week 24 visit (92.4%, 85/92 patients) (Figure 3A).
The proportion of patients in the tenofovir arm achieving HBV DNA < 300 copies/mL at week 104 was similar in those who required telbivudine add-on therapy at week 24 (100%, 11/11 patients) to those who were in the tenofovir monotherapy group following the week 24 visit (99.1%, 105/106 patients) (Figure 3B).
Percentage of patients achieving HBV DNA < 169 copies/mL (29 IU/mL) at weeks 24, 52 and 104 in the rITT population: The rate of patients achieving HBV DNA < 169 copies/mL at weeks 24, 52 and 104 was consistent with that observed for the endpoint of HBV DNA < 300 copies/mL (Figure 2B).
Percentage of patients achieving HBV DNA < 169 copies/mL at week 104 in the rITT population according to the requirement for add-on therapy at week 24: The proportion of patients in the telbivudine and tenofovir arms achieving HBV DNA < 169 copies/mL at week 104 and receiving add-on therapy were 7.6 and 0.9 percentage points greater, respectively, than patients who received monotherapy (Figure 3).
Maintained virologic responses at week 156 in the mITT population: The percentage of patients who maintained HBV DNA < 300 copies/mL at week 156 was similar in the telbivudine and tenofovir arms: 91.1% (72/79 patients) and 100% (89/89 patients), respectively, using LOCF imputation. Similar results were found in patients maintaining HBV DNA < 169 copies/mL [91.1% (72/79 patients) and 96.6% (86/89 patients), respectively].
HBsAg loss and HBsAg seroconversion: HBsAg loss and HBsAg seroconversion were not observed in any patient from either treatment arm at weeks 52, 104 or 156. Telbivudine treatment progressively reduced serum HBsAg levels (mean ± SD) from baseline in the rITT population [-0.116 ± 0.581 log10 IU/mL at week 52 (P = 0.0368) and -0.179 ± 0.633 log10 IU/mL at week 104 (P = 0.0032)]. In contrast, no change was reported in quantitative HBsAg during therapy with tenofovir [-0.038 ± 0.349 log10 IU/mL at week 52 (P = 0.2399) and -0.030 ± 0.385 log10 IU/mL at week 104 (P = 0.4063)]. At week 156, change from baseline in HBsAg levels in the mITT population was -0.204 ± 0.759 log10 IU/mL (P = 0.0193) in the telbivudine arm and -0.031 ± 0.412 log10/mL (P = 0.4760) in the tenofovir arm.
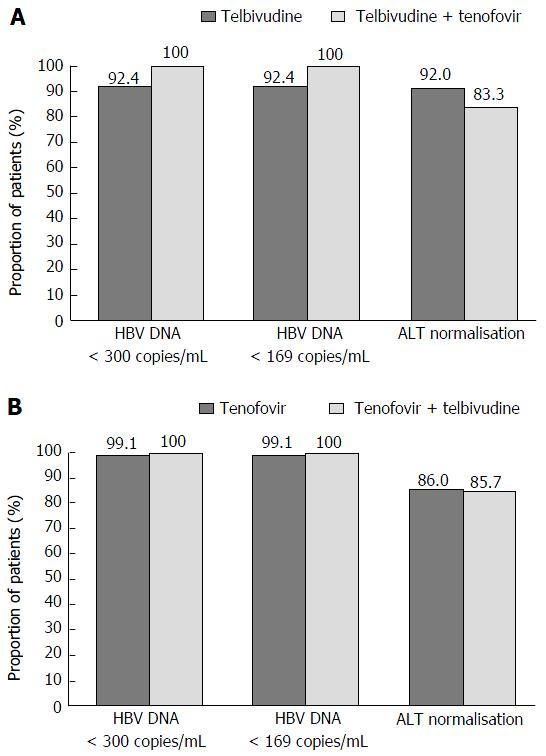
Biochemical response: ALT normalisation at weeks 52 and 104 in the rITT population: ALT levels significantly improved vs baseline in both treatment arms, with over 82% of patients in both arms achieving ALT normalisation at week 52 that was sustained up until week 104 (89.7% and 85.9% in the telbivudine and tenofovir arms, respectively) (Figure 4).
The results at week 104 by baseline viral load are presented in Figure 4.
ALT normalisation at week 104 in the rITT population according to the requirement for add-on therapy at week 24: The proportion of patients who achieved ALT nornalization at week 24 was higher (telbivudine arm) or similar (tenofovir arm) in patients who recevived add-on therapy (Figure 3).
Maintained biochemichal response at week 156 in the mITT population: ALT normalisation was maintained in 92.0% of patients in the telbivudine arm and 91.1% of patients in the tenofovir arm.
Patients experiencing VB and emergence of resistance in the rITTand mITT populations: At weeks 52 and 104, respectively, in the rITT population, cumulative rates of VB were reported in 2.7% (3/113) and 9.7% (11/113) of patients in the telbivudine arm (3.3% and 12.4% in the monotherapy group, none in the add-on treatment group). In the tenfovir arm, no patients developed VB cumulatively at week 52 and 1.7% (2/117) of patients developed VB cumulatively at week 104.
At week 52, cumulative emergence of resistance was reported in 2.7% (3/113) of patients in the telbivudine arm (3.3% in the monotherapy group, none in the add-on treatment group) and no treatment-emergent resistance was observed in the tenofovir arm. At week 104, cumulative emergence of resistance was reported in 7.4% (8/108) of patients in the telbivudine arm (9.2% in the monotherapy group, none in the add-on treatment group) and none in the tenofovir arm.
In the telbivudine arm, 10 patients experienced VB and 5 had emergence of resistance between weeks 104 and 156 in the mITT population. In the tenofovir arm, only 1 patient had VB and none developed viral resistance. The cumulative rate of VB at week 156 was 16.5% (13/79) in the telbivudine arm, and 1.1% (1/89) in the tenofovir arm. Cumulative rates of resistance were 10.8% (8/74) in the telbivudine arm (14.0% in the monotherapy group, none in the add-on treatment group) and none in the tenfovir arm.
No patients died or experienced ALT flare during the study. The overall incidence of serious AEs (SAEs) was similar in the telbivudine arm and in the tenofovir arm [11 (9.2%) patients and 13 (10.8%) patients, respectively]. One patient in the tenofovir arm reported drug-related SAEs [moderately increased blood creatine phosphokinase (CPK), mild arthralgia, and moderate fatigue], which led to temporary interruption of the study drug (Table 3). There were no cases of myositis or myopathy.
| Safety parameters | Telbivudine | Tenofovir | ||||
| Monotherapy (n = 98) | Intensification with tenofovir (n = 22) | Overall (n = 120) | Monotherapy (n = 109) | Intensification with telbivudine (n = 11) | Overall (n = 120) | |
| Any AE | 69 (70.4) | 17 (77.3) | 86 (71.7) | 75 (68.8) | 8 (72.7) | 83 (69.2) |
| AE related to drug | 36 (36.7) | 11 (50.0) | 47 (39.2) | 21 (19.3) | 6 (54.5) | 27 (22.5) |
| AE leading to drug discontinuation | 2 (2.0) | 0 (0.0) | 2 (1.7) | 5 (4.6) | 0 (0.0) | 5 (4.2) |
| Any SAE | 6 (6.1) | 5 (22.7) | 11 (9.2) | 11 (10.1) | 2 (18.2) | 13 (10.8) |
| SAE related to drug | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 1 (0.8) |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AEs related to drug occurring in ≥ 2% of patients in any treatment arm | ||||||
| Blood CPK increased | 23 (23.5) | 8 (36.4) | 31 (25.8) | 13 (11.9) | 3 (27.3) | 16 (13.3) |
| Nausea | 6 (6.1) | 2 (9.1) | 8 (6.7) | 0 (0.0) | 2 (18.2) | 2 (1.7) |
| Myalgia | 7 (7.1) | 1 (4.5) | 8 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alanine aminotransferase increased | 2 (2.0) | 0 (0.0) | 2 (1.7) | 3 (2.8) | 1 (9.1) | 4 (3.3) |
| Proteinuria | 2 (2.0) | 0 (0.0) | 2 (1.7) | 4 (3.7) | 0 (0.0) | 4 (3.3) |
| Aspartate aminotransferase increased | 3 (3.1) | 0 (0.0) | 3 (2.5) | 2 (1.8) | 0 (0.0) | 2 (1.7) |
| Any AE of special interest | 35 (35.7) | 11 (50.0) | 46 (38.3) | 23 (21.1) | 4 (36.4) | 27 (22.5) |
| AEs of special interest occurring in ≥ 2% of patients in any treatment arm | ||||||
| Blood CPK increased | 24 (24.5) | 10 (45.5) | 34 (28.3) | 17 (15.6) | 3 (27.3) | 20 (16.7) |
| Myalgia | 10 (10.2) | 2 (9.1) | 12 (10.0) | 2 (1.8) | 1 (9.1) | 3 (2.5) |
| Alanine aminotransferase increased | 5 (5.1) | 0 (0.0) | 5 (4.2) | 5 (4.6) | 1 (9.1) | 6 (5.0) |
| Proteinuria | 3 (3.1) | 0 (0.0) | 3 (2.5) | 4 (3.7) | 0 (0.0) | 4 (3.3) |
| Any patient with muscle event | 12 (12.2) | 2 (9.1) | 14 (11.7) | 2 (1.8) | 1 (9.1) | 3 (2.5) |
| Experiencing new-onset Grade 3/4 abnormal CPK within the study | 4 (4.1) | 1 (4.5) | 5 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Experiencing new-onset Grade 1/2 abnormal CPK within the study | 6 (6.1) | 1 (4.5) | 7 (5.8) | 1 (0.9) | 1 (9.1) | 2 (1.7) |
| Any patient with new-onset Grade 3/4 CPK episode within the study | 17 (17.3) | 2 (9.1) | 19 (15.8) | 3 (2.8) | 2 (18.2) | 5 (4.2) |
| Episode not resolved | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Two patients in the telbivudine arm and 5 patients in the tenofovir arm discontinued due to AEs [myalgia and hepatocellular carcinoma (HCC) for telbivudine; headache, HCC, hepatic cirrhosis, cholestatic jaundice, and breast cancer for tenofovir], which were assessed by the investigator as unrelated to the study drugs. Most AEs were mild to moderate in severity. The proportion of patients reporting at least 1 AE, regardless of study drug relationship, was similar for telbivudine and tenofovir arms. The overall incidence of AEs suspected to be related to study drug was somewhat higher in the telbivudine arm compared with the tenofovir arm. The most frequent (≥ 2%) drug-related AEs reported in both arms are described in Table 3. Increased blood CPK levels [31 (25.8%) patients], myalgia [8 (6.7%) patients, and nausea 8 (6.7%) patients] were the drug-related AEs that were observed more frequently in the telbivudine arm compared with the tenofovir arm [16 (13.3%), 0, and 2 (1.7%) patients, respectively]. AEs of special interest were observed in 46 (38.3%) patients in the telbivudine arm and 27 (22.5%) patients in the tenofovir arm. These included elevated blood CPK and myalgia as the most commonly reported AEs in the telbivudine arm, and elevated blood CPK and ALT as the most commonly reported AEs in the tenofovir arm. Myalgia suspected to be drug related was reported in the telbivudine arm. The number of patients experiencing at least 1 muscle event along with 1 new-onset abnormal CPK episode during the study was greater in the telbivudine arm (Table 3).
The telbivudine arm showed a higher incidence of Grade 3/4 CPK elevations during the study than the tenofovir arm [19 (15.8%) patients vs 5 (4.2%) patients, respectively]. All Grade 3/4 CPK elevations were resolved (Table 3).
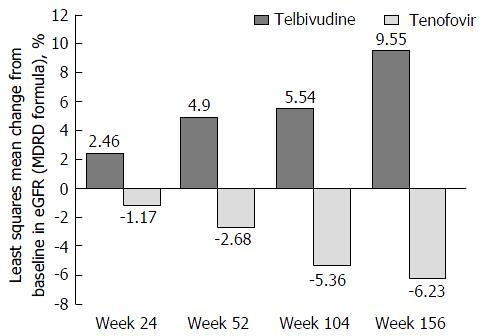
Telbivudine monotherapy (as of week 24) was associated with a significant improvement in eGFR as compared with tenofovir monotherapy (as of week 24). At week 24, the telbivudine monotherapy showed a statistically significant (P = 0.0798) improvement from baseline in eGFR compared to worsening with tenofovir monotherapy, with least squares mean percentage changes from baseline of 2.46% vs -1.17%, respectively. Further improvement in eGFR in the telbivudine monotherapy group (as of week 24) was observed at weeks 52 (4.90% vs -2.68% with tenofovir, P = 0.0098), 104 (5.54% vs -5.36%, P < 0.0001, respectively), and 156 (9.55% vs -6.23%, P < 0.0001, respectively) (Figure 5).
There was no significant change in vital signs from baseline for either treatment arm.
NAs given as a single daily oral dose are considered the mainstay of CHB treatment[13]. In clinical practice, attaining optimal efficacy with a low emergence of drug resistance remains an important goal[14]. The roadmap concept utilizing add-on therapy for patients who do not achieve HBV DNA < 300 copies/mL at week 24 (in particular for agents with lower barriers to resistance) has been identified as a strategy to achieve this goal. This study was the first prospective, randomised clinical trial using the roadmap concept in HBeAg-negative CHB patients comparing efficacy and safety of telbivudine with tenofovir. As previously reported[15], early detection of virologic response may be a useful guide to individualize CHB treatment. This study confirmed that monitoring virologic response at week 24 is a strong predictor of the treatment response by week 104[16]. These data were consistent with an earlier study comparing telbivudine with lamivudine[15].
In the real-world setting, use of the roadmap concept may offer several advantages such as early identification of patients with suboptimal responses to initiate an appropriate change in therapy[10,11], and to provide clinicians with options for individualized treatment decisions[5]. Although emergence of resistance had been identified as an issue for HBeAg-negative CHB patients treated with telbivudine monotherapy[15,17], the data from our study suggest that the risk for resistance is lower if telbivudine is administered using the roadmap concept, as compared to the GLOBE trial showing higher rates of resistance[15]. Moreover, despite a somewhat higher percentage of patients requiring add-on therapy in the telbivudine arm, the overall efficacy profile of the 2 roadmap approach arms was comparable, as assessed by the percentages of patients achieving HBV DNA levels < 300 or < 169 copies/mL, and ALT normalisation at weeks 52, 104 and 156. Moreover, telbivudine treatment resulted in a statistically significant reduction in serum HBsAg levels from baseline while no change was reported in quantitative HBsAg during therapy with tenofovir.
Overall, both treatments based on the roadmap concept were well tolerated over the 156 wk treatment period in HBeAg-negative patients. Although myalgia and elevated blood CPK levels were reported in a higher number of patients in the telbivudine arm, the rates were consistent with the findings reported earlier in the literature[12,15,18,19]. It is recommended that serum CPK levels should be monitored closely during treatment with telbivudine[20].
Renal safety issues with oral NAs have been well-documented[21-23]. Particularly, adefovir is considered to have high potential for nephrotoxicity and tenofovir has been associated with this risk[24]. In our study, telbivudine was associated with improvement in eGFR from baseline to week 156 compared to the increasing deterioration over time with tenofovir. The finding of improvement in eGFR with telbivudine treatment was consistent with that reported in previous studies where telbivudine significantly improved while adefovir and lamivudine worsened renal function[25,26]. CHB patients with impaired renal function at baseline have also shown an eGFR improvement after 1 year[27] and 2 years of treatment with telbivudine[11,28]. Similar results for telbivudine have also been reported in patients with cirrhosis, patients with compensated cirrhosis, or patients with no cirrhosis[29,30]. These findings imply that telbivudine may offer benefit in patients with known or at risk of renal impairment. Although telbivudine improves renal function, the mechanism of this renal protective effect remains to be determined[31].
The main limitations of the study are related to its design (open-label) and the relatively small sample size.
In conclusion, this study was the first prospective, randomised, comparative study of telbivudine-roadmap vs tenofovir-roadmap concept in HBeAg-negative patients with CHB. Both treatments based on the roadmap concept were effective over the 156 wk treatment period. Moreover, telbivudine showed an improvement in eGFR from baseline while a deterioriaton was observed with tenofovir; this could be an important consideration for long term therapy in CHB patients especially in those with a high risk for renal impairment.
The authors acknowledge the work of the OPTIMA investigators and participating institutions located in various countries. The investigators included Peter Ferenci and Wolfgang Vogel (Austria); Rozalina Balabanska, Jordan Genov, and Krum Katzarov (Bulgaria); Thomas Berg, Peter Buggisch, Heinz Hartmann, Hartwig Klinker, Jens Rasenack, Hans Wedemeyer, and Stefan Zeuzem (Germany); Evangelos Akriviadis, Alexandra Alexopoulou, Ioannis Elefsiniotis, and Konstantinos Mimidis (Greece); Evangelista Sagnelli (Italy); Djamal Abdurakhmanov, Pavel Bogomolov, Vladimir Chulanov, Marina Maevskaya, Maria Matsievich, Igor Nikitin, Olga Znoiko, and Konstantin Zhdanov (Russia); Maria Buti Ferret, Jose Luis Calleja, Albert Pardo, and Ricard Sola Lamoglia (Spain); Ulus Akarca, Iftihar Koksal, and Fehmi Tabak (Turkey). The authors would like to thank Krassimir Antonov, Deian Jelev, Lyudmila Mateva, and Dimitar Popov (Bulgaria) for their technical assistance. Medical writing support was provided by Farid Khalfi (Novartis Ireland Ltd., Dublin, Ireland).
Hepatitis B virus (HBV) infection is the major cause of chronic hepatitis worldwide. Emergence of resistance due to prolonged nucleos(t)ide analogue use or incomplete suppression of HBV still remains an important concern. Therefore, early virologic response at week 24 of therapy has been used to predict better outcomes and to reduce the risk of antiviral resistance.
This study used the response-guided add-on strategy (roadmap concept). For patients with HBV DNA ≥ 300 copies/mL (≥ 51 IU/mL) at week 24, tenofovir was added to telbivudine by week 26 in the telbivudine arm, and telbivudine was added to tenofovir by week 26 in the tenofovir arm. For patients with HBV DNA < 300 copies/mL at week 24, telbivudine and tenofovir monotherapies in the respective arms were continued.
This was the first prospective, randomised, 2-arm, open-label, non-inferiority study in hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) patients that compared telbivudine and tenofovir administered as per the roadmap concept. The safety of the combination of telbivudine and tenofovir, for which limited data are currently available, was also evaluated.
Efficacy was shown for both telbivudine-roadmap and tenofovir-roadmap regimens in HBeAg-negative CHB patients over 156 wk. Both treaments showed acceptable safety profiles. In addition, the telbivudine arm was associated with renal improvement.
This is an extensive randomised study to compare the roadmap treatment strategy between telbivudine and tenofovir in patients with HBeAg-negative CHB patients. As antiviral treatment may be life-long, renal protection becomes an important consideration. The current manuscript should be of benefit to the hepatologists and liver transplantation specialists worldwide.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B, B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Balaban YH, Chiu KW, Cholongitas EC, Chuang QL, Gong ZJ, Montasser MF, Romero MR, Wong GLH, Zhu Z S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2001] [Article Influence: 200.1] [Reference Citation Analysis (4)] |
| 2. | Tang CM, Yau TO, Yu J. Management of chronic hepatitis B infection: current treatment guidelines, challenges, and new developments. World J Gastroenterol. 2014;20:6262-6278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Hepatitis B Fact Sheet No. 204. July 2015. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. |
| 4. | Zoulim F, Locarnini S. Optimal management of chronic hepatitis B patients with treatment failure and antiviral drug resistance. Liver Int. 2013;33 Suppl 1:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Gu EL, Yu YQ, Wang JL, Ji YY, Ma XY, Xie Q, Pan HY, Wu SM, Li J, Chen CW. Response-guided treatment of cirrhotic chronic hepatitis B patients: multicenter prospective study. World J Gastroenterol. 2015;21:653-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Ryu HJ, Lee JM, Ahn SH, Kim do Y, Lee MH, Han KH, Chon CY, Park JY. Efficacy of adefovir add-on lamivudine rescue therapy compared with switching to entecavir monotherapy in patients with lamivudine-resistant chronic hepatitis B. J Med Virol. 2010;82:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Yang YJ, Shim JH, Kim KM, Lim YS, Lee HC. Assessment of current criteria for primary nonresponse in chronic hepatitis B patients receiving entecavir therapy. Hepatology. 2014;59:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Shin JW, Jung SW, Park BR, Kim CJ, Eum JB, Kim BG, Du Jeong I, Bang SJ, Park NH. HBV DNA level at 24 weeks is the best predictor of virological response to adefovir add-on therapy in patients with lamivudine resistance. Antivir Ther. 2012;17:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Lo AO, Wong GL. Current developments in nucleoside/nucleotide analogues for hepatitis B. Expert Rev Gastroenterol Hepatol. 2014;8:607-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Gane EJ. The Roadmap concept: using early on-treatment virologic responses to optimize long-term outcomes for patients with chronic hepatitis B. Hepatol Int. 2008;2:304-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Sun J, Xie Q, Tan D, Ning Q, Niu J, Bai X, Fan R, Chen S, Cheng J, Yu Y. The 104-week efficacy and safety of telbivudine-based optimization strategy in chronic hepatitis B patients: a randomized, controlled study. Hepatology. 2014;59:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Piratvisuth T, Komolmit P, Tanwandee T, Sukeepaisarnjaroen W, Chan HL, Pessoa MG, Fassio E, Ono SK, Bessone F, Daruich J. 52-week efficacy and safety of telbivudine with conditional tenofovir intensification at week 24 in HBeAg-positive chronic hepatitis B. PLoS One. 2013;8:e54279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Liu F, Wang X, Wei F, Hu H, Zhang D, Hu P, Ren H. Efficacy and resistance in de novo combination lamivudine and adefovir dipivoxil therapy versus entecavir monotherapy for the treatment-naive patients with chronic hepatitis B: a meta-analysis. Virol J. 2014;11:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Yu HC, Lin KH, Hsu PI, Tsay FW, Wang HM, Tsai TJ, Lai KH. Real-world application of the roadmap model in chronic hepatitis B patients with telbivudine therapy. Clin Ther. 2013;35:1386-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 16. | Zeuzem S, Gane E, Liaw YF, Lim SG, DiBisceglie A, Buti M, Chutaputti A, Rasenack J, Hou J, O'Brien C. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Thongsawat S, Gane EJ, Liaw YF, Jia J, Hou J, Chan HL, Papatheodoridis G, Wan M, Niu J. Efficacy and safety of continuous 4-year telbivudine treatment in patients with chronic hepatitis B. J Viral Hepat. 2013;20:e37-e46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 602] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 19. | Seto WK, Lai CL, Fung J, Wong DK, Yuen JC, Hung IF, Yuen MF. Significance of HBV DNA levels at 12 weeks of telbivudine treatment and the 3 years treatment outcome. J Hepatol. 2011;55:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Wang YH, Wu BQ, Liu H. Continuous venovenous hemodiafiltration for hyperlactatemia caused by telbivudine in a patient with chronic hepatitis B: a case report and update review. J Dig Dis. 2015;16:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Chan HL, Chen YC, Gane EJ, Sarin SK, Suh DJ, Piratvisuth T, Prabhakar B, Hwang SG, Choudhuri G, Safadi R. Randomized clinical trial: efficacy and safety of telbivudine and lamivudine in treatment-naive patients with HBV-related decompensated cirrhosis. J Viral Hepat. 2012;19:732-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Liaw YF, Raptopoulou-Gigi M, Cheinquer H, Sarin SK, Tanwandee T, Leung N, Peng CY, Myers RP, Brown RS, Jr . Jeffers L, Tsai N, Bialkowska J, Tang S, Beebe S, Cooney E. Efficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label study. Hepatology (Baltimore, Md). 2011;54:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Mallet V, Schwarzinger M, Vallet-Pichard A, Fontaine H, Corouge M, Sogni P, Pol S. Effect of nucleoside and nucleotide analogues on renal function in patients with chronic hepatitis B virus monoinfection. Clin Gastroenterol Hepatol. 2015;13:1181-1188.e1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 25. | Qi X, Wang JY, Mao RC, Zhang JM. Impact of nucleos(t)ide analogues on the estimated glomerular filtration rate in patients with chronic hepatitis B: a prospective cohort study in China. J Viral Hepat. 2015;22:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Gane EJ, Deray G, Liaw YF, Lim SG, Lai CL, Rasenack J, Wang Y, Papatheodoridis G, Di Bisceglie A, Buti M. Telbivudine improves renal function in patients with chronic hepatitis B. Gastroenterology. 2014;146:138-146.e135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Tsai MC, Chen CH, Hung CH, Lee CM, Chiu KW, Wang JH, Lu SN, Tseng PL, Chang KC, Yen YH. A comparison of efficacy and safety of 2-year telbivudine and entecavir treatment in patients with chronic hepatitis B: a match-control study. Clin Microbiol Infect. 2014;20:O90-O100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Lee M, Oh S, Lee HJ, Yeum TS, Lee JH, Yu SJ, Kim HY, Yoon JH, Lee HS, Kim YJ. Telbivudine protects renal function in patients with chronic hepatitis B infection in conjunction with adefovir-based combination therapy. J Viral Hepat. 2014;21:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Amarapurkar DN, Patel N. Increased eGFR with telbivudine in combination therapy of chronic hepatitis B infection. Indian J Gastroenterol. 2014;33:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Tsai MC, Yu HC, Hung CH, Lee CM, Chiu KW, Lin MT, Tseng PL, Chang KC, Yen YH, Chen CH. Comparing the efficacy and clinical outcome of telbivudine and entecavir naive patients with hepatitis B virus-related compensated cirrhosis. J Gastroenterol Hepatol. 2014;29:568-575. [PubMed] |
| 31. | Liang KH, Chen YC, Hsu CW, Chang ML, Yeh CT. Decrease of serum Angiotensin converting enzyme levels upon telbivudine treatment for chronic hepatitis B virus infection and negative correlations between the enzyme levels and estimated glumerular filtration rates. Hepat Mon. 2014;14:e15074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |