Published online Sep 18, 2016. doi: 10.4254/wjh.v8.i26.1110
Peer-review started: May 3, 2016
First decision: June 17, 2016
Revised: July 12, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: September 18, 2016
Processing time: 138 Days and 10 Hours
Hepatocellular adenoma (HCA) was recently classified into four pathological subtypes. There have been few studies describing the findings of contrast-enhanced ultrasonography (CEUS) of each type. Our case concerns a 78-year-old man who had undergone routine medical check-ups for hepatitis C for 11 years. Abdominal ultrasonography showed a 28 mm, hypo-echoic mass in the segment 4 of the liver. His integrating amount of drinking was 670 kg convert into ethanol. CEUS with Sonazoid demonstrated mild uniform hypo-enhancement with inflow of microbubbles from the periphery of the tumor in the arterial phase, and heterogeneously hypo-enhancement in the post vascular phase. Because the mass increased in size within 3 mo, a well differentiated hepatocellular carcinoma was suspected, and hepatic resection was performed. Microscopic findings showed homogeneous cell proliferation with low grade atypia, infiltration of inflammatory cells, ductular reactions, fatty deposit in part, and sinusoidal dilation. Immunohistochemistry revealed geographic positive for serum amyloid A (SAA), focal positive for glutamine synthetase, diffuse and strong positive for C-reactive protein, and positive for liver-type fatty acid binding protein. These pathological features corresponded to that of an inflammatory HCA. However, we could not make a clear diagnosis, because HCAs were defined as not to arise in cirrhotic liver. Finally, this tumor was diagnosed as a SAA positive hepatocellular neoplasm.
Core tip: Hepatocellular adenoma (HCA) was classified into four pathological subtypes. And HCA usually arises in the absence of significant fibrosis. Recently, some reports about serum amyloid A (SAA) positive hepatocellular neoplasm were published. All tumors shared features with inflammatory HCA arising in alcoholic cirrhosis. We describe the contrast-enhanced ultrasonographic findings of SAA positive HCA.
- Citation: Kumagawa M, Matsumoto N, Watanabe Y, Hirayama M, Miura T, Nakagawara H, Ogawa M, Matsuoka S, Moriyama M, Takayama T, Sugitani M. Contrast-enhanced ultrasonographic findings of serum amyloid A-positive hepatocellular neoplasm: Does hepatocellular adenoma arise in cirrhotic liver? World J Hepatol 2016; 8(26): 1110-1115
- URL: https://www.wjgnet.com/1948-5182/full/v8/i26/1110.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i26.1110
Hepatocellular adenoma (HCA) was recently classified into four pathological subtypes; hepatocyte nuclear factor 1 alpha inactivated HCA, beta catenin activated HCA, inflammatory HCA, and unclassified HCA[1-4]. Although contrast-enhanced ultrasonographic features of HCA have been reported in several literatures till now[5-7], there have been little studies that described those of each type of HCA[8]. HCAs usually arise in the liver without steatosis, because a nodule arising in fibrotic/cirrhotic liver was not to be a HCA according to World Health Organization classification 2010[1]. Recently, some reports about serum amyloid A (SAA) positive hepatocellular neoplasm were published. All nodules shared features with inflammatory HCA arising in alcoholic cirrhosis[9-11]. In this report, we describe contrast-enhanced ultrasonographic findings of SAA positive hepatocellular neoplasm which had features similar to inflammatory HCAs.
A 78-year-old man had undergone routine medical check-ups for hepatitis C over 21 years. He received interferon therapy 21 years ago, but could not achieve complete remission. In these years, he had compensatory hepatic cirrhosis and was gave medication of glycyrrhizin formulation. Abdominal ultrasonography showed 20 mm, hypo-echoic in the segment 4 of the liver 3 mo ago. Because the tumor increased in diameter to 28 mm, he was admitted to our hospital for further examinations. He had no history of other disease. He drank two glasses of whisky and one glass of beer from 20 till 66-year-old. His integrating amount of drinking was 670 kg convert into ethanol. He had no symptoms. Physical examination showed untoward features. Blood examination demonstrated thrombocytopenia, mild hyper-bilirubinemia, elevated liver enzymes (aspartate aminotransferase, 36 IU/L; alanine aminotransferase, 35 IU/L), positive for HCV-antibody, and 6.4 log IU/mL for HCV-RNA (Table 1).
| WBC (3.3-8.6) | 6200/μL | Total protein (6.6-8.1) | 8.0 g/dL |
| Hgb (13.7-16.8) | 15.0 g/dL | Albumin (4.1-5.1) | 4.7 g/dL |
| Platelet (148-348) | 108 × 103 /μL | HBs antibody | (-) |
| PT% (> 80) | 99% | HBc antigen | (-) |
| INR | 0.92 | HCV antibody | (+) |
| Total bilirubin (0.4-1.5) | 1.41 mg/dL | HCV-RNA | 6.4 logIU/mL |
| Direct bilirubin (0.05-0.4) | 0.37 mg/dL | Alpha-fetoprotein (< 20) | 6.4 ng/mL |
| Aspartate transaminase (13-30) | 36 IU/L | PIVKA-II (< 40) | 91 mAU/mL |
| Alanine transaminase (10-42) | 35 IU/L | ICG (15 s) (< 15.0) | 14.0% |
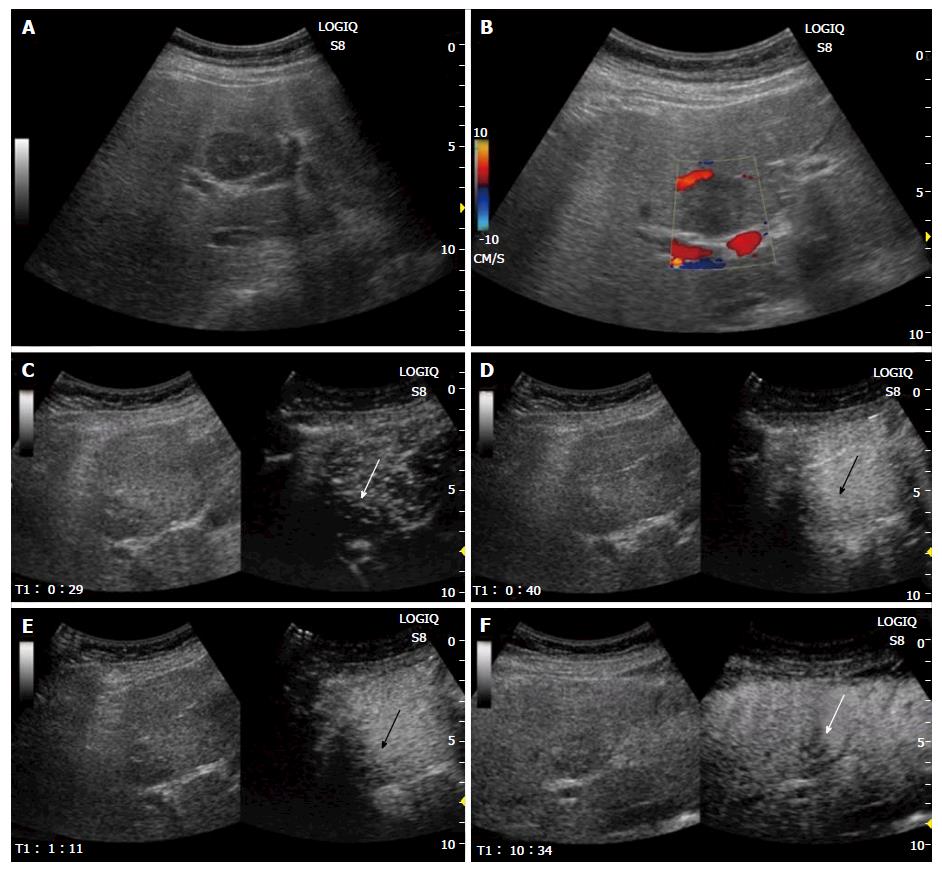
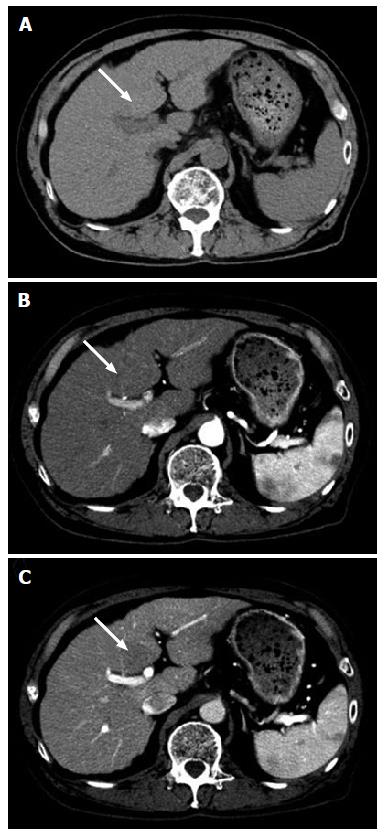
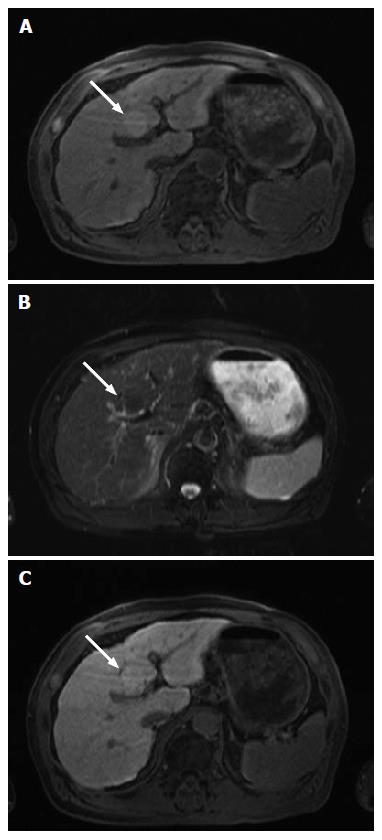
Sonographic examination showed a homogenous, hypo-echoic, round mass in the segment 4b of the liver (Figure 1A). Color Doppler sonography revealed no signals in the lesion (Figure 1B). Contrast-enhanced ultrasonography (CEUS) with 0.5 mL of Sonazoid (Daiichi Sankyo, Tokyo, Japan) demonstrated mild global hyper-enhancement with inflow of microbubbles from the periphery of the tumor in the arterial phase (Figure 1C and D), persist enhancement in the portal venous phase (Figure 1E), and heterogeneous hypo-enhancement in the post vascular phase (Figure 1F). Plain computed tomography (CT) showed a hypodense tumor (Figure 2A). Contrast-enhanced CT showed iso-enhancement in the arterial phase (Figure 2B) and slight hypo-enhancement in the portal phase (Figure 2C). Magnetic resonance imaging (MRI) demonstrated slightly high intensity in the T1 weighted image (Figure 3A), and slightly low intensity in the T2 weighted image (Figure 3B). Contrast-enhanced MRI using Gadolinium ethoxybenzyl diethylene triamine pentaacetic acid revealed slightly high intensity in the hepatobiliary phase (Figure 3C).
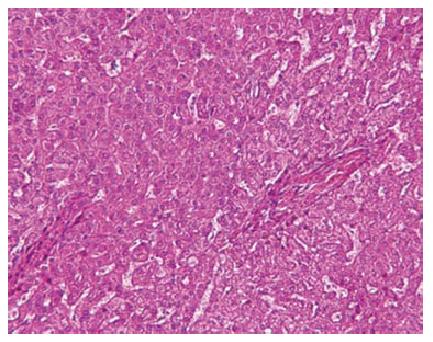
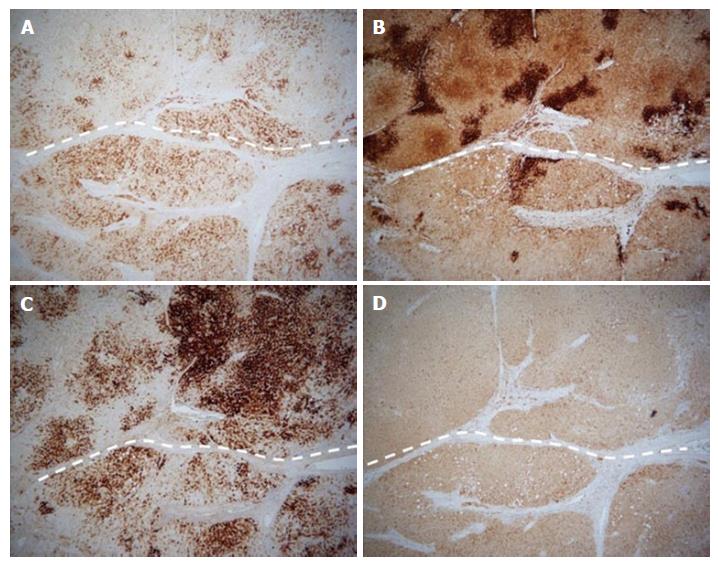
Because the mass increased in size, it was suspected as being a well differentiated hepatocellular carcinoma. Considering the risk of hemorrhage and dissemination, partial segment 4 resection was performed without biopsy. Because the mass was adjacent to horizontal portion of the left portal vein and pathological diagnosis was needed, percutaneous ablation was not chosen. Microscopic findings showed homogeneous cell proliferation with low grade atypia, infiltration of inflammatory cells, ductular reactions, fatty deposit in part, and sinusoidal dilation (Figure 4). Immunohistochemistry revealed geographic positive for SAA, focal positive for glutamine synthetase, diffuse and strong positive for C-reactive protein, and positive for liver-type fatty acid binding protein (Figure 5). These pathological features corresponded to that of an inflammatory HCA.
Classification of HCA is based on molecular, pathologic, and immunohistochemical features[1]. Inflammatory HCA accounts for 45%-60% of all HCAs[1-4], and has mutations of the IL6ST gene[12-14]. Alcohol intake and obesity are association with inflammatory HCA[9,15-17]. The rate of malignant transformation is unknown.
CEUS findings of HCA were described in previous studies[5-8,18]. In one study which investigated 18 lesions, which were iso-hypoechoic in 9, hyper-enhancing in all in the arterial phase, and iso or hypo-enhancement in all in the late phase[18]. Ricci et al[19] emphasized homogeneous and centripetal enhancement during artery phase was showed in almost all. In accordance with previous studies[19-21], HCA lesions had some typical features, including early, homogeneous, centripetal, and strong enhancement in the arterial phase and the lack of a portal vein supply. According to a study[22], a number of HCAs demonstrated persistent enhancement. Dong et al[22] said “slow wash-out” (persistent enhancement during portal venous and late phase) may be a discriminant sign for HCAs in CEUS. In our case, the arterial phase findings were not so strong but homogenous and centripetal hyper-enhancement and some peripheral vessels were showed. The contrast medium that used in that study was Sonovue (Bracco, Milan, Italy) whereas Sonazoid was administered to our patient. Sonovue and Sonazoid are phagocytosed by Kupffer cells, and visualize clearly malignant tumor as defect. CEUS using Sonazoid revealed hypo-enhancement in the post vascular phase in our patient which was interpreted as lack of Kupffer cells in the tumor.
Recently SAA-positive hepatocellular neoplasms were proposed[10,11]. Generally, HCA arises from normal liver[1,23]. Although SAA-positive hepatocellular neoplasms have similar features to inflammatory HCA, they arise from alcoholic cirrhosis. Sasaki et al[10,11] suggested, considering that the patient exposed to alcohol in inflammatory HCA, it may be not be surprising that inflammatory HCA arise in alcoholic hepatic disease or cirrhosis. Our case had liver cirrhosis with HCV infection, moreover had a history of excessive amounts-alcohol consumption. We considered that our case is SAA-positive hepatocellular neoplasm.
In conclusion, CEUS revealed homogeneous mild hyper-enhancement in the arterial phase and heterogeneous hypo-enhancement in the post vascular phase in our case. Some of CEUS findings corresponded to features of HCA. Our patient had both HCV infection and alcohol abuse, and it was not typical for inflammatory HCA. It may be a case of so-called SAA-positive hepatocellular neoplasm.
A 78-year-old man with hepatitis C and hepatic cirrhosis received abdominal ultrasonography, in which 20 mm, homogenous, hypo-echoic, round mass was shown in the segment 4b of the liver.
Because the mass increased in size and he had hepatic cirrhosis, a well differentiated hepatocellular carcinoma was suspected.
Dysplastic nodule, large regenerative nodule, hepatocellular adenoma (HCA), and focal nodular hyperplasia.
Hepatic pre-cirrhosis with early hepatocellular carcinoma.
A well differentiated hepatocellular carcinoma was suspected.
Inflammatory HCA.
Segment 4 partial resection.
HCA was classified into four pathological subtypes and usually arises in the absence of significant fibrosis. Serum amyloid A (SAA)-positive hepatocellular neoplasm shares features with inflammatory HCA arising in alcoholic cirrhosis.
SAA-positive hepatocellular neoplasms have similar features to inflammatory HCA, they arise from alcoholic cirrhosis.
Considering that the patient exposed to alcohol in inflammatory HCA, it may be not be surprising that it arise in alcoholic hepatic disease or cirrhosis. Recognizing SAA-positive hepatocellular neoplasm as differential diagnosis is important in the case that had both HCV infection and alcohol abuse.
This is a very interesting case, well investigated and presented with also pathological comparison images.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cosgrove D, Lagadinou M, Lee SY, Mauri G, Negrei C S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Bioulac-Sage P, Balabaud C, Wanless I. Focal nodular hyperplasia and hepatocellular adenoma, In: WHO classification of tumours of the digestive system, Edited by F Bosman, F Carneiro, H Hruban, et al. 4th ed. IARC, Lyon, 2010: 198-204. . |
| 2. | van Aalten SM, Verheij J, Terkivatan T, Dwarkasing RS, de Man RA, Ijzermans JN. Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J Hepatol. 2011;55:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 535] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 397] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Kong WT, Wang WP, Huang BJ, Ding H, Mao F, Si Q. Contrast-enhanced ultrasound in combination with color Doppler ultrasound can improve the diagnostic performance of focal nodular hyperplasia and hepatocellular adenoma. Ultrasound Med Biol. 2015;41:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Ricci P, Cantisani V, D’Onofrio M, Sahani D, Pagliara E, Calliada F, Mehmet E, Sanjeva K, Faccioli N, Pozzi-Mucelli R. Behavior of hepatocellular adenoma on real-time low-mechanical index contrast-enhanced ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2008;27:1719-1726. [PubMed] |
| 7. | Roche V, Pigneur F, Tselikas L, Roux M, Baranes L, Djabbari M, Costentin C, Calderaro J, Laurent A, Rahmouni A. Differentiation of focal nodular hyperplasia from hepatocellular adenomas with low-mechanical-index contrast-enhanced sonography (CEUS): effect of size on diagnostic confidence. Eur Radiol. 2015;25:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Laumonier H, Cailliez H, Balabaud C, Possenti L, Zucman-Rossi J, Bioulac-Sage P, Trillaud H. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: correlation with MRI findings. AJR Am J Roentgenol. 2012;199:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Sasaki M, Yoneda N, Kitamura S, Sato Y, Nakanuma Y. A serum amyloid A-positive hepatocellular neoplasm arising in alcoholic cirrhosis: a previously unrecognized type of inflammatory hepatocellular tumor. Mod Pathol. 2012;25:1584-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Sasaki M, Kondo F, Sawai Y, Imai Y, Kadowaki S, Sano K, Fukusato T, Matsui O, Nakanuma Y. Serum amyloid A-positive hepatocellular neoplasms in the resected livers from 3 patients with alcoholic cirrhosis. Histol Histopathol. 2013;28:1499-1505. [PubMed] |
| 11. | Sasaki M, Yoneda N, Sawai Y, Imai Y, Kondo F, Fukusato T, Yoshikawa S, Kobayashi S, Sato Y, Matsui O. Clinicopathological characteristics of serum amyloid A-positive hepatocellular neoplasms/nodules arising in alcoholic cirrhosis. Histopathology. 2015;66:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Nault JC, Fabre M, Couchy G, Pilati C, Jeannot E, Tran Van Nhieu J, Saint-Paul MC, De Muret A, Redon MJ, Buffet C. GNAS-activating mutations define a rare subgroup of inflammatory liver tumors characterized by STAT3 activation. J Hepatol. 2012;56:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 373] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 14. | Pilati C, Amessou M, Bihl MP, Balabaud C, Nhieu JT, Paradis V, Nault JC, Izard T, Bioulac-Sage P, Couchy G. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med. 2011;208:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Sasaki M, Yoneda N, Kitamura S, Sato Y, Nakanuma Y. Characterization of hepatocellular adenoma based on the phenotypic classification: The Kanazawa experience. Hepatol Res. 2011;41:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Paradis V, Champault A, Ronot M, Deschamps L, Valla DC, Vidaud D, Vilgrain V, Belghiti J, Bedossa P. Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology. 2007;46:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Forsberg F, Piccoli CW, Liu JB, Rawool NM, Merton DA, Mitchell DG, Goldberg BB. Hepatic tumor detection: MR imaging and conventional US versus pulse-inversion harmonic US of NC100100 during its reticuloendothelial system-specific phase. Radiology. 2002;222:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Ricci P, Laghi A, Cantisani V, Paolantonio P, Pacella S, Pagliara E, Arduini F, Pasqualini V, Trippa F, Filpo M. Contrast-enhanced sonography with SonoVue: enhancement patterns of benign focal liver lesions and correlation with dynamic gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol. 2005;184:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Dong Y, Zhu Z, Wang WP, Mao F, Ji ZB. Ultrasound features of hepatocellular adenoma and the additional value of contrast-enhanced ultrasound. Hepatobiliary Pancreat Dis Int. 2016;15:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 1.5] [Reference Citation Analysis (0)] |