Published online Jul 28, 2016. doi: 10.4254/wjh.v8.i21.891
Peer-review started: February 27, 2016
First decision: May 13, 2016
Revised: June 2, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: July 28, 2016
Processing time: 101 Days and 17.6 Hours
Currently, partial hepatectomy is the treatment of choice for a wide variety of liver and biliary conditions. Among the possible complications of partial hepatectomy, acute kidney injury (AKI) should be considered as an important cause of increased morbidity and postoperative mortality. Difficulties in the data analysis related to postoperative AKI after liver resections are mainly due to the multiplicity of factors to be considered in the surgical patients, moreover, there is no consensus of the exact definition of AKI after liver resection in the literature, which hampers comparison and analysis of the scarce data published on the subject. Despite this multiplicity of risk factors for postoperative AKI after partial hepatectomy, there are main factors that clearly contribute to its occurrence. First factor relates to large blood losses with renal hypoperfusion during the operation, second factor relates to the occurrence of post-hepatectomy liver failure with consequent distributive circulatory changes and hepatorenal syndrome. Eventually, patients can have more than one factor contributing to post-operative AKI, and frequently these combinations of acute insults can be aggravated by sepsis or exposure to nephrotoxic drugs.
Core tip: In the specific scenario of liver resections, there are limited and heterogeneous data regarding the occurrence of acute kidney injury (AKI) in the postoperative period, and its clinical relevance (mortality, morbidity and hospital stay) were not conclusively explored and clarified. Difficulties in the data analysis related to postoperative AKI after liver resections are mainly due the scarce data published on the subject.
- Citation: Peres LAB, Bredt LC, Cipriani RFF. Acute renal injury after partial hepatectomy. World J Hepatol 2016; 8(21): 891-901
- URL: https://www.wjgnet.com/1948-5182/full/v8/i21/891.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i21.891
Currently, partial hepatectomy is the treatment of choice for a wide variety of primary liver tumors (benign or malignant), tumors of the bile ducts and secondary malignant liver tumors. The partial liver resections may also be necessary in the management of complex cystic liver diseases, benign biliary structures, some cases of hepatic trauma and more recently with living donor liver transplantation[1]. With the refinement of surgical techniques, improved selection of patients to procedure, advances in anesthetic support and perioperative care, this traditionally complex and feared operation has become a routine procedure in the past 20 years, with acceptable mortality rates ranging from 3.1% to 4.5%[2-4].
Among the possible complications of major surgical procedures, including the partial hepatectomy, acute kidney injury (AKI) should be considered as an important cause of increased morbidity and postoperative mortality[5,6], with an incidence ranging from 10% to 30% after major operations[7,8]. Literature data report an incidence of 1% of AKI in the postoperative major non-cardiac surgery without liver resection[6] about 20% after cardiac surgery[9-11] and 50% after liver transplantation[12-18].
In the specific scenario of liver resections, there are limited and heterogeneous data regarding the occurrence of AKI in the postoperative period, with an incidence ranging from 0.9% to 15.1% of the patients[19-23], and its clinical relevance (mortality, morbidity and hospital stay) were not conclusively explored and clarified.
Difficulties in the data analysis related to postoperative AKI after liver resections are mainly due to the multiplicity of factors to be considered in this surgical patients, such as general medical conditions and comorbidities, nutritional disorders, metastatic malignancy with low physiological reserve systems, immunological disorders, chemotherapy treatment, functional capacity and volume of liver parenchyma to be preserved, and the perioperative hemodynamic effects of the different modalities of partial hepatectomy. Moreover, there is no consensus of the exact definition of AKI after liver resection in the literature, which hampers comparison and analysis of the scarce data published on the subject[22].
Despite this multiplicity of risk factors for postoperative AKI after partial hepatectomy, there are main factors that clearly contribute to its occurrence. First factor relates to large blood losses with renal hypoperfusion during the operation[20], that very often can be associated by the deleterious renal effects of red blood cell transfusion[23], and in some occasions this renal hypoperfusion occurs in patients with increased renal susceptibility to ischemia, usually elderly patients with underlying cardiovascular or renal disorders, or eventually it may be drug-induced[21-24]. Second factor relates to the occurrence of post-hepatectomy liver failure (PLF) with consequent distributive circulatory changes and hepatorenal syndrome (HRS)[20]. Eventually, patients can have more than one factor contributing to post-operative AKI, and frequently these combinations of acute insults can be aggravated by sepsis[20-24] or exposure to nephrotoxic drugs, such as aminoglycosides[25].
The aim of this review is to present the definition of postoperative AKI after partial hepatectomy, the different pathophysiological mechanisms for its occurrence and methods for preventing these events.
AKI is characterized by the deterioration of kidney function over a period of hours to days, resulting in the failure of the kidney to excrete nitrogenous waste products and to maintain fluid and electrolyte homeostasis[26]. In recent years, several criteria have been proposed for the diagnosis of AKI in general population, particularly the “Risk, Injury, Failure, Loss of Renal Function and End-Stage Renal Disease” (RIFLE) criteria[27], the “Acute Kidney Injury Network” (AKIN) criteria[28] and more recently, the criteria suggested by a panel of experts, which combine the AKIN and the RIFLE criteria, thus proposing a new classification: The “Kidney Disease Improving Global Outcomes” criteria[29] (Table 1).
| RIFLE criteria[27] | AKIN criteria[28] | KDIGO criteria[29] | |
| Diagnostic criteria | Increase in SCr to ≥ 1.5 times baseline, within 7 d; or GFR decrease > 25%; or urine volume < 0.5 mL/kg per hour for 6 h | Increase in sCr by ≥ 0.3 mg/dL (26.5 mmol/L) within 48 h; or increase in sCr ≥ 1.5 times baseline within 48 h; or urine volume < 0.5 mL/kg per hour for 6 h | Increase in sCr by ≥ 0.3 mg/dL (26.5 mmol/L) within 48 h; or increase in SCr to ≥ 1.5 times baseline, which is known or presumed to have occurred within the prior 7 d; or urine volume < 0.5 mL/kg per hour for 6 h |
| Risk: sCr increase 1.5-1.9 times baseline; or GFR decrease 25%-50%; or urine output < 0.5 mL/kg per hour for 6 h | Stage 1: sCr increase 1.5-1.9 times baseline; or sCr increase ≥ 0.3 mg/dL (26.5 mmol/L); or urine output < 0.5 mL/kg per hour for 6 h | Stage 1: sCr increase 1.5-1.9 times baseline; or sCr increase ≥ 0.3 mg/dL (26.5 mmol/L); or urine output < 0.5 mL/kg per hour for 6-12 h | |
| Staging | Injury: sCr increase 2.0-2.9 times baseline; or GFR decrease 50%-75%; or urine output < 0.5 mL/kg per hour for 12 h | Stage 2: sCr increase 2.0-2.9 times baseline; or urine output < 0.5 mL/kg per hour for 12 h | Stage 2: sCr increase 2.0-2.9 times baseline; or urine output < 0.5 mL/kg per hour for ≥ 12 h |
| Failure: sCr increase ≥ 3.0 times baseline: or GFR decrease 50%-75%; or sCr increase ≥ 4.0 mg/dL (353.6 mmol/L) with an acute increase of at least 0.5 mg/dL (44 mmol/L); or urine output < 0.3 mL/kg per hour for ≥ 24 h; or anuria for ≥ 12 h | Stage 3: sCr increase 3.0 times baseline; or sCr increase ≥ 4.0 mg/dL (353.6 mmol/L) with an acute increase of at least 0.5 mg/dL (44 mmol/L); or urine output < 0.3 mL/kg per hour for ≥ 24 h; or anuria for ≥ 12 h | Stage 3: sCr increase 3.0 times baseline; or sCr increase to ≥ 4.0 mg/dL (353.6 mmol/L); or initiation of renal replacement therapy; or urine output < 0.3 mL/kg per hour for ≥ 24 h; or Anuria for ≥ 12 h |
The first question regarding the definition of post-operative AKI after partial hepatectomy, would be determining which of these proposed AKI criteria is most appropriate for these patients undergoing liver resection. Whereas acute tubular necrosis (ATN), resulting from hypoxic damage to the renal medulla, is considered as a major cause of postoperative AKI[30], different from general population, liver resections are often performed in the presence of functional deficit of the hepatic parenchyma, as in fibrosis, steatosis, cirrhosis, chemotherapy-induced injury and also in biliary obstruction[2]. Moreover, the recent technical improvements in liver surgery have resulted in an expansion and more liberal indications for major hepatectomies in patients with these underlying liver conditions[2,3,31-34], however, the risk of postoperative complications, such as AKI, have remained important concerns[3,31,35].
In the specific case of hepatocellular carcinoma, the tumor generally appears in a cirrhotic liver, which is a contributor to unfavorable postoperative results in large procedures[36], regarding renal dysfunction, AKI is a common and potentially fatal event in patients with cirrhosis[37-39], with a reported prevalence of 14%-50% in patients with cirrhosis[40-45], this wide range in prevalence is likely due to different study populations and varying definitions of renal dysfunction. Studies evaluating survival predictors in cirrhosis, renal dysfunction was a powerful predictor of death, as Child-Pugh score[46-48].
Along with parenchymal dysfunction, the portal hypertension levels and its hemodynamic consequences are directly related to the degree of underlying liver injury[49-51], as it is observed in cirrhosis and others conditions, such as severe steatosis and chemotherapy-induced injury[52]. The types of chemotherapy-induced liver toxicity include steatosis[53], sinusoidal changes[54], steatohepatitis[55], and hemorrhagic central lobular necrosis[52]. Steatosis represents fatty changes in the liver, with the presence of fat droplets within the hepatocytes[56], and it has been shown that steatosis may interfere with circulation through sinusoids and impair regeneration, and in addition the liver’s protective mechanism against oxidative stress appear to be impaired[57,58]. The morbidity following liver resection associated with steatosis has been reported by Belghiti et al[2], in this study with 747 patients, the mortality rate was higher in patients having steatosis than in those with no steatosis, 22% vs 8%, respectively (P = 0.003). Likewise, according to Behrns et al[32] in 135 liver resections, morbidity was seen in 29% and 10% of the patients with steatosis and without steatosis, respectively.
Besides the fact that a significant portion of patients eligible for partial hepatectomy have underlying chronic liver disease or were exposed to systemic therapies with liver toxicity, the hemodynamic changes in patients after major liver resections may have similarities with those of patients with cirrhosis or acute liver failure, and depending on the remnant liver volume and functional quality of parenchyma (steatosis/cirrhosis) the clinical effects may be more evident[59].
In 1953, Kowalski and Abelmann[60] reported the results of a study which have demonstrated that cardiac output in cirrhotic patients was significantly higher compared with healthy volunteers. The reason for this so-called hyperdynamic state is that patients with cirrhosis develop portal hypertension with resultant splanchnic vasodilation and pooling of blood secondary to increased resistance to portal flow. This is due to (1) vasodilators such as nitric oxide, carbon monoxide, and endogenous cannabinoids[61,62]; and (2) vasodilation from inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 induced by bacterial translocation from the gut[63]. As a result, the concentration of cyclic guanosine monophosphate cyclic is increased, resulting in splanchnic vasodilation, decrease in central and arterial blood volume, low capillary pressure, low central venous pressure (LCVP), low systemic vascular resistance, and reduction of mean arterial pressure[64]. This compensatory increase in cardiac output via activation of the sympathetic nervous system by carotid baroreceptors maintains sufficient renal perfusion, however, with decompensation of cirrhosis and increasing severity of portal hypertension, the compensatory increase in cardiac output is inadequate to maintain circulatory blood volume and adequate renal perfusion[65]. Therefore, it would be reasonable that diagnostic and staging AKI criteria that consider this circulatory impairment could be better applied in patients undergoing liver resections, particularly large resections and those with chronic liver disease.
It is extremely important to point out that in the case of patients with chronic liver disease, isolated dosages of serum creatinine (sCr) levels can not reveal the actual renal function of the patient, because: (1) there is decreased creatine formation in the secondary muscles loss of muscle mass[66]; (2) is increased renal tubular secretion of creatinine (Cr)[67]; (3) increasing the circulating volume of distribution in cirrhosis can dilute the sCr[68]; and (4) interference in the measurement of Cr due to elevated bilirubin[69]. As a result, the serum levels of Cr in patients with cirrhosis overestimate glomerular filtration rate (GFR). Therefore, a dynamic definition referring to the elevation of serum Cr of ≥ 50% of preoperative levels to a final value ≥ 1.5 mg/dL (133 mol/L) could be more suitable for these patients, and clinical studies have shown that AKI according to these criteria was a strong predictor of hospital mortality in patients with liver disease[70-72].
Another situation relates to the measurement of urine output of patients with chronic liver disease and ascites, since these patients can often present oliguria with high sodium retention, but they can still maintain a relatively normal GFR[73]. On the other hand, these patients can also have an increased diuresis because of diuretics therapy.
Thus, the current criteria suggested by the “International Ascites Club” for definition of AKI in cirrhotic patients do not include unreal measurements for these patients[68] (Table 2), and apparently would be the most appropriate criteria for the diagnosis and management of AKI after partial hepatectomy, especially in cases of large resections and underlying chronic liver disease.
| Baseline sCr | A value of sCr obtained in the previous 3 mo, when available, can be used as baseline sCr. In patients with more than one value within the previous 3 mo, the value closest to the admission time to the hospital should be used. In patients without a previous sCr value, the sCr on admission should be used as baseline |
| Definition of AKI | Increase in sCr ≥ 0.3 mg/dL (≥ 26.5 mmol/L) within 48 h; or a percentage increase sCr ≥ 50% from baseline which is known, or presumed, to have occurred within the prior 7 d |
| Staging of AKI | Stage 1: Increase in sCr ≥ 0.3 mg/dL (26.5 mmol/L) or an increase in sCr ≥ 1.5-fold to twofold from baseline Stage 2: Increase in sCr > two to threefold from baseline Stage 3: Increase of sCr > threefold from baseline or sCr ≥ 4.0 mg/dL (353.6 mmol/L) with an acute increase ≥ 0.3 mg/dL (26.5 mmol/L) or initiation of renal replacement therapy |
| Progression of AKI | Progression: Progression of AKI to a higher stage and/or need for RRT |
| Regression: Regression of AKI to a lower stage | |
| Response to treatment | No response: No regression of AKI |
| Partial response: Regression of AKI stage with a reduction of sCr to ≥ 0.3 mg/dL (26.5 mmol/L) above the baseline value | |
| Full response: Return of sCr to a value within 0.3 mg/dL (26.5 mmol/L) of the baseline value |
Although the extent of liver resection correlates with the magnitude of the procedure, and patients undergoing resection of more than three segments or an additional extrahepatic procedure have an increased risk of complications[74-76], this is not a rigid rule. For example, an isolated resection of segment I is technically more demanding than a right hepatectomy, similarly, resection of segments IV, V, VIII or posterior right segments (segments VI, VII) may be technically more difficult than the left or right hepatectomy, although the transection area is larger. Therefore, a minor hepatectomy should not be considered as an operation of less magnitude, and most important, the prevention of intraoperative hemorrhage should not be neglected. If excessive blood loss persists and a reduction in oxygen delivery is not corrected, the renal medulla may be susceptible to ischemic ATN[77], and as a result, patients may suffer from AKI. The results of two large studies[3,31] suggest that a blood loss of 1250 mL is the cutoff value for major complications after liver resections, such as AKI. Furthermore, red blood cell transfusion, that can be necessary in the case of haemorrhage, can be an additional risk factor for postoperative AKI[78].
The kidneys are most vulnerable to moderate hypoperfusion when autoregulation is impaired. Factors increasing susceptibility to renal hypoperfusion may be seen in elderly patients or in patients with atherosclerosis, hypertension, or chronic renal failure, in whom hyalinosis and myointimal hyperplasia cause structural narrowing of the arterioles[79-81]. Increased susceptibility to renal ischemia may also occur in malignant hypertension because of intimal thickening and fibrinoid necrosis of the small arteries and arterioles[82]. In addition, in chronic kidney disease, afferent arterioles in the functioning glomeruli become dilated with impairment of the kidney’s ability to autoregulate the glomerular filtration rate in low-perfusion states[83].
Impaired decreasing of afferent arteriolar resistance can occur when a patient is receiving nonsteroidal anti-inflammatory drugs or cyclooxygenase-2 inhibitors, which reduce the synthesis of prostaglandins in the kidneys, as consequence a decreasing in glomerular capillary pressure occurs in occasions of low-perfusion states[82,84-86]. In other situations, calcineurin inhibitors[87], and radiocontrast agents[88] can act through various vasoconstrictor mediators to increase afferent arteriolar resistance, the later may have direct toxic effects on the tubules as well[81,82,88-92]. Decreased renal perfusion may also may have an exaggerated drop in the GFR in low-perfusion states as a consequence of not raising efferent arteriolar resistance by angiotensin II in patients who are receiving angiotensin-receptor blockers or angiotensin-converting-enzyme inhibitors.
Despite the deleterious effect of hemodynamic instability in renal perfusion, red blood cell transfusion, that can be necessary in the case of haemorrhage, can be an additional risk factor for postoperative AKI[78]. Although the exact causal link between red blood cell transfusion and postoperative AKI is not fully elucidated, there are several mechanisms that may be implicated: Deficiency in 2,3-diphosphoglycerate with impaired oxygen unloading from hemoglobin, less deformability of stored red blood cells with obstruction of smaller capillaries[93] stored red blood cells hemolysis with an increase in circulating free iron[94]. Other mechanisms might include loss of the ability to generate nitric oxide, release of procoagulant phospholipids, increased adhesiveness to vascular endothelium, and accumulation of proinflammatory phospholipids[93,95-98].
Apart from blood loss, that can leads to ATN because of severe hemodynamic instability, others risk factors for postoperative AKI after partial hepatectomy would be those that favor PLF, characterized by jaundice, coagulopathy, encephalopathy, ascites, and renal and pulmonary failure, all of which may become apparent only 3 to 5 d after surgery[1]. These risk factors for PLF are well described, such as a small volume of remaining liver with marked volume reduction of organ parenchyma[35,99,100] associated to parenchymal cell injury due portal hyperperfusion[59,101], liver cirrhosis or steatosis[102,103], and liver toxicity induced by chemotherapy[104]. In patients with liver cirrhosis, the postoperative liver failure may occur due the compromised liver microcirculation, with less resistance to ischemia-reperfusion injury[105] and impaired regeneration[106], in addition, portal hypertension, if present, is associated with a poor outcome because of compromised portal flow and the risk of postoperative upper gastrointestinal bleeding[107].
Liver steatosis is usually related to obesity, the presence of metabolic disorders, or the intake of alcohol or drugs, and this liver disorder increases the operative risk of partial hepatectomy[2,53,108]. The extent of liver resection in these patients with steatosis in order to avoid PLF is unclear, but the severity of fatty infiltration must be considered: Mild steatosis (up to 30% of hepatocytes containing fat) represents a minimal additional risk, in moderate steatosis (30% to 60% containing fat) caution is necessary, thus, a conservative resection should be favored, and patients with severe steatosis (more than 60% of hepatocytes containing fat) should undergo only limited resection[108].
Regarding the chemotherapy-induced liver aggression, the rates of complications and death after major liver resection are likely to be increased[55,109]. Oxaliplatin can induce a veno-occlusive syndrome, occasionally associated with nodular regenerative hyperplasia, these vascular obstructions result in a bluish appearance of the liver (blue liver syndrome)[54,110,111], and irinotecan can cause chemotherapy associated steatohepatitis[112], and liver impairment can be amplified after partial hepatectomy in both situations, triggering PLF[113].
A major concern regarding PLF is the onset of HRS. HRS is a reversible functional renal impairment that occurs in patients with advanced liver cirrhosis or hepatic failure. It is characterized by marked decrease in GFR and renal plasma flow in the absence of other cause of renal failure[114] (Table 3). The pathophysiological alterations of SHR consist of intravascular hypovolemia with activation of the renin-angiotensin-aldosterone system and vasoconstrictive sympathetic nervous system, leading to renal vasoconstriction of the afferent vessels and subsequent decrease in GFR[20]. Two subtypes of HRS have been identified: SHR type 1 is characterized by a rapidly progressive renal insufficiency defined as a doubling of the initial serum creatinine to a level greater than 2.5 mg/dL or 220 μmol/L in less than 2 wk, it is associated with very poor prognosis, and SHR Type 2 is characterized by a moderate renal insufficiency (Cr greater than 1.5 mg/dL or 133 μmol/L), follows a steady course or slowly progressive, often associated with refractory ascites[114].
| HRS-AKI |
| Diagnosis of cirrhosis and ascites |
| Diagnosis of AKI according to ICA-AKI criteria (Table 2) |
| No response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin 1 g/kg bodyweight |
| Absence of shock |
| No current or recent use of nephrotoxic drugs (NSAIDs, aminoglycosides, iodinated contrast media, etc.) |
| No macroscopic signs of structural kidney injury, defined as |
| Absence of proteinuria (> 500 mg/d) |
| Absence of microhaematuria (> 50 RBCs per high power field) |
| Normal findings on renal ultrasonography |
| Patients who fulfil these criteria may still have structural damage such as tubular damage. Urine biomarkers will become an important element in making a more accurate differential diagnosis between HRS and acute tubular necrosis |
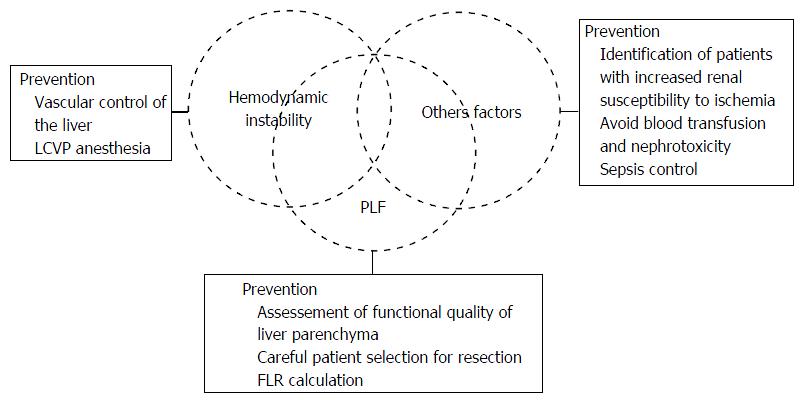
Despite the fact that patients can have more than one factor contributing to post-operative AKI after partial hepatectomy, eventually aggravated by sepsis[20,21-24] or exposure to nephrotoxic drugs[25], there are particular risk factors that must be controlled and specific operative and non-operative procedures that must be undertaken for prevention of post-operative renal injury in these patients (Figure 1).
For prevention of intraoperative blood loss with consequent hemodynamic instability during the partial hepatectomy, there are intraoperative maneuvers that may be crucial in the moment of parenchymal transaction, such as vascular control of the liver[21].
The vascular control of the liver is an effective method to reduce bleeding during the hepatectomy. While various techniques have been proposed, the two most widely used methods are the vascular inflow occlusion and complete vascular exclusion[115,116]. Occlusion of the hepatic vascular inflow[117] by the application of tourniquet in hepatoduodenal ligament[118] is the oldest and simplest way to reduce blood loss during hepatectomy. The “Pringle maneuver” can be used continuously to normal livers under normothermic conditions for a maximum of 60 min, and for 30 min in cirrhotic or steatotic livers, although longer periods have already been described[119-122]. According Belghiti et al[123] there is no significant difference in blood loss during surgery using the Pringle maneuver continuously or intermittently (15 min of ischemia for 5 min reperfusion). These concerns about longer periods of hepatic vascular inflow is mainly because that obstruction of the portal blood flow causes venous congestion of the bowel, and in combination with warm ischemic liver injury it results in a flush of anaerobic metabolites and cytokines back into the circulation on the clamp release[124]. In the total vascular exclusion[125], the occlusion of the hepatic vascular inflow is combined to hepatic venous exclusion. The complete hepatic ischemia can be associated to hypothermic perfusion with cooled preservation solution[126] and extracorporeal venovenous bypass, with “ex situ” liver resection[127] or “in situ” liver resection[128].
During the parenchymal transaction, a LCVP prevents the back bleeding from hepaticveins[19,129,130], and along with vascular control of the liver, these techniques test the patients cardiovascular reserve[21]. LCVP anesthesia is based on patients being maintained in hypovolaemic state until liver resection has been completed[19,129], this is in contrast to most other major surgical procedures, where patients receive large volumes of crystalloid and colloid during the peri-operative period[21]. Moreover, vasodilators are often used to further reduce central venous pressure (CVP), leading to distributive changes in blood flow[129], and whereas these techniques are applied for haemorrhage control and consequently promoting AKI prevention, a potential consequence of such circulatory changes is ATN, with subsequent renal impairment or failure[20]. The kidneys are at greater risk with abrupt fall in blood pressure, if the mean arterial pressure reaches values below 80 mmHg, there is a significant decrease in GFR[24].
In the study of Wang et al[131], the maintenance of CVP ≤ 4 mmHg has reduced blood loss during partial hepatectomy, and has shortened the length of hospital stay, with no detrimental effects on hepatic or renal function. According to Melendez et al[19], in 496 liver resections with an anesthetic protocol of fluid restriction, with the use of nitroglycerin, furosemide, and with the maintenance of a systolic blood pressure of 90 mmHg, the median volume blood loss was 645 mL and the incidence of AKI was 3.1%. A study with 2116 LCVP-assisted hepatectomies reported an estimated mean blood loss of 300 mL (IQR: 200-600 mL), 90-d mortality of 2%, and postoperative AKI of 16% in the whole cohort (13% at risk, 2% at injury and 1% experienced failure)[132]. A study reported a low incidence of AKI requiring renal replacement therapy after liver resection (< 1%), confirming that the routine use of LCVP anaesthesia in combination with intermittent inflow occlusion is safe[21].
Although there are strong evidences that LCVP during partial hepatectomy can minimize blood loss and mortality[19], it is not clear whether it would play a role in AKI prevention, as renal perfusion pressure can be decreased during relative hypovolemia, thus, further studies are required to prove this hypothesis.
In order to reduce the incidence of PLF, a careful preoperative planning and patient selection is mandatory. In the case of underlying cirrhosis, the best candidates for surgical resection are the exclusive Child-Pugh A patients with normal bilirubin values, the absence of clinical signs of portal hypertension (platelet count, splenomegaly and esophageal varices), only tumor diameter < 5 cm (without vascular invasion), asymptomatic and MELD < 8[107,133,134]. Hyperbilirubinemia, portal hypertension and clinical deterioration criteria are considered signs of poor postoperative course, despite the tumor resectability[135].
Analyzing the issue of remnant liver volume after partial hepatectomy, the functional quality of parenchyma should not be ignored. In obtaining the computed tomography images, it enables the calculation of the future liver remnant (FLR), in patients with normal liver function, it must be greater than 25% of the liver total volume, corresponding to 0.5 of the patient weight. In patients with cirrhosis, prolonged exposure to chemotherapy and biliary obstruction, this value is 40%, corresponding to 0.7 of the patient weight[136]. The occlusion of a branch of the portal vein can be performed in order to minimize the occurrence of hepatic insufficiency after major resections. This procedure makes possible the treatment of tumors previously classified as unresectable, providing contralateral liver hypertrophy, thereby increasing the FLR[137,138]. In some situations resectability only occurs when performing two sequential hepatectomies associated with portal ligation for manipulation of the FLR, the two-stage hepatectomy[139].
In the context of liver resections, the risk assessment of postoperative AKI requires the analysis of multiple variables involved in this complex universe, but probably there are main factors which significantly influence these patients for the occurrence of AKI: The massive blood loss during operation with or without an increased renal susceptibility to ischemia, and the occurrence of PLF. Certainly, the key interventions for preventing postoperative AKI after partial hepatectomy would be an appropriate preoperative work up, careful patient selection for surgery and rigorous perioperative control of the patient hemodynamic status by the surgical team.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Brazil
Peer-Review Report Classification
Grade A (Excellent): A, A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: He ST, Peltec A, Qin JM, Sirin G, Wang GY S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 2. | Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 798] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 4. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698-708; discussion 708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 529] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 5. | Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2362] [Article Influence: 118.1] [Reference Citation Analysis (0)] |
| 6. | Kheterpal S, Tremper KK, Englesbe MJ, O’Reilly M, Shanks AM, Fetterman DM, Rosenberg AL, Swartz RD. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 8. | Abelha FJ, Botelho M, Fernandes V, Barros H. Outcome and quality of life of patients with acute kidney injury after major surgery. Nefrologia. 2009;29:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Andersson LG, Ekroth R, Bratteby LE, Hallhagen S, Wesslén O. Acute renal failure after coronary surgery--a study of incidence and risk factors in 2009 consecutive patients. Thorac Cardiovasc Surg. 1993;41:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Bove T, Calabrò MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Landoni G, Bove T, Crivellari M, Poli D, Fochi O, Marchetti C, Romano A, Marino G, Zangrillo A. Acute renal failure after isolated CABG surgery: six years of experience. Minerva Anestesiol. 2007;73:559-565. [PubMed] |
| 12. | Rimola A, Gavaler JS, Schade RR, el-Lankany S, Starzl TE, Van Thiel DH. Effects of renal impairment on liver transplantation. Gastroenterology. 1987;93:148-156. [PubMed] |
| 13. | McCauley J, Van Thiel DH, Starzl TE, Puschett JB. Acute and chronic renal failure in liver transplantation. Nephron. 1990;55:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Ishitani M, Wilkowski M, Stevenson W, Pruett T. Outcome of patients requiring hemodialysis after liver transplantation. Transplant Proc. 1993;25:1762-1763. [PubMed] |
| 15. | Nuño J, Cuervas-Mons V, Vicente E, Turrión V, Pereira F, Mora NP, Barrios C, Millán I, Ardaiz J. Renal failure after liver transplantation: analysis of risk factors in 139 liver transplant recipients. Transplant Proc. 1995;27:2319-2320. [PubMed] |
| 16. | Bilbao I, Charco R, Balsells J, Lazaro JL, Hidalgo E, Llopart L, Murio E, Margarit C. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant. 1998;12:123-129. [PubMed] |
| 17. | Cabezuelo JB, Ramírez P, Ríos A, Acosta F, Torres D, Sansano T, Pons JA, Bru M, Montoya M, Bueno FS. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006;69:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Yalavarthy R, Edelstein CL, Teitelbaum I. Acute renal failure and chronic kidney disease following liver transplantation. Hemodial Int. 2007;11 Suppl 3:S7-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 367] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Saner F. Kidney failure following liver resection. Transplant Proc. 2008;40:1221-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Armstrong T, Welsh FK, Wells J, Chandrakumaran K, John TG, Rees M. The impact of pre-operative serum creatinine on short-term outcomes after liver resection. HPB (Oxford). 2009;11:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg. 2009;250:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 335] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 25. | Moore RD, Smith CR, Lipsky JJ, Mellits ED, Lietman PS. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984;100:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 244] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1162] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 27. | Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4448] [Cited by in RCA: 4698] [Article Influence: 223.7] [Reference Citation Analysis (0)] |
| 28. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4984] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 29. | KDIGO Board Members. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 30. | Noor S, Usmani A. Postoperative renal failure. Clin Geriatr Med. 2008;24:721-729, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 614] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 32. | Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 306] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 33. | Cohnert TU, Rau HG, Buttler E, Hernandez-Richter T, Sauter G, Reuter C, Schildberg FW. Preoperative risk assessment of hepatic resection for malignant disease. World J Surg. 1997;21:396-400; discussion 401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759-766. [PubMed] |
| 35. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 36. | Simons JP, Hill JS, Ng SC, Shah SA, Zhou Z, Whalen GF, Tseng JF. Perioperative mortality for management of hepatic neoplasm: a simple risk score. Ann Surg. 2009;250:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am. 2009;93:855-869, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, Garcia-Tsao G, Lee SS. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Hampel H, Bynum GD, Zamora E, El-Serag HB. Risk factors for the development of renal dysfunction in hospitalized patients with cirrhosis. Am J Gastroenterol. 2001;96:2206-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Péron JM, Bureau C, Gonzalez L, Garcia-Ricard F, de Soyres O, Dupuis E, Alric L, Pourrat J, Vinel JP. Treatment of hepatorenal syndrome as defined by the international ascites club by albumin and furosemide infusion according to the central venous pressure: a prospective pilot study. Am J Gastroenterol. 2005;100:2702-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Terra C, Guevara M, Torre A, Gilabert R, Fernández J, Martín-Llahí M, Baccaro ME, Navasa M, Bru C, Arroyo V. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med. 2005;31:1693-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Wu CC, Yeung LK, Tsai WS, Tseng CF, Chu P, Huang TY, Lin YF, Lu KC. Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol. 2006;65:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Montoliu S, Ballesté B, Planas R, Alvarez MA, Rivera M, Miquel M, Masnou H, Cirera I, Morillas RM, Coll S. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616-622; quiz e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Llach J, Ginès P, Arroyo V, Rimola A, Titó L, Badalamenti S, Jiménez W, Gaya J, Rivera F, Rodés J. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482-487. [PubMed] |
| 47. | Krag A, Bendtsen F, Henriksen JH, Møller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 48. | Lim YS, Larson TS, Benson JT, Kamath PS, Kremers WK, Therneau TM, Kim WR. Serum sodium, renal function, and survival of patients with end-stage liver disease. J Hepatol. 2010;52:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | de Franchis R, Dell’Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis. 2008;40:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford). 2013;15:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 52. | Tisman G, MacDonald D, Shindell N, Reece E, Patel P, Honda N, Nishimora EK, Garris J, Shannahan W, Chisti N. Oxaliplatin toxicity masquerading as recurrent colon cancer. J Clin Oncol. 2004;22:3202-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 53. | Kooby DA, Fong Y, Suriawinata A, Gonen M, Allen PJ, Klimstra DS, DeMatteo RP, D’Angelica M, Blumgart LH, Jarnagin WR. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 54. | Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 763] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 55. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 952] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 56. | Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist. 2007;12:825-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Maroş T, Seres-Sturm L, Lakatos O, Seres-Sturm M, Mody E, Blazsek V. Data regarding the restorative effects of the partial removal of the liver in advanced stages of toxic cirrhosis. Morphol Embryol (Bucur). 1975;21:213-217. [PubMed] |
| 58. | Haney A, Peacock EE, Madden JW. Liver regeneration and hepatic collagen deposition in rats with dimethjylnitrosamine-induced cirrhosis. Ann Surg. 1972;175:863-869. [PubMed] |
| 59. | Golriz M, Majlesara A, El Sakka S, Ashrafi M, Arwin J, Fard N, Raisi H, Edalatpour A, Mehrabi A. Small for Size and Flow (SFSF) syndrome: An alternative description for posthepatectomy liver failure. Clin Res Hepatol Gastroenterol. 2016;40:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Kowalski HJ, Abelmann WH. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953;32:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 398] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (33)] |
| 62. | Ros J, Clària J, To-Figueras J, Planagumà A, Cejudo-Martín P, Fernández-Varo G, Martín-Ruiz R, Arroyo V, Rivera F, Rodés J. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Navasa M, Follo A, Filella X, Jiménez W, Francitorra A, Planas R, Rimola A, Arroyo V, Rodés J. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology. 1998;27:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 295] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Albillos A, Rossi I, Cacho G, Martínez MV, Millán I, Abreu L, Barrios C, Escartín P. Enhanced endothelium-dependent vasodilation in patients with cirrhosis. Am J Physiol. 1995;268:G459-G464. [PubMed] |
| 65. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 66. | Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 173] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 386] [Article Influence: 38.6] [Reference Citation Analysis (1)] |
| 69. | Spencer K. Analytical reviews in clinical biochemistry: the estimation of creatinine. Ann Clin Biochem. 1986;23:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 235] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, Angeli P, Ruiz-Del-Arbol L, Planas R, Solà R. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 352] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 71. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 72. | Angeli P, Fasolato S, Mazza E, Okolicsanyi L, Maresio G, Velo E, Galioto A, Salinas F, D’Aquino M, Sticca A. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 73. | Angeli P, Gatta A, Caregaro L, Menon F, Sacerdoti D, Merkel C, Rondana M, de Toni R, Ruol A. Tubular site of renal sodium retention in ascitic liver cirrhosis evaluated by lithium clearance. Eur J Clin Invest. 1990;20:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Breitenstein S, DeOliveira ML, Raptis DA, Slankamenac K, Kambakamba P, Nerl J, Clavien PA. Novel and simple preoperative score predicting complications after liver resection in noncirrhotic patients. Ann Surg. 2010;252:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Oussoultzoglou E, Jaeck D, Addeo P, Fuchshuber P, Marzano E, Rosso E, Pessaux P, Bachellier P. Prediction of mortality rate after major hepatectomy in patients without cirrhosis. Arch Surg. 2010;145:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Andres A, Toso C, Moldovan B, Schiffer E, Rubbia-Brandt L, Terraz S, Klopfenstein CE, Morel P, Majno P, Mentha G. Complications of elective liver resections in a center with low mortality: a simple score to predict morbidity. Arch Surg. 2011;146:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 511] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 79. | Badr KF, Ichikawa I. Prerenal failure: a deleterious shift from renal compensation to decompensation. N Engl J Med. 1988;319:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Brady HR, Clarkson MR, Lieberthal W. Acute Renal Failure. Brenner and Rector’s The Kidney. Philadelphia: Saunders 2004; 1215-1292. |
| 81. | Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 82. | Abuelo JG. Diagnosing vascular causes of renal failure. Ann Intern Med. 1995;123:601-614. [PubMed] |
| 83. | Taal MW, Luyckx VA, Brenner BM. Adaptation to nephron loss. Brenner And Rector’s The Kidney. Philadelphia: Saunders 2004; 1955-1997. |
| 84. | Schlondorff D. Renal complications of nonsteroidal anti-inflammatory drugs. Kidney Int. 1993;44:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 180] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Perazella MA, Eras J. Are selective COX-2 inhibitors nephrotoxic? Am J Kidney Dis. 2000;35:937-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Braden GL, O’Shea MH, Mulhern JG, Germain MJ. Acute renal failure and hyperkalaemia associated with cyclooxygenase-2 inhibitors. Nephrol Dial Transplant. 2004;19:1149-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Klein IH, Abrahams A, van Ede T, Hené RJ, Koomans HA, Ligtenberg G. Different effects of tacrolimus and cyclosporine on renal hemodynamics and blood pressure in healthy subjects. Transplantation. 2002;73:732-736. [PubMed] |
| 88. | Oudemans-van Straaten HM. Contrast nephropathy, pathophysiology and prevention. Int J Artif Organs. 2004;27:1054-1065. [PubMed] |
| 89. | Schor N. Acute renal failure and the sepsis syndrome. Kidney Int. 2002;61:764-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Lee HY, Kim CH. Acute oliguric renal failure associated with angiotensin II receptor antagonists. Am J Med. 2001;111:162-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 91. | Johansen TL, Kjaer A. Reversible renal impairment induced by treatment with the angiotensin II receptor antagonist candesartan in a patient with bilateral renal artery stenosis. BMC Nephrol. 2001;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Toto RD. Renal insufficiency due to angiotensin-converting enzyme inhibitors. Miner Electrolyte Metab. 1994;20:193-200. [PubMed] |
| 93. | Tinmouth A, Fergusson D, Yee IC, Hébert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 94. | Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, Crowther MA, Hozhabri S, Beattie WS. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology. 2012;116:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | van de Watering L. Red cell storage and prognosis. Vox Sang. 2011;100:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 96. | Almac E, Ince C. The impact of storage on red cell function in blood transfusion. Best Pract Res Clin Anaesthesiol. 2007;21:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 97. | Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radic Biol Med. 2002;32:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 98. | Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063-17068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 487] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 99. | Lo CM, Fan ST, Liu CL, Chan JK, Lam BK, Lau GK, Wei WI, Wong J. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112-1116. [PubMed] |
| 100. | Nishizaki T, Ikegami T, Hiroshige S, Hashimoto K, Uchiyama H, Yoshizumi T, Kishikawa K, Shimada M, Sugimachi K. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575-580. [PubMed] |
| 101. | Wang F, Pan KT, Chu SY, Chan KM, Chou HS, Wu TJ, Lee WC. Preoperative estimation of the liver graft weight in adult right lobe living donor liver transplantation using maximal portal vein diameters. Liver Transpl. 2011;17:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 103. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 821] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 104. | Fong Y, Bentrem DJ. CASH (Chemotherapy-Associated Steatohepatitis) costs. Ann Surg. 2006;243:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 105. | Seifalian AM, Piasecki C, Agarwal A, Davidson BR. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68:780-784. [PubMed] |
| 106. | Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, Furukawa K, Tanaka T, Tomoda F, Tanaka W. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology. 1993;18:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 621] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 108. | McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 109. | Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 110. | Bilchik AJ, Poston G, Curley SA, Strasberg S, Saltz L, Adam R, Nordlinger B, Rougier P, Rosen LS. Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol. 2005;23:9073-9078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Aloia T, Sebagh M, Plasse M, Karam V, Lévi F, Giacchetti S, Azoulay D, Bismuth H, Castaing D, Adam R. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983-4990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 392] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 112. | Morris-Stiff G, Tan YM, Vauthey JN. Hepatic complications following preoperative chemotherapy with oxaliplatin or irinotecan for hepatic colorectal metastases. Eur J Surg Oncol. 2008;34:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 113. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1723] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 114. | Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 115. | Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am. 2004;84:563-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Smyrniotis V, Farantos C, Kostopanagiotou G, Arkadopoulos N. Vascular control during hepatectomy: review of methods and results. World J Surg. 2005;29:1384-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 117. | Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541-549. [PubMed] |
| 118. | Li AK, Mok SD. Simplified hepatectomy: the tourniquet method. Aust N Z J Surg. 1989;59:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Huguet C, Gavelli A, Chieco PA, Bona S, Harb J, Joseph JM, Jobard J, Gramaglia M, Lasserre M. Liver ischemia for hepatic resection: where is the limit? Surgery. 1992;111:251-259. [PubMed] |
| 120. | Hannoun L, Borie D, Delva E, Jones D, Vaillant JC, Nordlinger B, Parc R. Liver resection with normothermic ischaemia exceeding 1 h. Br J Surg. 1993;80:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Midorikawa Y, Kubota K, Takayama T, Toyoda H, Ijichi M, Torzilli G, Mori M, Makuuchi M. A comparative study of postoperative complications after hepatectomy in patients with and without chronic liver disease. Surgery. 1999;126:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 122. | Smyrniotis VE, Kostopanagiotou GG, Contis JC, Farantos CI, Voros DC, Kannas DC, Koskinas JS. Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study. World J Surg. 2003;27:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [PubMed] |
| 124. | Choukèr A, Schachtner T, Schauer R, Dugas M, Löhe F, Martignoni A, Pollwein B, Niklas M, Rau HG, Jauch KW. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 125. | Delva E, Nordlinger B, Parc R, Lienhart A, Hannoun L, Huguet C. Hepatic vascular exclusion (HVE) for major liver resections. Int Surg. 1987;72:78-81. [PubMed] |
| 126. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. [PubMed] |
| 127. | Hannoun L, Balladur P, Delva E, Panis Y, Camus Y, Honiger J, Levy E, Parc R. [“Ex situ-in vivo” surgery of the liver: a new technique in liver surgery. Principles and preliminary results]. Gastroenterol Clin Biol. 1991;15:758-761. [PubMed] |
| 128. | Delrivière L, Hannoun L. In situ and ex situ in vivo procedures for complex major liver resections requiring prolonged hepatic vascular exclusion in normal and diseased livers. J Am Coll Surg. 1995;181:272-276. [PubMed] |
| 129. | Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 121] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 130. | Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 131. | Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 169] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 132. | Correa-Gallego C, Berman A, Denis SC, Langdon-Embry L, O’Connor D, Arslan-Carlon V, Kingham TP, D’Angelica MI, Allen PJ, Fong Y. Renal function after low central venous pressure-assisted liver resection: assessment of 2116 cases. HPB (Oxford). 2015;17:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 134. | Bellavance EC, Lumpkins KM, Mentha G, Marques HP, Capussotti L, Pulitano C, Majno P, Mira P, Rubbia-Brandt L, Ferrero A. Surgical management of early-stage hepatocellular carcinoma: resection or transplantation? J Gastrointest Surg. 2008;12:1699-1708. [PubMed] |
| 135. | Emond JC, Samstein B, Renz JF. A critical evaluation of hepatic resection in cirrhosis: optimizing patient selection and outcomes. World J Surg. 2005;29:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Liau KH, Blumgart LH, DeMatteo RP. Segment-oriented approach to liver resection. Surg Clin North Am. 2004;84:543-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Malinowski M, Geisel D, Stary V, Denecke T, Seehofer D, Jara M, Baron A, Pratschke J, Gebauer B, Stockmann M. Portal vein embolization with plug/coils improves hepatectomy outcome. J Surg Res. 2015;194:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 138. | Broering DC, Hillert C, Krupski G, Fischer L, Mueller L, Achilles EG, Schulte am Esch J, Rogiers X. Portal vein embolization vs. portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg. 2000;6:905-913; discussion 913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |