Published online Sep 28, 2016. doi: 10.4254/wjh.v8.i27.1149
Peer-review started: April 15, 2016
First decision: May 19, 2016
Revised: June 19, 2016
Accepted: July 29, 2016
Article in press: August 1, 2016
Published online: September 28, 2016
Processing time: 162 Days and 13.8 Hours
To evaluate risk of recidivism on a case-by-case basis.
From our center’s liver transplant program, we selected patients with alcoholic liver disease who were listed for transplant based on Ohio Solid Organ Transplantation Consortium (OSOTC) exception criteria. They were considered to have either a low or medium risk of recidivism, and had at least one or three or more months of abstinence, respectively. They were matched based on gender, age, and Model for End-Stage Liver Disease (MELD) score to controls with alcohol-induced cirrhosis from Organ Procurement and Transplant Network data.
Thirty six patients with alcoholic liver disease were approved for listing based on OSOTC exception criteria and were matched to 72 controls. Nineteen patients (53%) with a median [Inter-quartile range (IQR)] MELD score of 24 (13) received transplant and were followed for a median of 3.4 years. They were matched to 38 controls with a median (IQR) MELD score of 25 (9). At one and five years, cumulative survival rates (± standard error) were 90% ± 7% and 92% ± 5% and 73% ± 12% and 77% ± 8% in patients and controls, respectively (Log-rank test, P = 0.837). Four (21%) patients resumed drinking by last follow-up visit.
Compared to traditional criteria for assessment of risk of recidivism, a careful selection process with more flexibility to evaluate eligibility on a case-by-case basis can lead to similar survival rates after transplantation.
Core tip: For the first time, we report the rates of liver transplant and survival for patients with alcohol-induced cirrhosis who were deemed eligible for liver transplant and listed based on approval under the Ohio Solid Organ Transplantation Consortium medically urgent exception criteria. These criteria allow patients with low to medium risk of recidivism, to receive a liver transplant after only one to three months of abstinence. We showed that transplant rate and short and long term survival after transplant is comparable between these patients and United States general population of patients with alcohol-induced cirrhosis who received liver transplant.
- Citation: Hajifathalian K, Humberson A, Hanouneh MA, Barnes DS, Arora Z, Zein NN, Eghtesad B, Kelly D, Hanouneh IA. Ohio solid organ transplantation consortium criteria for liver transplantation in patients with alcoholic liver disease. World J Hepatol 2016; 8(27): 1149-1154
- URL: https://www.wjgnet.com/1948-5182/full/v8/i27/1149.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i27.1149
Cirrhosis due to alcoholic liver disease is an important cause of morbidity and mortality both globally and in the United States. Globally, in 2010 cirrhosis due to alcoholic liver disease led to more than 493000 deaths[1]. In United States in 2011 liver cirrhosis was responsible for 34860 deaths, 48% of which were related to alcohol consumption[2]. Among patients with cirrhosis due to alcoholic liver disease mortality rates vary based on presence or absence of complications of cirrhosis but it is generally high with a one-year mortality ranging from 17% to 64% and five-year mortalities ranging from 58% to 85%[3].
Liver transplantation imparts great survival benefit to appropriately selected patients with advanced and de-compensated cirrhosis due to alcohol consumption, which is comparable to survival benefit of transplant in other types of chronic liver disease[4,5]. The definition of “appropriately selected patients” in this context remains controversial[5], with the most important factor being minimum duration of abstinence. Conventionally, most liver transplant programs in United States require patients to be abstinent for at least 6 mo and participate in an alcohol rehabilitation program to be considered for transplant[6,7]; while it is known that delayed referral for transplant and longer waiting times, even for a few months, will significantly decrease the probability of patient’s survival in the pre-transplant period[8]. There are data suggesting that careful evaluation of patients for transplant on an individual basis instead of using general and inflexible enrollment rules might lead to favorable outcomes in highly selected patients with alcoholic liver disease[9,10]. The state of Ohio Solid Organ Transplantation Consortium (OSOTC) provides such a mechanism for case-by-case evaluation based on clinical guidelines for medically urgent patients with cirrhosis due to alcoholic liver disease. Based on factors such as estimated risk of recidivism, severity of their alcohol use history and previous attempts to remain sober, social support, insight into alcohol use, and willingness of the patient to comply with OSOTC regulations, these patients can be approved as an exception and listed for transplant after only one to three months of abstinence.
The aim of this study was to determine the effect of using OSOTC transplant eligibility criteria on patients’ survival compared with conventional criteria for assessment of risk of recidivism, in patient with cirrhosis due to alcoholic liver disease.
The study protocol was approved by the Cleveland Clinic Institutional Review Board. Since 2009 we selected patients with alcoholic cirrhosis for consideration of liver transplantation based on OSOTC exception criteria. No donor organs were obtained from executed prisoners or other institutionalized persons. As defined below these are medically urgent patients with low to medium risk of recidivism, who were approved for a medically urgent exception to be transplanted either during the time they were completing alcohol treatment, or some completed treatment after their transplant.
Transplant rates were compared between these patients and matched patients with alcoholic cirrhosis from Organ Procurement and Transplant Network (OPTN) data records who had complete data to calculate their Model for End-Stage Liver Disease (MELD) score[11,12] at the time of listing. To compare survival after transplant we used the OPTN patients with alcoholic cirrhosis who had complete data to calculate their MELD score at the time of transplant as well as follow-up data on survival after transplant. Patients from OPTN dataset were matched to our patients randomly and according to the following predetermined variables: 10-year age category, gender, and MELD score category same as the case patient’s category (< 10, 10-19, 20-29, 30-39, ≥ 40)[13]. MELD score was calculated as (9.57 × log creatinine mg/dL) + (3.78 × log bilirubin mg/dL) + (11.20 × log international normalized ratio) + 6.43. All laboratory values which were less than 1 were set to 1 and serum creatinine for patients with values of more than 4 or on dialysis was set to 4 in order to calculate MELD score. The MELD score was truncated at 40 for patients with a MELD score of more than 40 (http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=98).
OSOTC follows standard criteria for patients in need of liver transplant who are diagnosed with substance use disorder at the time of evaluation (Table 1). This includes patients with alcohol-induced cirrhosis who are diagnosed with alcohol use disorder. The standard criteria includes demonstrating abstinence for at least 12 mo before listing, or at least three months of abstinence plus three months of current participation in an active recovery program and negative random toxicology screens prior to listing confirmed by collateral information (http://www.osotc.org/resources/chemical-dependency-criteria/). In addition, patients must show insight into substance use and understanding of the effects of substance use on their health.
| Ohio Solid Organ Transplantation Consortium Criteria | |
| Low-risk | 1 mo confirmed abstinence, a signed contract and commitment to begin a rehabilitation program and finish it either before or after transplant |
| No previous failure with substance rehabilitation; never been told that substance was affecting health; and good social support | |
| Medium-risk | Three month confirmed abstinence, a signed contract and commitment to begin a rehabilitation program and/or finish it either before or after transplant |
| One or more failures with rehabilitation; and minimal support system | |
| High-risk | Two or more failures to remain abstinent despite medical complication |
| Refusal to sign contract | |
| Minimal to poor social support | |
| Must complete standard criteria treatment plan, not eligible for an exception | |
| Other barriers | No insight into their alcohol use consequences |
| No recognition that alcohol caused their liver failure | |
| Refusal to start treatment | |
| No sober support network |
OSOTC also provides exception criteria for medically urgent patients who have not been abstinent for 12 mo and are too ill to complete the recovery program participation conditions in the standard criteria. These exception criteria apply to patients with MELD score of more than 22 (calculated or eligible for exception). According to OSOTC exception criteria, and after signing a contract and showing commitment to rehabilitation, patients at low risk of recidivism - defined as no previous failure with substance rehabilitation, never having been told that substance was affecting health, and good social support - can be listed for transplant after one month of abstinence. Patients at medium risk of recidivism - defined as one or more failures with rehabilitation, and minimal support system - can be listed after a minimum of three months of abstinence, and after signing a contract and showing commitment to rehabilitation. All patients’ records are reviewed by OSOTC chemical disorder committee representatives and discussed in a committee conference call, in addition to our liver transplant patient selection committee, in order to decide approval or not of these exception criteria. Patients at high risk for recidivism - defined as two or more failures to remain abstinent despite medical complications, refusal to sign a contract, and minimal or poor social support - do not qualify for OSOTC exception criteria.
All analysis was done with Stata Data Analysis and Statistical Software (version 11.2 SE, StataCrop LP). Variables are reported as number (percentage) or median (IQR). Survival probabilities are reported as percentage ± SE. Categorical variables are compared between patients and controls with χ2 test. Waiting time was defined as the period from the day an individual is listed for liver transplant to the day the transplant is done. Waiting time was compared between cases and controls with a Cox proportional hazards model containing patient group as the only independent variable to predict waiting time (i.e., time to liver transplant). Follow-up time after transplant was defined as the period from the day an individual receives a liver transplant until death or the last follow-up visit. Data for patients who remained alive by the end of the follow-up period was censored at the time of last follow-up visit. Survival probabilities were estimated with Kaplan-Meier method and were compared between groups with Log-Rank test. All P-values are two-sided.
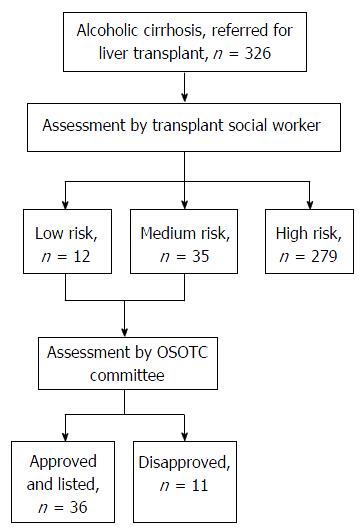
Between 2009 and 2013, 326 patients with alcoholic liver disease were evaluated for liver transplant at the Cleveland Clinic, of whom 279 (85%) patients were considered high-risk for recidivism or alcohol relapse based on the OSOTC criteria (Figure 1). These high-risk patients underwent the standard chemical dependency requirements defined above before being considered eligible for liver transplant. Forty-seven (15%) patients were considered by our social workers and liver transplant committee at the Cleveland Clinic to be at medium or low-risk for recidivism or alcohol relapse based on the OSOTC criteria, but only 36 (13%) patients were approved by the consortium.
Thirty-six patients with alcoholic cirrhosis were approved for liver transplant at the Cleveland Clinic based on OSOTC exception criteria. They were matched based on age, gender, and MELD score category to a random sample of 72 controls from OPTN database with alcohol-induced cirrhosis that underwent liver transplant following conventionally used criteria of alcohol rehabilitation. Table 2 represents the baseline characteristics of patients and control groups. Sixty four percent of patients and controls were male. At the time of listing five patients had a MELD score of 10-19 (14%), 18 had a MELD score of 20-29 (50%), 12 had a MELD score of 30-39 (33%), and one patient (3%) had a MELD score of 40 or more. These were individually matched to controls with the same MELD score category, leading to a median (IQR) MELD score of 27 (11) among patients and 24 (11) among controls (Table 1). At the time of liver transplant one patient had a MELD score of less than 10 (5%), two had MELD scores of 10-19 (11%), 11 had MELD scores of 20-29 (58%), four had MELD scores of 30-39 (21%), and one patient had a MELD score of 40 or more (5%). Again, these patients were individually matched to controls with the same MELD score category leading to a median (IQR) MELD score of 24 (13) among patients and 25 (9) among controls.
| Patients | Controls | |
| At listing | ||
| n | 36 | 72 |
| Age, median (IQR) | 58 (14) | 60 (11) |
| Male, n (%) | 23 (64) | 46 (64) |
| INR, median (IQR) | 1.8 (0.6) | 1.9 (0.7) |
| Total Bilirubin, median (IQR) | 8.9 (19.3) | 5 (7.7) |
| Creatinine, median (IQR) | 2.1 (2.4) | 1.5 (2) |
| Albumin, median (IQR) | 3.2 (0.9) | 2.9 (1) |
| MELD, median (IQR) | 27 (11) | 24 (11) |
| At transplant | ||
| n | 19 | 38 |
| Age, median (IQR) | 56 (17) | 55 (13) |
| Male, n (%) | 13 (68) | 26 (68) |
| INR, median (IQR) | 1.6 (0.5) | 1.9 (0.8) |
| Total Bilirubin, median (IQR) | 8.3 (12.1) | 6 (5.7) |
| Creatinine, median (IQR) | 2.6 (2.9) | 1.3 (1.3) |
| Albumin, median (IQR) | 2.9 (0.8) | 2.8 (1) |
| MELD, median (IQR) | 24 (13) | 25 (9) |
Nineteen out of 36 (53%) patients received a liver transplant and 17 dropped off the transplant list. The most common cause of drop-off transplant list was infection and the vast majority of dropped off patients died (n = 15, 88%). The transplant drop-off rate was not different for controls of whom 41 (57%) received a transplant (P-value = 0.681). Patients in the OSOTC group received their liver after a median waiting time of 19 d after listing, and controls received their transplant after a median 21 d of waiting time (P-value = 0.648). Although the majority of both patients and controls received their transplant in less than 2 mo (Table 3), 10% of controls had to wait more than five months while all patients received their transplants before the five months mark.
| Patients | Controls | P-value | |
| After listing | |||
| No. listed | 36 | 72 | |
| No. transplanted (%) | 19 (53) | 41 (57) | 0.6871 |
| Waiting time for transplant, d, median (IQR) | 19 (7-65) | 21 (5-54) | 0.6482 |
| After transplant | |||
| No. transplanted | 19 | 38 | |
| Follow-up after transplant, months, median (IQR) | 41 (29-58) | 37 (14-61) | |
| 1-yr survival, % ± SE | 90 ± 7 | 92 ± 5 | 0.8373 |
| 5-yr survival, % ± SE | 73 ± 12 | 77 ± 8 |
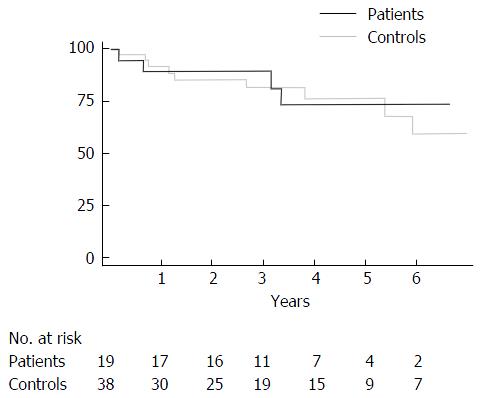
Both patients and controls had a median follow-up of more than three years after transplant (Table 3). One year after transplant 90% ± 7% of patients were alive compared with 92% ± 5% of controls. At five years, 73% ± 12% of patients was still alive compared with 77% ± 8% of controls (Figure 2). Survival rates after transplant did not differ significantly between patients and controls (log rank test, P-value = 0.837).
Among 19 patients who received their transplant based on OSOTC medically urgent exception criteria, four patients had resumed drinking by last follow-up visit for a 21% relapse rate after a median follow up of 3.4 years.
For the first time, we report the rates of liver transplant and survival for patients with alcohol-induced cirrhosis who were deemed eligible for liver transplant and listed based on approval under the OSOTC medically urgent except criteria. These criteria allow patients with low to medium risk of recidivism, to receive a liver transplant after only one to three months of abstinence. These patients all committed to begin an alcohol treatment program before or during listing and to finish the program, even if it was after their transplant. We showed that transplant rate and short and long term survival after transplant is comparable between these patients and United States general population of patients with alcohol-induced cirrhosis who received their transplant after being evaluated for risk of recidivism based on conventionally used criteria. The risk of recidivism in our patients was comparable to previously published rates ranging from 15% to more than 20%[14-17].
Our findings challenge the notion of a defined abstinence period as the only criterion for liver transplant eligibility in patient with alcoholic liver cirrhosis[18]. However, the stringency of OSOTC process resulted in our selecting a very small number of patients with alcoholic cirrhosis for liver transplantation. Numerous studies have observed that the enforcement of sobriety period delays listing for transplantation in a significant number of patients with a low probability of alcohol relapse following liver transplant[17,19-23]. Indeed, the duration of alcohol abstinence before liver transplant is a poor indicator of relapse of alcoholism following transplantation[24].
Although our results are encouraging, the study has several limitations. The number of patients included in the study was small. Matched controls may not have been comparable to OSOTC patients in terms of family support, intention to remain abstinent from alcohol, or availability of counseling services at transplant center in the event of alcohol relapse. Future studies will benefit from a control group of patients with alcoholic liver disease undergoing liver transplantation that are matched to OSOTC patients on the basis of social and familial support.
In summary, liver transplantation may be an appropriate rescue option for selected patients with alcoholic liver disease after only one to three months of abstinence. Our results show that OSOTC transplant eligibility criteria provide a valid method to identify these patients who may benefit from liver transplantation with low to medium risk of recidivism.
A minimum of 12 mo of abstinence, or three months of abstinence and participation in an alcohol rehabilitation program, are the standard requirements before patients with alcoholic liver disease are eligible for transplantation in Ohio. Some patients are too ill to participate in a rehab program. The Ohio Solid Organ Transplantation Consortium (OSOTC) has a mechanism to evaluate risk of recidivism on a case-by-case basis.
Liver transplantation imparts great survival benefit to appropriately selected patients with advanced and de-compensated cirrhosis due to alcohol consumption, which is comparable to survival benefit of transplant in other types of chronic liver disease.
The aim of this study was to determine the effect of using OSOTC transplant eligibility criteria on patients’ survival compared with conventional criteria for assessment of risk of recidivism, in patient with cirrhosis due to alcoholic liver disease.
Patients can be approved as an exception and listed for transplant after only one to three months of abstinence.
They were matched based on gender, age, and MELD score to controls with alcohol-induced cirrhosis from Organ Procurement and Transplant Network data.
The manuscript “Ohio solid organ transplantation consortiumcriteria for liver transplantation in patients with alcoholic liver disease” by Hajifathalian et al is an interesting paper and the important contribution from OSOTC group supporting an update of the Criteria for Liver Transplantation based on alcohol abstinence duration potentially help to obtain the transplant for larger group of patients with a low probability of alcohol relapse.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bubnov RV, Gatselis NK, Jin B, Penkova-Radicheva MP, Sirin G S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 2. | Yoon YH, Chen CM, Yi HY. Surveillance Report #100: Liver Cirrhosis Mortality in the United States: National, State, and Regional Trends, 2000-2011. Available from: http://pubs.niaaa.nih.gov/publications/Surveillance100/Cirr11.htm. |
| 3. | Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 4. | Bellamy CO, DiMartini AM, Ruppert K, Jain A, Dodson F, Torbenson M, Starzl TE, Fung JJ, Demetris AJ. Liver transplantation for alcoholic cirrhosis: long term follow-up and impact of disease recurrence. Transplantation. 2001;72:619-626. [PubMed] |
| 5. | Webb K, Shepherd L, Day E, Masterton G, Neuberger J. Transplantation for alcoholic liver disease: report of a consensus meeting. Liver Transpl. 2006;12:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Everhart JE, Beresford TP. Liver transplantation for alcoholic liver disease: a survey of transplantation programs in the United States. Liver Transpl Surg. 1997;3:220-226. [PubMed] |
| 7. | Watt KD, McCashland TM. Transplantation in the alcoholic patient. Semin Liver Dis. 2004;24:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Everhart JE, Lombardero M, Detre KM, Zetterman RK, Wiesner RH, Lake JR, Hoofnagle JH. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64:1300-1306. [PubMed] |
| 9. | Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology. 2012;55:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 658] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 11. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2067] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 12. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 605] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 13. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1864] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 14. | Dumortier J, Dharancy S, Cannesson A, Lassailly G, Rolland B, Pruvot FR, Boillot O, Faure S, Guillaud O, Rigole-Donnadieu H. Recurrent alcoholic cirrhosis in severe alcoholic relapse after liver transplantation: a frequent and serious complication. Am J Gastroenterol. 2015;110:1160-1166; quiz 1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Jauhar S, Talwalkar JA, Schneekloth T, Jowsey S, Wiesner RH, Menon KV. Analysis of factors that predict alcohol relapse following liver transplantation. Liver Transpl. 2004;10:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Foster PF, Fabrega F, Karademir S, Sankary HN, Mital D, Williams JW. Prediction of abstinence from ethanol in alcoholic recipients following liver transplantation. Hepatology. 1997;25:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Miguet M, Monnet E, Vanlemmens C, Gache P, Messner M, Hruskovsky S, Perarnau JM, Pageaux GP, Duvoux C, Minello A. Predictive factors of alcohol relapse after orthotopic liver transplantation for alcoholic liver disease. Gastroenterol Clin Biol. 2004;28:845-851. [PubMed] |
| 18. | Lucey MR, Brown KA, Everson GT, Fung JJ, Gish R, Keeffe EB, Kneteman NM, Lake JR, Martin P, McDiarmid SV. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628-637. [PubMed] |
| 19. | Bird GL, O’Grady JG, Harvey FA, Calne RY, Williams R. Liver transplantation in patients with alcoholic cirrhosis: selection criteria and rates of survival and relapse. BMJ. 1990;301:15-17. [PubMed] |
| 20. | Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl. 2001;7:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Kumar S, Stauber RE, Gavaler JS, Basista MH, Dindzans VJ, Schade RR, Rabinovitz M, Tarter RE, Gordon R, Starzl TE. Orthotopic liver transplantation for alcoholic liver disease. Hepatology. 1990;11:159-164. [PubMed] |
| 22. | Osorio RW, Ascher NL, Avery M, Bacchetti P, Roberts JP, Lake JR. Predicting recidivism after orthotopic liver transplantation for alcoholic liver disease. Hepatology. 1994;20:105-110. [PubMed] |
| 23. | Yates WR, Martin M, LaBrecque D, Hillebrand D, Voigt M, Pfab D. A model to examine the validity of the 6-month abstinence criterion for liver transplantation. Alcohol Clin Exp Res. 1998;22:513-517. [PubMed] |
| 24. | DiMartini A, Day N, Dew MA, Javed L, Fitzgerald MG, Jain A, Fung JJ, Fontes P. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |