Published online Oct 28, 2016. doi: 10.4254/wjh.v8.i30.1262
Peer-review started: April 23, 2016
First decision: June 12, 2016
Revised: August 13, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: October 28, 2016
Processing time: 185 Days and 2.1 Hours
To apply the Frontal Assessment Battery to cirrhotic patients with or without overt hepatic encephalopathy (OHE) and controls.
The frontal assessment battery (FAB) was applied to 87 patients with liver cirrhosis (16 with and 71 without OHE) and 40 control subjects without cirrhosis treated at the alcohol and liver outpatient clinics and the gastroenterology ward of the Cassiano Antônio de Moraes University Hospital (Hospital Universitário Cassiano Antônio de Moraes - HUCAM), Espírito Santo, Brazil.
The average FAB score was lower for the cirrhotic than for the non-cirrhotic patients (10.6 ± 3.67 vs 12.25 ± 2.72, P = 0.015). The FAB score was lower for the cirrhotic patients with OHE than for the patients without OHE (8.25 ± 4.55 vs 11.14 ± 3.25, P = 0.027). The total FAB score was lower for the cirrhotic patients without OHE than for the non-cirrhotic patients, although this difference was not significant (11.14 ± 3.25 vs 12.25 ± 2.72, P = 0.067). Nevertheless, the difference in the scores on the subtest that assessed the ability to inhibit a response previously conditioned to a stimulus was significant (1.72 ± 0.93 vs 2.2 ± 0.85, P = 0.011).
The present study indicates that the FAB is a promising tool for outpatient minimal HE screening and the assessment of HE severity.
Core tip: The diagnosis of hepatic encephalopathy is based on the West Haven classification. Minimal hepatic encephalopathy is defined by cognitive changes in patients with liver cirrhosis or portosystemic shunting without changes in their physical examination. The diagnosis is performed by neurophysiological and/or neuropsychological tests that are difficult to apply and are expensive. The frontal assessment battery (FAB), which is quick and easy to apply, can be used by the clinician. In the present study, the FAB score was lower in cirrhotic patients, especially those with hepatic encephalopathy. The FAB is a promising test for minimal hepatic encephalopathy screening at the bedside and in outpatient clinics.
- Citation: de Souza KZ, Zago-Gomes MP. Frontal assessment battery: A tool for screening minimal hepatic encephalopathy? World J Hepatol 2016; 8(30): 1262-1268
- URL: https://www.wjgnet.com/1948-5182/full/v8/i30/1262.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i30.1262
Hepatic encephalopathy (HE) comprises a heterogeneous group of neuropsychiatric disorders that occur in patients with liver cirrhosis (LC) or portosystemic shunting in the absence of other known brain diseases[1]. The traditional HE classification comprises four grades based on the West Haven criteria for the semi-quantitative grading of mental status[2,3]. Overt HE (OHE) is characterized by the presence of clinical manifestations that are easily recognizable in clinical interviews and physical examinations and corresponds to grades 2 to 4. Subclinical HE includes forms of the disease that are not easily recognizable in the clinical interview and physical examination, such as West Haven grade 1 and minimal HE (MHE), in which the cognitive deficits can only be detected using specialized tests[4,5]. The cognitive deficits caused by MHE have negative impacts on the social and occupational lives of the patients and thus impair their quality of life[2] and are associated with a higher risk of accidents[6]. Because MHE cannot be recognized during a physical examination, its diagnosis is established through the application of tests to apparently normal individuals who exhibit risk factors for the development of this condition[7].
Traditionally, the MHE diagnosis is established based on neurophysiological and/or neuropsychological tests[4]. The majority of these tests are extensive and require a significant amount of time to perform; additionally, they are difficult to interpret and require the expertise of neuropsychiatry professionals[8]. The frontal assessment battery (FAB) developed by Dubois et al[9] is a quick tool; its application requires approximately 10 min. It is well accepted by patients and is useful for identifying the presence and assessing the severity of dysexecutive syndromes that affect cognition and motor behavior[10]. The FAB comprises six subtests (conceptualization, lexical fluency, motor series, conflicting instructions, inhibitory control, and automatic behavior)[9,11]. Originally, the FAB was used to assess patients with Parkinson’s disease who exhibited abnormalities on the Wisconsin Card Sorting Test, Trail Making Test and verbal fluency tests[9,12] and patients with abnormal perfusion of the frontal lobe on imaging tests[13-17]. These studies suggest that the FAB evaluates executive functions of the frontal lobe. Additionally, it has proven useful for distinguishing between Alzheimer’s disease (AD) and frontotemporal dementia (FTD)[18], detecting subclinical dysexecutive alterations in alcoholic subjects, formulating differentiated therapeutic strategies for the management of alcoholic patients on an individual basis[19], and correlating the use of crack cocaine with a decline in the frontal executive functions as a function of the duration of drug use[20]. The present study represents the first application of the FAB in patients with chronic liver disease. The results are compared between individuals with OHE and subclinical HE and the controls.
The aim of the present study was to determine whether the FAB could detect differences between patients with LC and the controls and between cirrhotic patients with and without OHE and to investigate whether this tool might be indicated for outpatient screening for MHE.
The present study assessed 127 individuals treated at the gastroenterology ward and liver and alcohol outpatient clinics of Cassiano Antônio de Moraes University Hospital, Federal University of Espírito Santo (Hospital Universitário Cassiano Antônio de Moraes, da Universidade Federal do Espírito Santo), Espírito Santo, Brazil. A total of 87 patients had LC, including 16 with OHE and 71 without HE. The remaining 40 patients were defined as controls and were matched according to gender and the cirrhosis etiology (alcoholism, hepatitis B, or hepatitis C). All LC patients were assessed and classified based on the Child-Pugh classification for the severity of liver disease. Individuals with clinical manifestations of psychiatric diseases, those who had consumed alcohol in the past 15 d, and those under 18 years of age were excluded from the study.
The LC diagnosis was established based on the combination of clinical criteria and the results of imaging and/or histopathological tests.
Patients with LC and neuropsychiatric disorders detected during the clinical interviews and physical examinations in the absence of other known brain diseases were diagnosed with OHE[1]. The following clinical manifestations were considered: Behavioral alterations, sleep disorders, irritability, and depression. The psychomotor abnormalities included asterixis, bradykinesia, tremors, and rigidity. Additionally, mental confusion and acute temporal-spatial disorientation were considered[21].
The FAB was applied to patients individually by the same examiner using only a paper and pencil; the tests were applied to inpatients at the bedside and at the outpatient clinics. The FAB is described in Table 1.
| Frontal assessment battery |
| (1) Similarities |
| In what way are they alike? |
| (a) A banana and an orange |
| (b) A table and a chair |
| (c) A tulip, a rose, and a daisy |
| 3 correct: 3 |
| 2 correct: 2 |
| 1 correct: 1 |
| None correct: 0 |
| (2) Lexical fluency |
| Say as many words as you can begin with the letter "S", except for proper nouns |
| (3) Motor series |
| Fist, palm, edge (first together, then alone) |
| 6 correct consecutive series alone: 3 |
| At least 3 correct consecutive series alone: 2 |
| 3 correct consecutive series with the examiner: 1 |
| Cannot perform 3 correct consecutive series even with the examiner: 0 |
| (4) Conflicting instructions |
| Tap twice when I tap once |
| Tap once when I tap twice |
| No errors: 3 |
| 1-2 errors: 2 |
| More than 2 errors: 1 |
| Patient imitates the taps of the examiner at least 4 consecutive times: 0 |
| (5) Go/No Go |
| Tap once when I tap once |
| Do not tap when I tap twice |
| No errors: 3 |
| 1-2 errors: 2 |
| More than 2 errors: 1 |
| Patient imitates the taps of the examiner at least 4 consecutive times: 0 |
| (6) Behavior |
| Do not touch my hands |
| The patient’s hands should be on his/her knees with the palm up. Without saying anything, the examiner brings his/her own hands close to the patient’s. If the patient touches the examiner’s hands, the examiner says: Now, do not touch my hands. Then, a new attempt begins |
| Patient does not touch the examiners hands: 3 |
| Patient hesitates and asks what he/she has to do: 2 |
| Patient touches the examiner’s hands without hesitation: 1 |
| Patient touches the examiner’s hands even after he/she was told not to do so: 0 |
To compare the FAB scores to dichotomous categorical variables, the scores were categorized as high (> 11) or low (≤ 11) based on the median scores of the LC patients.
The present study was approved by the ethics committee of the Center of Health Sciences, Federal University of Espírito Santo (Universidade Federal do Espírito Santo), which is associated with the National Commission of Research Ethics (Comissão Nacional de Ética em Pesquisa - CONEP). After receiving a detailed explanation of the study, the participants signed an informed consent form approved by the institutional medical ethics committee.
The statistical analysis was performed with the Epi Info 6.04 e BioEstat 5.3 software. The data were subjected to a descriptive analysis, including the frequency distribution (in the case of qualitative variables), are expressed as absolute numbers (n) and percentages (%), and the means and standard deviations (SD) were calculated. The means of data with a normal distribution were compared using Student’s t-test. Categorical variables were subjected to a cross-tabulation analysis with a χ2 test. Fisher’s exact test was used to compare two variables when the expected frequency according to the null hypothesis was less than five, and the maximum likelihood ratio was used when the exposure variable comprised more than two categories.
The non-parametric Mann-Whitney test was used for continuous variables with a non-normal distribution. Associations with a value of P < 0.05 were considered statistically significant.
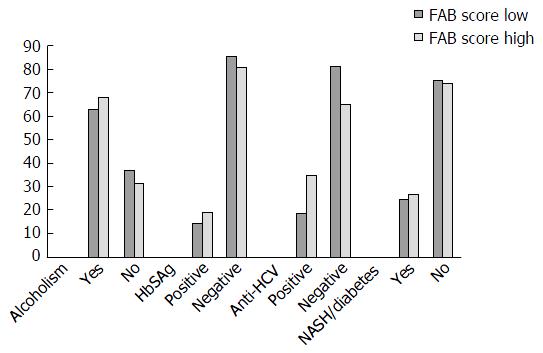
The demographic characteristics of the sample are described in Table 2. The predominant cause of cirrhosis was alcoholism (65.5%). The cirrhosis etiology and the underlying diseases of the control subjects are described in Table 3. No significant differences were detected in the FAB scores related to the liver cirrhosis etiology.
| Variables | Cirrhotic (n = 87) | Non-cirrhotic (n = 40) | P-value |
| Gender | 0.814 | ||
| Male | 68 (78) | 32 (80) | |
| Female | 19 (22) | 8 (20) | |
| Age range | 0.001 | ||
| 50 yr or older | 69 (79) | 20 (50) | |
| Under 50 yr | 18 (21) | 20 (50) | |
| Formal schooling | 0.577 | ||
| None | 5 (6) | 3 (7.5) | |
| 1 to 3 yr | 15 (17) | 3 (7.5) | |
| 4 to 7 yr | 33 (38) | 18 (45) | |
| 8 to 11 yr | 28 (32) | 12 (30) | |
| 12 or more years | 6 (7) | 4 (10) |
| Variables | Cirrhotic (n = 87) | Non-cirrhotic (n = 40) | P-value |
| Alcoholism | 0.434 | ||
| Yes | 57 (65.5) | 29 (72.5) | |
| No | 30 (34.5) | 11 (27.5) | |
| Hepatitis B surface antigen | 0.864 | ||
| Positive | 14 (16) | 7 (17.5) | |
| Negative | 72 (84) | 33 (82.5) | |
| Anti-hepatitis C virus antibodies | 0.316 | ||
| Positive | 22 (25.6) | 7 (17.5) | |
| Negative | 64 (74) | 33 (82.5) | |
| Non-alcoholic steatohepatitis/diabetes | 0.007 | ||
| Yes | 22 (25) | 2 (5) | |
| No | 65 (75) | 38 (95) |
Among the 87 cirrhotic patients, 33 were classified as Child-Pugh class A, 36 were classified as class B, and 17 were classified as class C. Most of the cirrhotic patients with OHE were classified as Child-Pugh class C (56% vs 11% of the cirrhotic patients without OHE, P < 0.001).
Figure 1 shows that no difference was observed in the FAB scores as a function of the cause of disease when the cirrhotic patients and controls were compared.
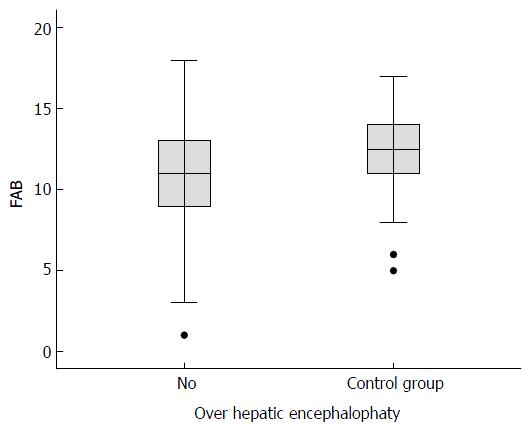
The FAB score was lower (mean 10.6, SD ± 3, maximum score 18) for the cirrhotic patients than for the controls (mean 12.5, SD ± 2.72, P = 0.015). For the cirrhotic patients with OHE, the average score was 8.25 (SD ± 4.55) and the median score was 7.5. In comparison, the scores of the cirrhotic patients without HE were higher (mean 11.4, SD ± 3.25, median 11, P = 0.027). The FAB score was lower for the cirrhotic patients without OHE than for the controls, although the difference was not significant (P = 0.067) (Figure 2).
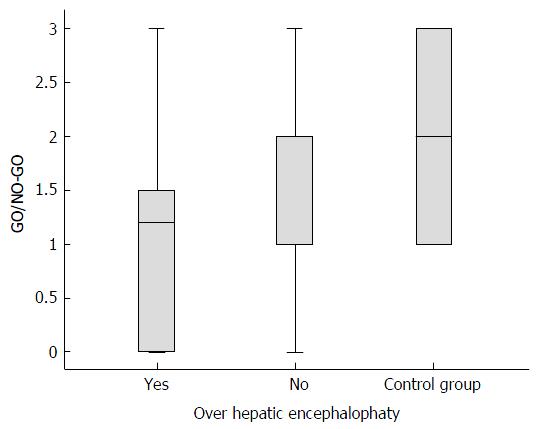
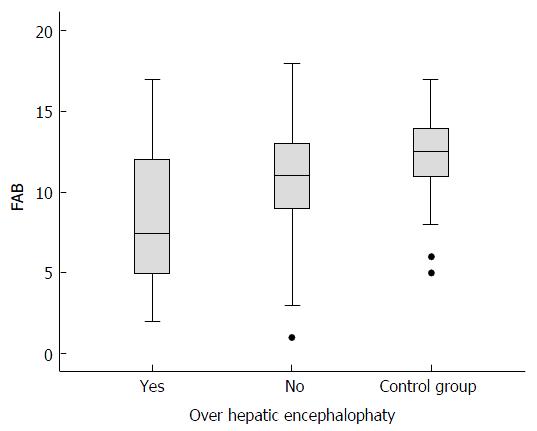
The poorest scores in the cirrhotic group corresponded to the inhibitory control subtest (GO/NO-GO) (mean 1.61, SD ± 0.98) compared with the controls (mean 2.2, SD ± 0.85, P = 0.02). The GO/NO-GO scores were lower for the cirrhotic group without OHE (mean 1.72; SD ± 0.93) than for the controls (mean 2.2, SD ± 0.85, P = 0.011). The GO/NO-GO scores were also lower for the cirrhotic group with OHE (mean 1.13, SD ± 1.09) than for the cirrhotic group without OHE (mean 1.72, SD ± 0.93, P = 0.02), as shown in Figure 3. In this subtest, the individuals were required to inhibit a learned behavior (clap once when the examiner claps twice) and then perform a different task (do not clap when the examiner claps twice). No significant differences were detected in any of the other subtests in the comparison between the cirrhotic patients without OHE and the control group (Figure 4).
Of the 16 cirrhotic patients with OHE, 12 exhibited low FAB scores (12/16, 75%). Low FAB scores were exhibited by 37/71 (52%) cirrhotic patients without HE and 17/40 (43%) controls. The linear association test detected a significant difference among the groups (P = 0.038).
Several studies have shown that MHE causes abnormalities in the attention, social interactions, behavior, and quality of sleep of patients, with consequent impairment of the performance of the activities of daily living. Additionally, interference with more complex activities, such as driving ability or planning a trip, impairs the quality of life of patients and may increase the risk of accidents involving themselves and others[2].
The investigation of patients with cirrhosis by the West Haven test is not sufficient to identify subclinical forms of encephalopathy[22]. Traditionally, the MHE diagnosis is established based on the detection of neurological dysfunctions in neurophysiological and/or neuropsychological tests[4]. However, these tests are rather long, time consuming, and difficult to interpret[8]. Dhiman et al[3] suggested that the Psychometric Hepatic Encephalopathy Score, which is a battery of neuropsychological tests that can detect abnormalities such as alterations of motor function, visuospatial orientation, visual perception, visual construction, attention, concentration, and (with somewhat lower efficacy) memory disorders, should be considered the gold standard for the assessment of MHE, whereas computer-based tests such as Critical Flicker Frequency and Inhibitory Control Test should be used for screening. However, neurophysiological tests are difficult to apply in an outpatient setting because they require modern facilities and equipment[2].
A study was conducted in which the Mini-Mental State Examination was applied to patients with LC and OHE, MHE, or without HE to establish whether this test might be used as a screening method for MHE and HE West Haven grades 1 and 2. However, a significant difference was not found between the scores of patients with MHE and those without HE. Moreover, alteration of the mental status was only detected in patients with OHE West Haven grade 3, which could be clinically detected without additional assessment methods[21].
Citro et al[22] conducted a study that applied the Trail Making Test (a simple inexpensive test) in a recent series evidenced a poor psychometric performance in more than half of the patients who were free of manifest encephalopathy. The authors also observed that subclinical hepatic encephalopathy was mostly present in patients with HCV-related cirrhosis. In the present study, there was no difference in FAB scores related to the cirrhosis etiology.
The present study used the FAB described by Dubois et al[9] as a useful and practical tool to establish the presence and severity of dysexecutive syndromes affecting cognition and motor behavior[9] and to assess patients with and without LC.
Some studies have indicated that the FAB evaluates the executive functions of the frontal lobe. In clinical practice, the FAB has also been used to distinguish between AD and FTD at the bedside, even in the earliest stages of disease[23]. One case-control study compared a group of 170 alcoholic subjects to a group of 40 non-alcoholic controls to assess frontal functions in different categories of alcoholism according to the Lesch typology. The use of the FAB as an assessment instrument allowed the detection of subclinical dysexecutive abnormalities among the alcoholic subjects. These data might serve to formulate differentiated therapeutic strategies for the management of alcoholic patients on an individual basis[20].
The FAB was also used as a tool in a descriptive cross-sectional case series study of 72 crack cocaine users in which their patterns of drug use, global cognition, and frontal executive functions were assessed. The results indicated a decline in executive functions associated with the duration of drug use, especially for the functions investigated by the Automatic behavior subtest[20].
No reports exist in the literature of studies investigating the frontal executive functions in cirrhotic patients. Therefore, no reference values for normality existed to compare with the results of the cirrhotic and non-cirrhotic patients. In the present case-control study, the FAB was applied to cirrhotic and non-cirrhotic patients. The performance of the former group was poorer, suggesting a considerable difference in the performance on the FAB between these two patient groups. The lower scores exhibited by the cirrhotic patients might be attributed to the presence of MHE, which is a subclinical condition that affects less than 15% of cirrhotic Child-Pugh class A patients and approximately 50% of patients classified as Child-Pugh class B or C[10]. Among patients with advanced liver disease according to the Child-Pugh classification, the scores of patients classified as Child-Pugh class C were not significantly different from the overall group of cirrhotic patients who exhibited the lowest FAB scores. However, the cirrhotic patients classified as Child-Pugh class C exhibited poorer performance in the GO/NO-GO and Automatic behavior subtests.
Abnormalities in the FAB GO/NO-GO subtest indicate difficulty in inhibiting inappropriate responses due to injury to the ventral part of the frontal lobe[9]. In the present study, the scores exhibited by cirrhotic patients were significantly lower than the scores of the non-cirrhotic patients. This finding might be explained by the executive dysfunction caused by MHE[8].
No other FAB subtest scores (conceptualization, verbal fluency, motor series, conflicting instructions, and automatic behavior) were significantly different between the cirrhotic and non-cirrhotic patients.
Detecting minimal hepatic encephalopathy in patients with cirrhosis may help improve their quality of life[22]. The FAB score was lower for patients with OHE than for cirrhotic patients without OHE. This finding indicates that the FAB can detect the psychomotor abnormalities characteristic of OHE[1].
Regarding the FAB subtests, patients with OHE exhibited lower scores on the Motor series subtest, which indicated injury to the frontal lobe that impaired temporal organization and the maintenance and execution of successive actions. Additionally, this group of patients exhibited lower scores on the GO/NO-GO subtest, in which abnormalities indicated difficulty inhibiting inappropriate responses. These findings show that the FAB detects HE-related cognitive dysfunctions in the early subclinical stage of disease[2] via the GO/NO-GO subtest as well as the psychomotor abnormalities characteristic of OHE, which are recognizable in the physical examination[3] via the Motor series subtest.
Although the FAB score exhibited by the cirrhotic patients without MHE was lower than the average score of the controls, the difference was not significant. Further studies with larger sample sizes are needed to thoroughly investigate this difference. The score on the GO/NO-GO subtest was significantly lower for the cirrhotic patients without OHE than for the non-cirrhotic patients. We may infer that this difference is due to the presence of MHE-related executive dysfunctions in some cirrhotic patients who do not have clinical OHE manifestations.
Currently, no studies have applied the FAB to cirrhotic patients with or without complications such as HE to establish the cutoff point for defining normality. However, the differences in the FAB scores between the cirrhotic and non-cirrhotic patients and between patients with and without HE in the present study demonstrate its value. Further studies are needed to determine whether the FAB could be used as a screening tool for all grades of OHE severity and particularly as a helpful tool for the assessment of West Haven grade 1 and MHE in clinical practice[1].
In conclusion, the FAB is a promising tool with an easy and quick application that may be used by trained general practitioners in both the outpatient setting and at the bedside as a screening method for West Haven grade 1 HE and MHE. Further studies are needed to validate this tool and compare it to other neuropsychometric batteries currently used to detect MHE.
I thank my professor, Dr. Maria da Penha Zago-Gomes; my patients; and the institution Universidade Federal do Espirito Santo, where I graduated and developed my study.
The usual diagnostic minimal hepatic encephalopathy (MHE) tests are time consuming and require the participation of specialized professionals. The delay in diagnosis has a negative impact on the quality of life of patients and leads to a higher risk of progression to overt HE. The availability of an easily applicable screening test for MHE, such as the frontal assessment battery (FAB), allows early detection of MHE and its treatment.
The FAB is a battery of tests with easy and quick application that was originally used for the evaluation of patients with dysexecutive syndromes of the frontal lobe, such as Parkinson’s disease, chronic alcohol abusers and crack users. The FAB can also be useful in differentiating the frontotemporal dementia of Alzheimer’s disease. Therefore, the FAB would be a useful tool for evaluating the presence and severity of dysexecutive syndromes that affect cognition and motor behavior in liver cirrhosis patients.
Previous articles in the literature unsuccessfully proposed simple and inexpensive screening tests for MHE, such as the Mini Mental and Trail Making Test. However, the application of the FAB for the evaluation of MHE and HE in patients with liver cirrhosis is unique. Their study suggests that the FAB should be considered a promising tool for screening MHE and HE by the clinician.
The FAB is a promising tool for screening HE and MHE in patients both at the bedside and in outpatient clinics.
It is a good paper.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tarantino G S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1410] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 2. | Dhiman RK, Saraswat VA, Sharma BK, Sarin SK, Chawla YK, Butterworth R, Duseja A, Aggarwal R, Amarapurkar D, Sharma P. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Dhiman RK, Chawla YK. Minimal hepatic encephalopathy. Indian J Gastroenterol. 2009;28:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology. 2012;55:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, Gibson DP, Hoffmann RG, Stravitz RT, Heuman DM. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Sharma P. Minimal hepatic encephalopathy. J Assoc Physicians India. 2009;57:760-763. [PubMed] |
| 8. | Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2634] [Cited by in RCA: 2801] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 10. | Moorhouse P, Gorman M, Rockwood K. Comparison of EXIT-25 and the Frontal Assessment Battery for evaluation of executive dysfunction in patients attending a memory clinic. Dement Geriatr Cogn Disord. 2009;27:424-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Beato RG, Nitrini R, Formigoni AP, Caramelli P. Brazilian version of the frontal assessment battery (FAB). Dement Neuropsychol. 2007;1:59-65. |
| 12. | Cohen OS, Vakil E, Tanne D, Molshatzki N, Nitsan Z, Hassin-Baer S. The frontal assessment battery as a tool for evaluation of frontal lobe dysfunction in patients with Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Guedj E, Allali G, Goetz C, Le Ber I, Volteau M, Lacomblez L, Vera P, Hitzel A, Hannequin D, Decousus M. Frontal Assessment Battery is a marker of dorsolateral and medial frontal functions: A SPECT study in frontotemporal dementia. J Neurol Sci. 2008;273:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Kim TH, Huh Y, Choe JY, Jeong JW, Park JH, Lee SB, Lee JJ, Jhoo JH, Lee DY, Woo JI. Korean version of frontal assessment battery: psychometric properties and normative data. Dement Geriatr Cogn Disord. 2010;29:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Oshima E, Terada S, Sato S, Ikeda C, Nagao S, Takeda N, Honda H, Yokota O, Uchitomi Y. Frontal assessment battery and brain perfusion imaging in Alzheimer’s disease. Int Psychogeriatr. 2012;24:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yoshida H, Terada S, Sato S, Kishimoto Y, Ata T, Ohshima E, Honda H, Ishihara T, Kuroda S. Frontal assessment battery and brain perfusion imaging in early dementia. Dement Geriatr Cogn Disord. 2009;27:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Benke T, Karner E, Delazer M. FAB-D: German version of the Frontal Assessment Battery. J Neurol. 2013;260:2066-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Slachevsky A, Villalpando JM, Sarazin M, Hahn-Barma V, Pillon B, Dubois B. Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch Neurol. 2004;61:1104-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Zago-Gomes MP, Nakamura-Palacios EM. Cognitive components of frontal lobe function in alcoholics classified according to Lesch's typology. Alcohol Alcohol. 2009;44:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Moscon J. Avaliação dos padrões de consumo, cognição global e de funções executivas em usuários de crack em ambulatório especializadode alta demanda. Tese de mestrado apresentada ao programa de pós-graduação em ciências fisiológicas do centro de ciências da saúde da Universidade Federal do Espírito Santo, ES. 2013;. |
| 21. | Koziarska D, Wunsch E, Milkiewicz M, Wójcicki M, Nowacki P, Milkiewicz P. Mini-Mental State Examination in patients with hepatic encephalopathy and liver cirrhosis: a prospective, quantified electroencephalography study. BMC Gastroenterol. 2013;13:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Citro V, Milan G, Tripodi FS, Gennari A, Sorrentino P, Gallotta G, Postiglione A, Tarantino G. Mental status impairment in patients with West Haven grade zero hepatic encephalopathy: the role of HCV infection. J Gastroenterol. 2007;42:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Lipton AM, Ohman KA, Womack KB, Hynan LS, Ninman ET, Lacritz LH. Subscores of the FAB differentiate frontotemporal lobar degeneration from AD. Neurology. 2005;65:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |