Published online Jan 28, 2016. doi: 10.4254/wjh.v8.i3.191
Peer-review started: November 3, 2015
First decision: November 24, 2015
Revised: December 1, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: January 28, 2016
Processing time: 79 Days and 7.5 Hours
Ablative treatment methods have emerged as safe and effective therapies for patients with primary and secondary liver tumors who are not surgical candidates at the time of diagnosis. This article reviews the current literature and describes the techniques, complications and results for radiofrequency ablation, microwave ablation, cryoablation, and irreversible electroporation.
Core tip: Innovative ablation techniques, including radiofrequency ablation, microwave ablation, cryoablation and irreversible electroporation have become accepted as treatment modalities for patients with early stage tumor or for single metastases. This review paper describes the available ablation techniques and summarizes the evidence supporting the use of each modality.
- Citation: Ryan MJ, Willatt J, Majdalany BS, Kielar AZ, Chong S, Ruma JA, Pandya A. Ablation techniques for primary and metastatic liver tumors. World J Hepatol 2016; 8(3): 191-199
- URL: https://www.wjgnet.com/1948-5182/full/v8/i3/191.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i3.191
The liver is a common site for both primary malignancy and metastatic disease. Hepatocellular carcinoma (HCC) remains the fifth most common malignancy in the world and its incidence is rising[1,2]. Traditionally, the first line therapy for hepatic tumors has been surgical resection or transplantation. However, many patients are not surgical candidates at the time of diagnosis[2]. For this reason interest in minimally invasive, ablative treatment methods has grown[3]. Percutaneous ablative techniques include radiofrequency ablation (RFA), microwave ablation, cryoablation, and irreversible electroporation (IRE). This review focuses on the use of percutaneous ablative techniques in the treatment of HCC, as well as of metastatic disease from colorectal, neuroendocrine, and breast carcinomas.
RFA is a low risk alternative treatment for HCC and liver metastases in patients who cannot undergo surgery or transplant[4]. Unlike other non-surgical strategies (TACE, Y90), the goal of RFA is curative[4].
RFA creates a closed loop circuit which results in an alternating electric field causing agitation of ions within the target tissue[5]. The circuit is created using an RF generator, an electrode, grounding pads, and the patient[3]. The resultant ionic agitation creates heat leading to cell death from coagulative necrosis[6]. In order to ensure tumor destruction, the mass needs to be treated to a temperature of 50 °C-100 °C for approximately 4-5 min[6]. Temperatures higher than 100 °C can cause gas formation, also known as carbonization, which can reduce ablation effectiveness, and char adjacent tissues[7].
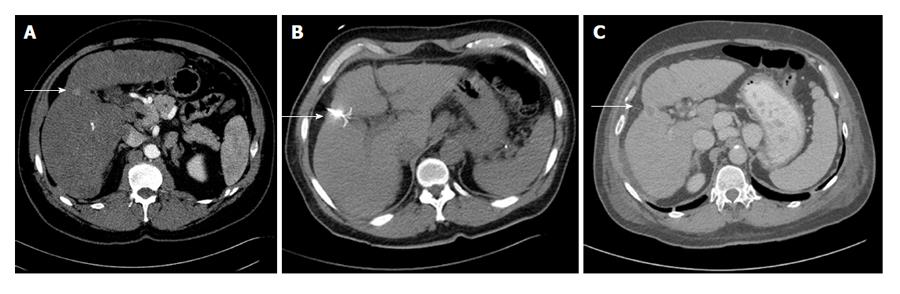
In order to achieve primary technical success, the entire tumor must be ablated as well as a sufficient margin around the tumor. Similar to surgical techniques, a 1 cm margin in all planes is needed to minimize the risk of residual disease or local recurrence[3]. Therefore, the planned target ablation diameter should be 2 cm larger than the tumor diameter[3]. If the tumor is small enough, this can be accomplished with one electrode (Figure 1). However, if the tumor is too large, multiple ablations can be performed[8], although there is a risk of local recurrence due to inadequate tumor destruction from the error inherent in positioning electrodes[3]. Other causes of inadequate tumor ablation include heterogeneous tissue composition (i.e., fibrosis, calcification) and adjacent blood flow, known as a “heat sink”, which can cool the tissue and reduce the maximum achieved temperature[9].
RFA can be performed with guidance by ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI) depending on lesion visibility and operator experience. Patients typically receive either conscious sedation or general anesthesia to control pain and minimize patient movement during the procedure. The decision to administer prophylactic antibiotics is somewhat controversial and institution dependent. A longer course of antibiotics may be warranted in patients who are at increased risk of liver abscess, including patients with a history of biliary-enteric anastomosis, biliary stents, or sphincterotomy[6]. This is thought to be due to retrograde movement of bacteria into the ablation cavity as a result of altered anatomy[10].
RFA has a low rate of major complications. The largest study on RFA complications by Koda et al[11] evaluated 13283 patients (16346 treated lesions) with a total of 579 complications (3.5%) and 5 deaths (0.04%). The rate of liver injury was 1.69% (276 patients) which included 75 (0.47%) hepatic infarcts, 32 (0.19%) liver abscesses, 110 (0.67%) bile duct injuries, and 37 (0.23%) bile leaks[11]. A more recent study from Lee et al[1] reported a similar major complication rate of 3.1% in 169 treated lesions, including 2 bile duct injuries. The overall reported complication rate ranges from 2.2% to 9.5%[6].
The rate of extrahepatic injury is also extremely rare. Koda et al[11] reported a total of 113 (0.69%) extrahepatic complications including, in order of decreasing frequency, pleural effusions, skin burns, pneumothorax, gastrointestinal injury, diaphragmatic injury, gallbladder injury, and cardiac tamponade. The risk of extrahepatic injury can be reduced by a technique called “hydrodissection”, which involves injecting D5W to create space between adjacent organs. Saline infusions are not used for hydrodissection due to the theoretical risk of conduction of the electrical current through this type of fluid. Another potential complication is seeding, either in the peritoneum or along the ablation track. The reported risk of tumor seeding ranges from 0.04%[11] to 0.6%[12]. The risk (0.95%) of tumor seeding has been described to be slightly increased when concomitant biopsy is performed[12].
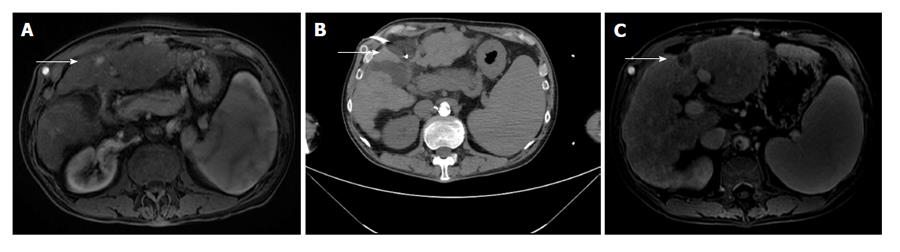
Cryoablation involves rapid cooling of a cryoprobe resulting in cell death[13]. Cryoablation has been historically used for both HCC (Figure 2) and hepatic metastases.
Cryoablation works by passing high pressure argon gas through a probe resulting in cooling of the metallic. As the probe cools, surrounding tissues are also cooled by convection and conduction[14]. Helium gas is then forced through the probe causing warming of the probe and thawing of the adjacent tissues. The cooling and subsequent thawing of the probe results in cell death by a variety of methods. The initial cooling results in intracellular ice crystal formation leading to cell membrane damage and death[15]. Larger ice crystals also form during slow thawing, resulting in a shearing effect and additional cell death[16]. Lastly, ice crystals develop in the small blood vessels feeding a tumor, leading to ischemia[16]. Like the other ablative techniques, cryoablation can be performed percutaneously or laparoscopically. Percutaneous cryoablation can be performed with CT, MRI or US guidance.
Although cryoablation has many uses for tumor ablation, including renal and osseous lesions, its utility in the liver is somewhat limited. The disadvantages of cryoablation include variable ablation size (resulting in the need for multiple cryoprobes), reduced cooling effect due to a heat sink from hepatic vessels, and the risk of major complications. An advantage of cryoablation over other ablative techniques is that the ice ball can be visualized during the procedure under both CT and ultrasound guidance, allowing for better adjustment.
The risk of complication within the liver is higher with cryoablation compared to RFA. Complications include hemorrhage, injury to adjacent organs, biliary injury, and “cryoshock”. Hemorrhage results from ice ball formation within the liver leading to shearing injury to the liver parenchyma and nearby blood vessels. Shearing forces can also cause biliary injury which can lead to late hemorrhage or hepatic abscess formation. Cryoablation of lesions near the liver edge risks damage to adjacent organs, usually bowel, kidney or adrenal glands. A complication unique to cryoablation is “cryoshock” which occurs due to the release of cytokines, resulting in a systemic syndrome characterized by fever, tachycardia, and tachypnea. A retrospective study by Adam et al[17] found increased complication rates among patients treated with cryoablation (29%) compared to those who underwent RFA (8%). Additional studies have found similar results including a study demonstrating a 41% complication rate for cryoablation patients compared to 3% in patients who underwent RFA[18].
However, a large study by Yang et al[19] found very low rates of major complications with cryoablation. In this study of 300 patients who underwent cryoablation, the major complication rate was 6.3%[19]. Major complications included cryoshock (6 patients), extensive hemorrhage (5 patients), gastric bleeding (4 patients), liver abscess (1 patient), intestinal fistula (1 patient), and liver failure (2 patients)[19]. The risk of minor complications is reported to be 48.6%[19]. These include fever, pain, skin frostbite, pleural effusion, and arterial-portal venous fistula. Pneumothorax is rarely reported in treated tumors located near the diaphragm[19].
Microwave ablation is an emerging technology with particular applicability in treating hepatic tumors in patients who are not surgical candidates. It has been used for larger tumors than those treated by RFA[20].
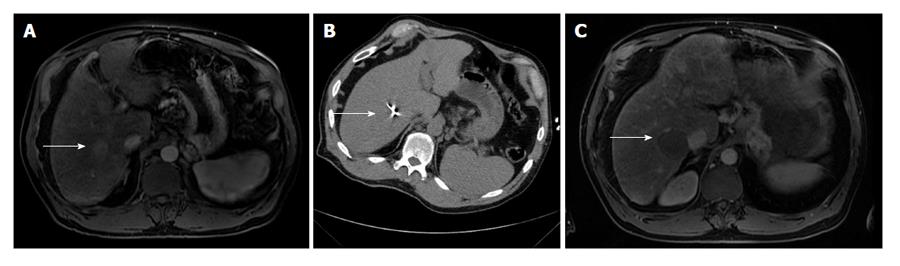
Microwave ablation utilizes an antenna to locally deliver a high frequency (915 MHz or 2.45 GHz) oscillating electromagnetic field to induce rapid realignment of polar molecules (typically water molecules) in a lesion (Figure 3). This results in markedly increased kinetic energy and subsequent tissue heating[21]. Tissues with a larger concentration of water, such as tumors, are particularly susceptible to microwave heating[21].
Microwave ablation can be performed with one or multiple antenna probes. Multiple antenna probes in close proximity allow for electrical and thermal synergy. Multiple probes can also be powered simultaneously which is not possible with RF ablation. Recent developments in microwave technology have produced high-powered water cooled systems which allow for smaller applicators and increased power.
Compared to RF ablation, microwave has several advantages. Microwave is capable of producing very high temperatures (greater than 150 °C) much faster than RF. In addition, microwave is more effective in propagating heating through charred and desiccated tissues which allows for a large ablation zone. Microwave does not require grounding pads or other similar devices[15]. Microwave ablation is not as susceptible to heat sink phenomena as RF ablation. This is particularly useful in the liver, which has a rich vascular supply. A recent study demonstrated larger zones of ablation and faster heating with microwave compared to RFA[22]. Additional studies have demonstrated larger and more consistent ablation zones with microwave without significant influence from adjacent hepatic vessels[23,24]. Ablation time is often less than 10 min, typically averaging 2-5 min, which improves overall efficiency and reduces anesthesia time.
Although microwave ablation is promising, several disadvantages have limited its widespread adoption. Compared to RFA, microwave power is more difficult to generate safely, mostly due to larger cables which are prone to heating issues[21]. In addition there remains still uncertainty about the size and shape of ablation zones with microwave[21].
Microwave ablation is typically performed under general anesthesia to reduce patient discomfort and for better control of patient breathing and motion. As with RF ablation, microwave can be performed under CT or ultrasound guidance. Ultrasound allows for real time monitoring of the ablation and shorter procedure time. CT guidance allows for localization of lesions which are difficult to visualize, and for better evaluation of adjacent structures. Hydrodissection can be used to displace adjacent structures, typically bowel or diaphragm.
A systematic review of the literature by Lahat et al[25] evaluated the safety of ablative techniques including microwave ablation. In the review of 16 studies, they reported a major complication rate of 4.6% for microwave ablation compared to 4.1% for RFA. The pooled mortality rate for microwave was 0.23% compared to 0.15% for RFA. The most common major complication was hemorrhage requiring blood transfusion. Additional complications included portal vein thrombosis, bile leak/biloma, liver abscess, pleural effusion, and tumor seeding.
IRE is a relatively new non-thermal ablative technique approved by the Food and Drug Administration in 2006 for soft tissue ablation[26]. It has been used for liver, pancreas, kidney and lung ablations. IRE has several advantages over current, more proven ablative techniques.
IRE utilizes multiple electrodes to deliver high voltage (2-3 kV) direct current pulses lasting microseconds to milliseconds[27]. The repeated electrical pulses cause damage to the cell membranes[26]. Initially the cell membrane damage is reversible, but it becomes irreversible after a period of time leading to apoptosis[26]. Because of the extremely short ablation time, care must be taken to ensure proper electrode positioning as mid treatment adjustment is not possible. Most IRE devices require simulation planning with the use of multiple probes placed in parallel to achieve the desired ablation zone.
IRE results in a well-defined ablation zone with sharp margins and relatively little damage to nearby tissues[27]. Because IRE does not utilize thermal methods for ablation, adjacent tissue architecture is well preserved[28]. The combination of fast ablation times and minimal damage to nearby tissues makes IRE well suited for treatment of lesions in sensitive locations, including those adjacent to blood vessels and bile ducts. In addition, this eliminates the problems with heat sink seen in other thermal ablative techniques. However, the use of multiple parallel probes results in a significant increase in procedural cost and complexity[29]. One potential drawback to IRE is that imaging changes related to the ablation zone may take several minutes to manifest by ultrasound[30]. IRE also requires general anesthesia with paralytic agents as the electrical current generated during the procedure can cause muscle spasms and arrhythmias[31]. To lessen this risk, the IRE generator is connected to an ECG triggering device and pulses are delivered to the target/treatment zone during the cardiac refractory period[27].
A recent large systematic review investigated the safety and efficacy of IRE in several organs. The reported overall complication rate was 16% in 129 treated patients[26]. The most common complications included pneumothorax, portal vein thrombosis, biliary occlusion, pleural effusion, and cholangitis[26]. There was no periprocedural mortality reported in treated liver lesions, although 3 patients died after pancreatic IRE[26]. Self-reported post-procedural pain scores were similar between patients treated with IRE and RFA. Arrhythmias were reported in 4% of cases[26]. Ventricular arrhythmias were seen without synchronized pulse delivery while only atrial arrhythmias were seen in patients who received synchronized pulses[26]. No uncontrolled muscle spasms were reported in any of the reviewed studies in patients who received paralytic agents[26].
Radiofrequency ablation: Numerous studies support the usage of RFA as a first line treatment for HCC in patients who are poor surgical candidates. One of the largest studies by Tateishi et al[32] evaluated RFA of 2140 nodules measuring less than 3 cm in 664 patients. Survival rates at 1-5 years post-treatment were similar for patients with first line RFA alone compared with those who underwent RFA as part of a combination therapy[32]. In addition, the rate of local progression of disease was similar for RFA alone when compared to ethanol treatment or hepatectomy[32]. A study by Lencioni et al[33] evaluated patients with early stage HCC (single lesion < 5 cm or up to 3 lesions < 3 cm each) who underwent RFA alone or palliative TACE or ethanol injection. Overall survival rates at 5 years were 48% with a median survival of 57 mo for the RFA group, which was not significantly different from the TACE or ethanol groups[33]. Histologic analysis of tumors which underwent RFA and subsequent transplantation found that 74% of ablated tumors were treated successfully by histologic criteria[34]. For tumors measuring less than 3 cm, the percentage successfully treated rose to 83%[34]. Another large study of 1502 HCC tumors in 1305 patients over 12 years by Kim et al[35] found survival rates of 59.7% and 32.3% at 5 and 10 years respectively. Additional studies have demonstrated similar overall recurrence and survival rates for patients who were poor surgical candidates using RFA as first line treatment[36].
Several recent studies have evaluated RFA as a first line treatment in tumors measuring more than 3 cm. A study by Lee et al[1] evaluated 162 patients who underwent RFA for up to three tumors with a maximum diameter of 5 cm. Overall 5 year survival and recurrence-free survival rates were 67.9% and 25.9% respectively[1]. The most significant predictors of poor survival were Child-Pugh class B, elevated serum α-fetoprotein level, and presence of portal-systemic collaterals[1]. The rate of local tumor progression at 5 years was 14.5% with tumor size being the only significant predictive factor[1]. Local tumor progression did have a significant negative effect on median recurrence free survival (28.0 mo vs 12.0 mo) and resulted in over two times more interventional procedures[1]. A study by Livraghi et al[37] evaluated RFA of 126 HCCs larger than 3 cm in 114 patients. Complete necrosis on follow up CT scan was observed in 47.6% of patients and near complete necrosis (90%-99%) was observed in 31.7% of patients. The observed complication rate was similar to other studies[37].
More recent studies have called into question the conclusion that RFA is equivalent to surgery in the treatment of HCC. A recent meta-analysis by Qi et al[38] evaluated 3 randomized control trials. Surgical resection was found to be superior to RFA with respect to overall survival (HR = 1.41) and recurrence free survival (HR = 1.41)[38]. However, surgical patients had a significantly higher incidence of complications and a significantly longer hospital stay than patients treated with RFA[38]. A more recent study by Miura et al[39] investigated 2804 patients who underwent ablation or surgical resection for a solitary HCC < 3 cm. Overall survival at 3 and 5 years was higher in the resection group (67%, 55%) than in the RFA group (52%, 36%)[39]. There were baseline differences between the two groups which somewhat limited the analysis. However, after propensity matching, the overall survival rate was still higher in the resection group (54%) vs RFA (37%)[39]. Surgical resection was also independently associated with improved survival (HR = 0.62)[39].
Cryoablation: Multiple studies have evaluated the utility of cryoablation in the treatment of HCC. Chen et al[40] performed percutaneous cryoablation in 76 lesions of unresectable HCC and 76 lesions of recurrent HCC. 1 and 3 year survival rates in the unresectable group were 81.4% and 60.3% while the disease-free survival rates were 67.6% and 20.8%[40]. Survival rates in the recurrent HCC group were 70.2% and 28.8% at 1 and 3 years respectively, while the disease-free survival rates were 53.8% and 7.7%. There was a low overall complication rate (12.1%) and there were no peri-procedural deaths[40]. A similar study by Wang et al[41] evaluated cryoablation of 156 patients with HCC < 5 cm in diameter. The reported 1, 2 and 3 years overall survival rates were 92%, 82% and 64%[41]. Disease free survival rates were 72%, 56% and 43% at 1, 2 and 3 years[41].
One of the largest studies evaluating cryoablation and HCC was performed by Yang et al[19] and looked at 300 patients with unresectable HCC. A total of 223 tumors were incompletely ablated while 185 tumors were completely ablated[19]. The rate of local progression of disease at a median 36.7 mo follow up time was 31%[19]. The most significant risk factors for tumor recurrence were size and tumor location. The mean survival of patients after cryoablation was 45.7, 28.4 and 17.7 mo, in increasing order of tumor stage[19]. A study by Adam et al[17] looked at cryoablation vs RFA for unresectable HCC. Despite similar initial post-treatment results, they found a significantly higher rate of local progression of disease in patients treated with cryoablation vs RFA (53% vs 18%)[17].
Microwave: Many studies have demonstrated the safety and effectiveness of microwave ablation in the treatment of HCC. Dong et al[42] studied 234 patients who underwent microwave ablation, showing 1, 2, 3, 4 and 5 years survival rates of 92.7%, 81.6%, 72.9%, 66.4% and 56.7%. The reported local recurrence rate was 7%[42]. A more recent study from Ziemlewicz et al[43] of microwave ablation in 107 HCC lesions found an overall survival rate of 76.0% at median 14 mo follow up. The primary effectiveness was 93.7% for tumors 4 cm or smaller and 75.0% for tumors greater than 4 cm[43], with an overall primary effectiveness of 91.6%. This illustrates the ability of microwave to effectively treat larger tumors measuring more than 4 cm in diameter. No major complications or mortality were reported[43]. A study of microwave ablation in 182 patients with a single HCC was performed by Sun et al[44]. The complete ablation rate was 93%[44]. The overall survival rates were 89%, 74% and 60% at 1, 2 and 3 years respectively, while the recurrence-free survival rates were 51%, 36%, 27% at 1, 2 and 3 years respectively. Tumor recurrence was associated with increasing patient age and tumor size. The major complication rate was 2.7%[44].
Microwave ablation also compares favorably to treatment with RFA. A study of 102 patients with HCC found similar complete ablation rates of 94.9% for microwave and 93.1% for RFA[45]. The local recurrence rate was better with microwave ablation (11.8%) when compared to RFA (20.9%)[45]. A similar study by Shibata et al[46] reported complete ablation rates of 89% for microwave ablation compared to 96% for RFA. Overall complication rates were also similar.
Irreversible electroporation: There is less data on the efficacy of IRE in comparison with other ablative techniques because the procedure is relatively new. However, several studies have demonstrated the efficacy of IRE in treating hepatocellular carcinoma. Cheung et al[47] evaluated IRE of 18 HCC lesions in 11 patients with a size range of 1.0-6.1 cm and a mean follow up of 18 mo. In tumors measuring less than 3 cm, complete ablation was achieved in 93%, with an overall 73% complete ablation rate. Cannon et al[48] reported a primary efficacy of 97% in 14 HCC lesions ranging in size from 1.1-5.0 cm. Thomson et al[31] performed IRE in 18 patients with HCC, achieving complete tumor ablation in 15 patients.
Radiofrequency ablation: Percutaneous RFA is also increasingly used to treat hepatic metastases, including metastases from colorectal carcinoma (CRCLM), neuroendocrine tumors, and breast cancer[4]. The requirements for surgical resection of metastases are similar to HCC and therefore only 10%-20% of patients are surgical candidates at the time of presentation[49]. The ideal candidate for RFA has biopsy proven hepatic metastases without underlying liver disease. A study of patients with colorectal metastases who were not surgical candidates and underwent RFA found survival rates of 86%-99%, 46%-68%, and 24%-44% at 1, 3 and 5 years respectively[9]. A study by Oshowo et al[50] of patients with a solitary CRCLM reported a 3-year survival rate of 52% in patients who underwent RFA vs 55% in patients who underwent surgery. Kim et al[51] found similar overall and disease free survival rates in patients who underwent resection vs RFA for a solitary CRCLM < 3 cm. The disease free survival rate was significantly lower in patients with metastases > 3 cm[51]. There is additional data supporting the role of RFA as an adjunctive therapy in palliative treatment of CRCLM vs chemotherapy alone. Berber et al[52] evaluated RFA in 135 patients with colorectal metastases and found a median survival of 28.9 mo, compared to 11-14 mo in patients who underwent chemotherapy alone.
RFA has also been successfully used in treating hepatic metastases from neuroendocrine tumors. As with HCC and colorectal metastases, only 10% of patients with neuroendocrine metastases are surgical candidates at the time of presentation. Berber et al[53] evaluated the role of RFA in treating patients with carcinoid syndrome, as well as other neuroendocrine metastases. Two hundred and thirty-four tumors in 34 patients were treated with RFA[53]. Symptoms were improved in 95% of patients with significant or complete symptom control seen in 80% of patients[53]. This was compared to a response rate of 90% with surgery and 50%-88% with somatostatin analogues[53]. The rate of local progression of disease was 26% during the follow up period (1.6 years) while 41% of patients had no evidence of disease progression during the same period[53]. Another study by Elvin et al[54] of 109 RFA treatments of neuroendocrine metastases showed a local recurrence rate of 10% during follow up (mean 3.2 years) with CT evidence of successful treatment in 90% of patients.
Cryoablation: The data on using cryoablation for metastatic disease is limited compared to the data for RFA since few centers use cryoablation for treating liver lesions. An older study by Kerkar et al[55] in 2004 evaluated 56 patients who underwent cryoablation for colorectal metastases. The 3 and 5 years overall survival rates in the colorectal metastases group was 43% and 22% with a median survival of 30 mo[55]. A more recent and larger study by Ng et al[56] reported the results of cryoablation in 293 patients with unresectable colorectal metastases. 1-, 3-, 5- and 10-year survival rates were 87%, 41.8%, 24.2% and 13.3%[56]. Disease-free survival rates were 37.9%, 17.2%, 13.4% and 10.8% at 1, 3, 5 and 10 years[56]. “Recurrences” were reported elsewhere in the liver in 73%, at the cryoablation site in 23%, and at the edge of the ablation cavity in 14%[56].
Seifert et al[57] reported results of cryoablation in 13 patients with metastatic neuroendocrine tumors. Twelve patients (93%) had complete ablations without reported local progression of disease on follow up imaging. Of additional clinical importance, 7 patients who had preoperative hormone-related symptoms experienced helpful palliative results[57].
Zhang et al[58] reported recent results with cryoablation of breast cancer metastases. They performed cryoablation of 39 liver metastases in 17 patients. Tumor response was 92% in the immediate post-op period and 87.1% at 1 mo. Local progression was seen in 6 lesions (15.4%) at 3 mo. The 1 year survival rate was 70.6%.
Microwave ablation: One of the first studies to evaluate microwave ablation in the treatment of metastatic disease was by Shibata et al[59]. They compared microwave ablation to surgical resection in patients with metastatic colorectal cancer and found similar 1, 2 and 3 years survival rates (71%, 57% and 14% for microwave and 69%, 56% and 23% for resection), and mean survival rates (27 mo for microwave vs 25 mo for resection)[59]. A study by Tanaka et al[60] also found similar survival and recurrence rates in patients who underwent microwave alone compared to microwave and eventual resection for colorectal metastases. Another study reported identical five-year survival rates (24%) for patients with colorectal metastases treated with microwave ablation vs microwave and surgery[61].
Irreversible electroporation: Silk et al[62] reported results of IRE in 9 patients with a total of 19 metastatic colorectal cancer lesions ranging from 1.0-4.7 cm. They reported an efficacy of 55% with local tumor recurrence in 5 of 9 patients at 9 mo[62]. Thomson et al[31] reported a primary efficacy of 67% in a total of 45 metastatic lesions (including colorectal, breast, and neuroendocrine cancers) treated with IRE. Kingham et al[63] evaluated IRE of 28 metastatic lesions including metastatic colorectal and neuroendocrine cancers. They reported a total local failure rate of 7.5% with time to recurrence ranging from 66-230 d.
The choice of ablation modality is important to potential treatment success. While each case is unique and modality choice is often driven by local expertise and operator experience, several general concepts prevail. RFA is very safe and effective in smaller hepatic tumors. However, RFA is less effective with larger tumors and tumors near blood vessels. In contrast, microwave ablation has been shown to be more effective with larger tumor sizes and is affected less by the heat sink effect. Although cryoablation has historically been avoided with hepatic tumors due to concerns about complications, it has been used very safely more recently following the development of smaller probes. Lastly, in limited studies, IRE has been shown to be safe and effective in the treatment of both HCC and metastatic disease especially near sensitive structures such as blood vessels and bile ducts, although continued research is needed to demonstrate long term efficacy.
Percutaneous ablation has become widely accepted as a curative technique in the treatment of HCC and hepatic metastatic disease. Specifically, ablation is useful in the treatment of patients who are not surgical candidates but in whom curative treatment is desired. Percutaneous ablation is safe and effective. Although additional studies are needed, percutaneous ablation continues to evolve as an option in the treatment of HCC and metastatic disease.
P- Reviewer: He JY, Kaya M, Qin JM S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, Han JK, Choi BI. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 3. | Rhim H, Goldberg SN, Dodd GD, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21 Spec No:S17-35; discussion S36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | McDermott S, Gervais DA. Radiofrequency ablation of liver tumors. Semin Intervent Radiol. 2013;30:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Lencioni R, Crocetti L, Cioni D, Della Pina C, Bartolozzi C. Percutaneous radiofrequency ablation of hepatic colorectal metastases: technique, indications, results, and new promises. Invest Radiol. 2004;39:689-697. [PubMed] |
| 6. | Venkatesan AM, Gervais DA, Mueller PR. Percutaneous radiofrequency thermal ablation of primary and metastatic hepatic tumors: current concepts and review of the literature. Semin Intervent Radiol. 2006;23:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 699] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16346 treated nodules in 13283 patients. Hepatol Res. 2012;42:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | O’Rourke AP, Haemmerich D, Prakash P, Converse MC, Mahvi DM, Webster JG. Current status of liver tumor ablation devices. Expert Rev Med Devices. 2007;4:523-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Walker K, Lindeque B. The application of cryoprobe therapy in orthopedic oncology. Orthopedics. 2014;37:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Yu H, Burke CT. Comparison of percutaneous ablation technologies in the treatment of malignant liver tumors. Semin Intervent Radiol. 2014;31:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95:1187-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Adam R, Hagopian EJ, Linhares M, Krissat J, Savier E, Azoulay D, Kunstlinger F, Castaing D, Bismuth H. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch Surg. 2002;137:1332-1339; discussion 1340. [PubMed] |
| 18. | Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, Granchi J, Curley SA. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592-599. [PubMed] |
| 19. | Yang Y, Wang C, Lu Y, Bai W, An L, Qu J, Gao X, Chen Y, Zhou L, Wu Y. Outcomes of ultrasound-guided percutaneous argon-helium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:674-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Liang HH, Chen MS, Peng ZW, Zhang YJ, Zhang YQ, Li JQ, Lau WY. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Lubner MG, Brace CL, Ziemlewicz TJ, Hinshaw JL, Lee FT. Microwave ablation of hepatic malignancy. Semin Intervent Radiol. 2013;30:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Fan W, Li X, Zhang L, Jiang H, Zhang J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol. 2012;198:W46-W50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA. Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB (Oxford). 2007;9:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Lahat E, Eshkenazy R, Zendel A, Zakai BB, Maor M, Dreznik Y, Ariche A. Complications after percutaneous ablation of liver tumors: a systematic review. Hepatobiliary Surg Nutr. 2014;3:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 26. | Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, Meijer S, van Kuijk C, van den Tol PM, Meijerink MR. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25:997-1011; quiz 1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 281] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 27. | Knavel EM, Brace CL. Tumor ablation: common modalities and general practices. Tech Vasc Interv Radiol. 2013;16:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 28. | Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010;4 Suppl 1:S99-S104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Adeyanju OO, Al-Angari HM, Sahakian AV. The optimization of needle electrode number and placement for irreversible electroporation of hepatocellular carcinoma. Radiol Oncol. 2012;46:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Schmidt CR, Shires P, Mootoo M. Real-time ultrasound imaging of irreversible electroporation in a porcine liver model adequately characterizes the zone of cellular necrosis. HPB (Oxford). 2012;14:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Thomson KR, Cheung W, Ellis SJ, Federman D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C, Haydon A. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 33. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 34. | Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 35. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 36. | N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 37. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 741] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 38. | Qi X, Tang Y, An D, Bai M, Shi X, Wang J, Han G, Fan D. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2014;48:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Miura JT, Johnston FM, Tsai S, Eastwood D, Banerjee A, Christians KK, Turaga KK, Gamblin TC. Surgical resection versus ablation for hepatocellular carcinoma ≤ 3 cm: a population-based analysis. HPB (Oxford). 2015;17:896-901. [PubMed] |
| 40. | Chen HW, Lai EC, Zhen ZJ, Cui WZ, Liao S, Lau WY. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg. 2011;9:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Wang C, Lu Y, Chen Y, Feng Y, An L, Wang X, Su S, Bai W, Zhou L, Yang Y. Prognostic factors and recurrence of hepatitis B-related hepatocellular carcinoma after argon-helium cryoablation: a prospective study. Clin Exp Metastasis. 2009;26:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Ziemlewicz TJ, Hinshaw JL, Lubner MG, Brace CL, Alexander ML, Agarwal P, Lee FT. Percutaneous microwave ablation of hepatocellular carcinoma with a gas-cooled system: initial clinical results with 107 tumors. J Vasc Interv Radiol. 2015;26:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Sun AX, Cheng ZL, Wu PP, Sheng YH, Qu XJ, Lu W, Zhao CG, Qian GJ. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol. 2015;21:2997-3004. [PubMed] |
| 45. | Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, Xu ZF, Liu GJ, Zheng YL. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 391] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 47. | Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013;12:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 48. | Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 49. | Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 601] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 50. | Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Kim KH, Yoon YS, Yu CS, Kim TW, Kim HJ, Kim PN, Ha HK, Kim JC. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Berber E, Flesher N, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg. 2002;26:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Elvin A, Skogseid B, Hellman P. Radiofrequency ablation of neuroendocrine liver metastases. Abdom Imaging. 2005;30:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Kerkar S, Carlin AM, Sohn RL, Steffes C, Tyburski J, Littrup P, Weaver D. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery. 2004;136:770-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Ng KM, Chua TC, Saxena A, Zhao J, Chu F, Morris DL. Two decades of experience with hepatic cryotherapy for advanced colorectal metastases. Ann Surg Oncol. 2012;19:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Seifert JK, Cozzi PJ, Morris DL. Cryotherapy for neuroendocrine liver metastases. Semin Surg Oncol. 1998;14:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Zhang W, Yu H, Guo Z, Li B, Si T, Yang X, Wang H. Percutaneous cryoablation of liver metastases from breast cancer: initial experience in 17 patients. Clin Radiol. 2014;69:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Shibata T, Niinobu T, Ogata N, Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276-284. [PubMed] |
| 60. | Tanaka K, Shimada H, Nagano Y, Endo I, Sekido H, Togo S. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Morita T, Shibata T, Okuyama M, Ikeda K, Tsukahara Y, Kitada M, Nishikubo M, Ishida T, Shimano T. [Microwave coagulation therapy for liver metastases from colorectal cancer]. Gan To Kagaku Ryoho. 2004;31:695-699. [PubMed] |
| 62. | Silk MT, Wimmer T, Lee KS, Srimathveeravalli G, Brown KT, Kingham PT, Fong Y, Durack JC, Sofocleous CT, Solomon SB. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Kingham TP, Karkar AM, D’Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, Sofocleous CT, Solomon SB, Jarnagin WR, Fong Y. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012;215:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |