Published online Oct 8, 2016. doi: 10.4254/wjh.v8.i28.1200
Peer-review started: May 17, 2016
First decision: June 14, 2016
Revised: June 26, 2016
Accepted: August 15, 2016
Article in press: August 16, 2016
Published online: October 8, 2016
Processing time: 137 Days and 3.2 Hours
To clarify the clinical factors associated with liver regeneration after major hepatectomy and the hypertrophic rate after portal vein embolization (PVE).
A total of 63 patients who underwent major hepatectomy and 13 patients who underwent PVE in a tertiary care hospital between January 2012 and August 2015 were included in the analysis. We calculated the remnant liver volume following hepatectomy using contrast-enhanced computed tomography (CT) performed before and approximately 3-6 mo after hepatectomy. Furthermore, we calculated the liver volume using CT performed 2-4 wk after PVE. Preoperative patient characteristics and laboratory data were analyzed to identify factors affecting postoperative liver regeneration or hypertrophy rate following PVE.
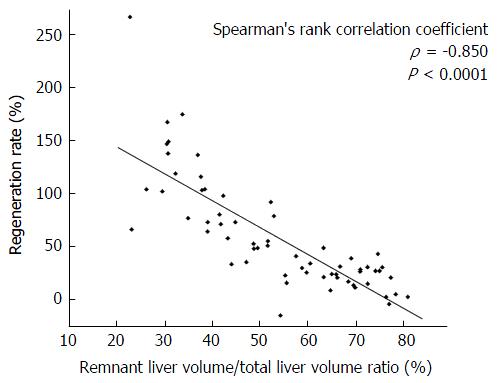
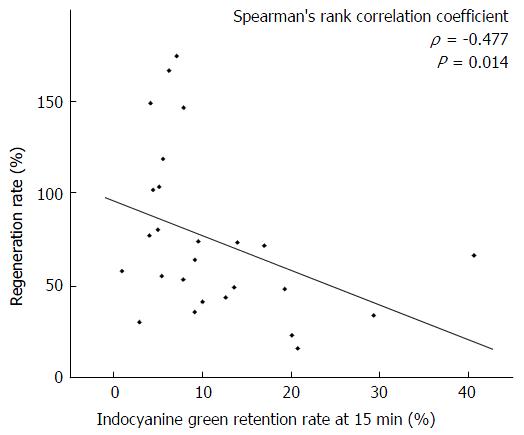
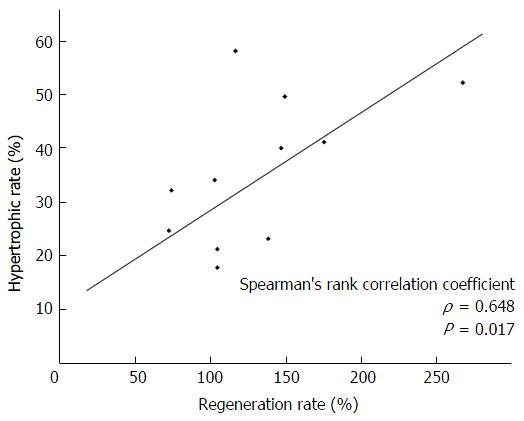
The remnant liver volume/total liver volume ratio negatively correlated with the liver regeneration rate after hepatectomy (ρ = -0.850, P < 0.001). The regeneration rate was significantly lower in patients with an indocyanine green retention rate at 15 min (ICG-R15) of ≥ 20% in the right hepatectomy group but not in the left hepatectomy group. The hypertrophic rate after PVE positively correlated with the regeneration rate after hepatectomy (ρ = 0.648, P = 0.017). In addition, the hypertrophic rate after PVE was significantly lower in patients with an ICG-R15 ≥ 20% and a serum total bilirubin ≥ 1.5 mg/dL.
The regeneration rate after major hepatectomy correlated with hypertrophic rate after PVE. Both of them were attenuated in the presence of impaired liver function.
Core tip: Little is known about the clinical factors associated with liver regeneration after major hepatectomy. In the present study, the liver regeneration rate after major hepatectomy correlated with the remnant liver volume and hypertrophic rate after portal vein embolization. The regeneration rate after major hepatectomy and hypertrophic rate after portal vein embolization were attenuated in the presence of impaired liver function.
- Citation: Kageyama Y, Kokudo T, Amikura K, Miyazaki Y, Takahashi A, Sakamoto H. Impaired liver function attenuates liver regeneration and hypertrophy after portal vein embolization. World J Hepatol 2016; 8(28): 1200-1204
- URL: https://www.wjgnet.com/1948-5182/full/v8/i28/1200.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i28.1200
Curative resection is the most effective treatment for liver cancer[1]. Although resection-related mortality and morbidity have substantially decreased in recent years, the postoperative mortality rate remain to be as high as 1%-5%[2-7]. The capacity of hepatic regeneration after hepatectomy and the hypertrophic rate after portal vein embolization (PVE) are important for allowing surgeons to determine the appropriate extent of resection[8-11]. Better regeneration after hepatectomy and liver hypertrophy after PVE may prevent posthepatectomy complications, including hepatic failure[12,13]. Little is known about preoperative clinical factors influencing postoperative liver regeneration.
The aim of this study was to clarify the relationship between preoperative clinical factors and the regenerative capacity of the remnant liver after hepatectomy. Furthermore, we examined the relationship between the regeneration rate after hepatectomy and hypertrophic rate after PVE and clinical factors that affect the hypertrophic rate after PVE.
A total of 63 patients who underwent major hepatectomy in the Division of Gastroenterological Surgery, Saitama Cancer Center, between January 2012 and August 2015 were included in the analysis. The liver volume was measured using enhanced computed tomography (CT) images taken before and approximately 3-6 mo after hepatectomy[14]. For volumetric analysis, a three-dimensional image analysis software was used (SYNAPSE VINCENT; Fuji Medical Systems, Tokyo, Japan). The regeneration rate was calculated as follows: [(liver volume after hepatectomy/estimated remnant liver volume before hepatectomy) × 100] - 100 (%). The indications for PVE were determined by the balance between the indocyanine green fractional disappearance rate (ICG-K) and the volumetric ratio of the future remnant liver volume. PVE was performed in patients whose values were estimated as follows: (ICG-K) × (remnant liver volume/total liver volume) < 0.05[15]. The liver volume after PVE was calculated using enhanced CT images taken 2-4 wk after PVE. The hypertrophic rate after PVE was estimated as follows: [(remnant liver volume after PVE/remnant liver volume before PVE) × 100] - 100 (%). Preoperative patient characteristics and laboratory data, including platelet count, total bilirubin, and indocyanine green retention rate at 15 min (ICG-R15), were analyzed to identify factors affecting postoperative liver regeneration. For the measurement of ICG-R15, Indocyanine green (Diagnogreen, Daiichi-Sankyo, Tokyo, Japan) was administered at dose of 0.5 mg/kg by the antecubital vein of the opposite arm. Then, venous peripheral blood samples were collected every 5 min for 15 min to measure the ICG absorbance. ICG-K and ICG-R15 were calculated by fitting the serum disappearance curve by a single-exponential decay equation.
Statistical analysis was performed using the JMP 11 software (SAS Institute, Inc., Cary, NC). Categorical variables were analyzed using the Wilcoxon rank sum test. Correlations between two parameters were examined by calculating the Spearman’s rank correlation coefficient. A 2-tailed P value of < 0.05 was considered statistically significant.
Among the 63 patients, 42 were men and 21 were women, with a mean age of 68.1 years (range: 45-89 years). The diseases indicating the need for hepatectomy were metastatic liver carcinoma (n = 31), intrahepatic cholangiocarcinoma (n = 14), hilar cholangiocarcinoma (n = 10), hepatocellular carcinoma (n = 4), gallbladder carcinoma (n = 2), hemangioma (n = 1), and neuroendocrine tumor (n = 1). A total of 22 patients had background liver diseases, including chronic viral hepatitis (n = 6), alcoholic hepatitis (n = 1), and obstructive jaundice (n = 15). Preoperative chemotherapy within 6 mo was performed in 18 patients and 13 patients underwent preoperative PVE. The operative procedures performed in the 63 patients included right hepatectomy or extended right hepatectomy (n = 26), left hepatectomy or extended left hepatectomy (n = 32), right trisegmentectomy (n = 3), and left trisegmentectomy (n = 2). The median remnant liver volume/total liver volume ratios after right hepatectomy and extended right hepatectomy, left hepatectomy and extended left hepatectomy, right trisegmentectomy, and left trisegmentectomy were 42.5%, 68.4%, 26.2% and 40.3%, respectively. Their median regeneration rates were 65.6%, 25.7%, 138.1% and 101.2%, respectively. The remnant liver volume/total liver volume ratio negatively correlated with the regeneration rate after hepatectomy (ρ = -0.850, P < 0.001; Figure 1).
Because the liver regeneration rates were significantly different between the patients who underwent right hepatectomy or extended right hepatectomy (right hepatectomy group) and left hepatectomy or extended left hepatectomy (left hepatectomy group), we analyzed these two groups separately. In the right hepatectomy group, regeneration rate was significantly lower in patients with an ICG-R15 of ≥ 20%. It was not associated with platelet count, total bilirubin, diabetes mellitus, viral hepatitis, obstructive jaundice, or preoperative chemotherapy. The ICG-R15 value negatively correlated with liver regeneration rate in the right hepatectomy group (ρ = -0.477, P = 0.014; Figure 2). In the left hepatectomy group, no factor was associated with the regeneration rate (Table 1). In the 13 patients who underwent preoperative PVE, the median hypertrophic rate was 32.2% (range: 2.7%-58.3%). The hypertrophic rate positively correlated with the regeneration rate after hepatectomy (ρ = 0.648, P = 0.017; Figure 3). The hypertrophic rate was significantly lower in patients with an ICG-R15 of ≥ 20% and total bilirubin of ≥ 1.5 mg/dL (Table 2).
| Right hepatectomy | Left hepatectomy | |||||||
| n | Regeneration rate (%)1 | P | n | Regeneration rate (%)1 | P | |||
| Age (mean) | 45-83 (69) | P = 0.891 | 46-89 (69) | P = 0.321 | ||||
| Sex (male/female) | 18/8 | 65.6/69.2 | P = 0.355 | 20/12 | 25.7/25.9 | P = 0.969 | ||
| Background liver disease (yes/no) | 6/20 | 51.0/70.2 | P = 0.248 | 14/18 | 24.0/25.7 | P = 0.621 | ||
| Platelet count (/mm3) ≥ 100 | 24 | 69.3 | P = 0.178 | 30 | 24.7 | P = 0.586 | ||
| < 100 | 2 | 41.6 | 2 | 28.8 | ||||
| Total bilirubin (mg/dL ) ≥ 1.5 | 1 | 71.8 | P = 0.842 | 4 | 28.2 | P = 0.724 | ||
| < 1.5 | 25 | 64.4 | 27 | 24.9 | ||||
| ICG-R15 (%) ≥ 20 | 4 | 28.5 | P < 0.05 | 4 | 28.2 | P = 0.724 | ||
| < 20 | 22 | 72.7 | 27 | 24.9 | ||||
| Diabetes mellitus (yes/no) | 4/22 | 63.7/65.6 | P = 0.570 | 4/28 | 37.8/24.7 | P = 0.459 | ||
| Preoperative chemotherapy (yes/no) | 10/16 | 60.0/74.5 | P = 0.317 | 8/24 | 29.7/24.1 | P = 0.361 | ||
| n | Hypertrophic rate (%)1 | P | ||
| Age (mean) | 50–80 (65) | P = 0.845 | ||
| Sex (male/female) | 10/3 | 30.5/40.1 | P = 0.612 | |
| Background liver disease (yes/no) | 6/7 | 23.1/40.1 | P = 0.087 | |
| Platelet count (/mm3) ≥ 100 | 12 | 30.5 | P = 0.593 | |
| < 100 | 1 | 40.1 | ||
| Total bilirubin (mg/dL) ≥ 1.5 | 4 | 19.7 | P < 0.05 | |
| < 1.5 | 9 | 40.0 | ||
| ICG-R15 (%) ≥ 20 | 2 | 12.0 | P < 0.05 | |
| < 20 | 11 | 34.2 | ||
| Diabetes mellitus (yes/no) | 3/10 | 32.2/31.4 | P = 1.000 | |
| Preoperative chemotherapy (yes/no) | 3/10 | 40.1/26.8 | P = 0.237 |
Our study demonstrated that the liver regeneration rate was significantly lower in patients with an ICG-R15 of ≥ 20% in the right hepatectomy group, but not in the left hepatectomy group. The hypertrophic rate after PVE positively correlated with the regeneration rate after hepatectomy. In addition, the hypertrophic rate after PVE was significantly lower in patients with an ICG-R15 of ≥ 20% and a serum total bilirubin of ≥ 1.5 mg/dL.
Although several studies reported factors affecting liver regeneration after hepatectomy, the factors vary among studies. Yamanaka et al[16] reported that the extent of resection and impaired liver function were associated with the liver regeneration, whereas Ogata et al[17] reported that serum hyaluronan was a predictor of liver regeneration in patients with hepatocellular carcinoma. In living-donor liver transplantation, remnant liver volume[18], sex[19], and age[20] have been reported to be associated with liver regeneration. Aoki et al[14] reported that sex and alanine aminotransferase values were associated with liver regeneration in the early phase, and the final regeneration rate was associated with the ratio of resected liver volume. In our study, the remnant liver volume in the right hepatectomy group was significantly larger than that in the left hepatectomy group, and together with previous reports, the regeneration rate was highly affected by remnant liver volume/total liver volume ratio. Therefore, left and right hepatectomy should be separately considered when analyzing liver regeneration.
The regeneration rate was significantly lower in patients with a higher ICG-R15 in the right hepatectomy group, whereas no variables related to liver regeneration were identified in the left hepatectomy group. These results also confirmed that liver regeneration after right and left hepatectomy should be separately considered.
Our study demonstrated the correlation between the hypertrophic rate after PVE and liver regeneration rate after hepatectomy. The hypertrophic rate positively correlated with the regeneration rate, and regeneration rate after major hepatectomy and hypertrophic rate after PVE were attenuated in the presence of impaired liver function.
In conclusion, the regeneration rate after major hepatectomy correlated with the remnant liver volume and hypertrophic rate after PVE. The regeneration rate after right hepatectomy and hypertrophic rate after PVE were attenuated in the presence of impaired liver function.
Although resection-related mortality and morbidity have substantially decreased in recent years, the postoperative mortality rate has remained as high as 1%-5%. Portal vein embolization (PVE) is proposed to induce hypertrophy of the anticipated liver remnant to reduce such complications. The capacity of hepatic regeneration after hepatectomy and the hypertrophic rate after PVE are important for allowing surgeons to determine the appropriate extent of resection. Better regeneration after hepatectomy and liver hypertrophy after PVE may prevent posthepatectomy complications, including hepatic failure.
Little is known about preoperative clinical factors influencing postoperative liver regeneration and liver hypertrophy after PVE.
In this study, the relationship between preoperative clinical factors and the regenerative capacity of the remnant liver after hepatectomy were clarified. Furthermore, the authors examined the relationship between the regeneration rate after hepatectomy and hypertrophic rate after PVE and clinical factors that affect the hypertrophic rate after PVE.
This study suggests that the regeneration rate after major hepatectomy correlated with the remnant liver volume and hypertrophic rate after PVE, and the regeneration rate after right hepatectomy and hypertrophic rate after PVE were attenuated in the presence of impaired liver function.
PVE: A procedure in the preoperative treatment of patients selected for major hepatic resection. PVE is performed via either the percutaneous transhepatic or the transileocolic route and is usually reserved for patients whose future liver remnants are too small to allow resection.
The manuscript is an interesting one. The authors, using 63 patients who underwent major hepatectomy and 13 patients who underwent portal vain embolization, calculated regeneration rate correlated with the remnant liver volume. In conclusion, they found that the regeneration rate after right hepatectomy and the hypertrophic rate after PVE were attenuated in the presence of impaired liver function. It is a well-written and presented manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW, Qin JM, Sazci A, Zhang X S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Venook AP. Treatment of hepatocellular carcinoma: too many options? J Clin Oncol. 1994;12:1323-1334. [PubMed] |
| 2. | Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 799] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 4. | Gomez D, Malik HZ, Bonney GK, Wong V, Toogood GJ, Lodge JP, Prasad KR. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Kaneko K, Shirai Y, Wakai T, Yokoyama N, Akazawa K, Hatakeyama K. Low preoperative platelet counts predict a high mortality after partial hepatectomy in patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:5888-5892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 7. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 8. | Okamoto E, Kyo A, Yamanaka N, Tanaka N, Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984;95:586-592. [PubMed] |
| 9. | Yamanaka N, Okamoto E, Kuwata K, Tanaka N. A multiple regression equation for prediction of posthepatectomy liver failure. Ann Surg. 1984;200:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Shimada M, Matsumata T, Maeda T, Itasaka H, Suehiro T, Sugimachi K. Hepatic regeneration following right lobectomy: estimation of regenerative capacity. Surg Today. 1994;24:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [PubMed] |
| 12. | Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolization in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, Saric J, Trillaud H, Petit P, Bartoli JM. Preoperative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Aoki T, Imamura H, Matsuyama Y, Kishi Y, Kobayashi T, Sugawara Y, Makuuchi M, Kokudo N. Convergence process of volumetric liver regeneration after living-donor hepatectomy. J Gastrointest Surg. 2011;15:1594-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Okochi O, Kaneko T, Sugimoto H, Inoue S, Takeda S, Nakao A. ICG pulse spectrophotometry for perioperative liver function in hepatectomy. J Surg Reserch. 2002;103:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, Furukawa K, Tanaka T, Tomoda F, Tanaka W. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology. 1993;18:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Ogata T, Okuda K, Ueno T, Saito N, Aoyagi S. Serum hyaluronan as a predictor of hepatic regeneration after hepatectomy in humans. Eur J Clin Invest. 1999;29:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Fulcher AS, Posner MP. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 240] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, Jenkins RL. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Yokoi H, Isaji S, Yamagiwa K, Tabata M, Sakurai H, Usui M, Mizuno S, Uemoto S. Donor outcome and liver regeneration after right-lobe graft donation. Transpl Int. 2005;18:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |