Published online Jun 8, 2016. doi: 10.4254/wjh.v8.i16.685
Peer-review started: March 2, 2016
First decision: March 22, 2016
Revised: April 7, 2016
Accepted: May 10, 2016
Article in press: May 11, 2016
Published online: June 8, 2016
Processing time: 92 Days and 9 Hours
AIM: To elucidate causes for false negative magnetic resonance imaging (MRI) exams by identifying imaging characteristics that predict viable hepatocellular carcinoma (HCC) in lesions previously treated with locoregional therapy when obvious findings of recurrence are absent.
METHODS: This retrospective institutional review board-approved and Health Insurance Portability and Accountability Act-compliant study included patients who underwent liver transplantation at our center between 1/1/2000 and 12/31/2012 after being treated for HCC with locoregional therapy. All selected patients had a contrast-enhanced MRI after locoregional therapy within 90 d of transplant that was prospectively interpreted as without evidence of residual or recurrent tumor. Retrospectively, 2 radiologists, blinded to clinical and pathological data, independently reviewed the pre-transplant MRIs for 7 imaging features. Liver explant histopathology provided the reference standard, with clinically significant tumor defined as viable tumor ≥ 1.0 cm in maximum dimension. Fisher’s exact test was first performed to identify significant imaging features.
RESULTS: Inclusion criteria selected for 42 patients with 65 treated lesions. Fourteen of 42 patients (33%) and 16 of 65 treated lesions (25%) had clinically significant viable tumor on explant histology. None of the 7 imaging findings examined could reliably and reproducibly determine which treated lesion had viable tumor when the exam had been prospectively read as without evidence of viable HCC.
CONCLUSION: After locoregional therapy some treated lesions that do not demonstrate any MRI evidence of HCC will contain viable tumor. As such even patients with a negative MRI following treatment should receive regular short-term imaging surveillance because some have occult viable tumor. The possibility of occult tumor should be a consideration when contemplating any action which might delay liver transplant.
Core tip: Hepatocellular carcinoma (HCC) is often treated with locoregional therapy such as transarterial chemoembolization as a bridge to transplantation. Detecting residual or recurrent tumor within these treated lesions is challenging and some treated lesions that do not demonstrate any magnetic resonance imaging (MRI) evidence of HCC will contain foci of viable tumor. Regular, short-term imaging surveillance is clinically important for patients being considered for liver transplantation even when prior MRIs have been negative and the possibility of a false negative MRI exam needs to be considered when managing these patients.
- Citation: Becker-Weidman D, Civan JM, Deshmukh SP, Roth CG, Herrine SK, Parker L, Mitchell DG. Hepatocellular carcinoma after locoregional therapy: Magnetic resonance imaging findings in falsely negative exams. World J Hepatol 2016; 8(16): 685-690
- URL: https://www.wjgnet.com/1948-5182/full/v8/i16/685.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i16.685
The American College of Radiology developed the liver imaging reporting and data system (LI-RADS) to standardize how hepatocellular carcinoma (HCC) is diagnosed[1]. These criteria were validated in untreated lesions and therefore do not apply to lesions after treatment with locoregional therapy. Although certain imaging findings are associated with the presence of viable HCC in a treated lesion there is currently no formal system to assess the probability of viable tumor.
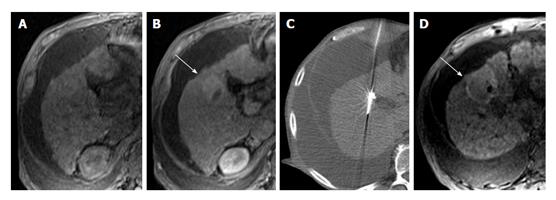
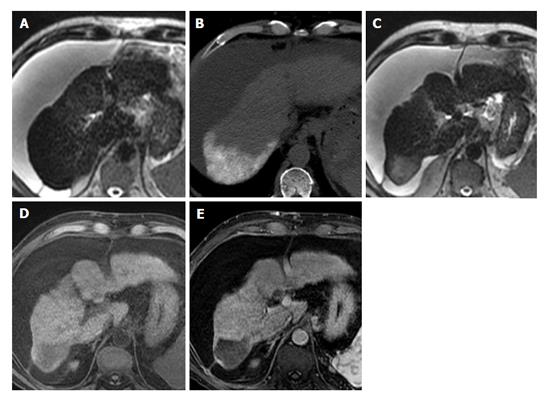
Magnetic resonance imaging (MRI) is commonly used status post locoregional therapy to evaluate for recurrent or residual viable tumor. Because the hallmark of HCC is avid arterial-phase enhancement, dynamic imaging following gadolinium-based contrast administration should be a core component of the examination. Arterial-phase enhancement following locoregional therapy has a reported sensitivity and specificity of 82% to 100% and 79% to > 90% respectively[2,3]. Subtle arterial-phase enhancement can be obscured in treated lesions as they often demonstrate heterogeneous high signal on T1-weighted images due to the presence of blood products (Figure 1). HCC is a very cellular tumor[4] and will typically restrict the diffusion of water molecules giving it high signal on diffusion-weighted imaging (DWI) and corresponding low signal on the computer generated apparent diffusion coefficient map. Diffusion restriction following locoregional therapy has a reported sensitivity and specificity of 61% to 75% and 88% to > 90% respectively[2,3]. Identifying restricted diffusion in treated areas is complicated by the fact that these areas typically demonstrate high signal on T2-weighted images due to fibrosis (Figure 2), appearing as T2 shine through on DWI. Signal intensity on T2-weighted images is not typically helpful as it is affected by treatment and can be variable, although it is typically mildly to moderately hyperintense. Signal intensity on precontrast T1-weighted images is quite variable and generally not helpful. HCC is usually hypointense or isointense but can be hyperintense with intratumoral fat.
Unresectable HCC is often treated with locoregional therapy to decrease disease burden and as a bridge to transplant. In these patients accurate assessment of tumor response is integral to directing patient care. False negative MRI exams are due to a number of factors including technical limitations and inherent MR signal alteration of the treated areas. In addition there is no formal system for evaluating treated lesions. The goal of this study was to retrospectively determine which MRI features were most predictive of histological findings of residual or recurrent HCC in a population that does not demonstrate obvious recurrence.
This retrospective study was approved by our institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Our study included patients with HCC who underwent liver transplantation at our center between 1/1/2000 and 12/31/2012 after being treated with locoregional therapy. Inclusion criteria selected patients that had a contrast-enhanced MRI after locoregional therapy within 90 d of transplant that was prospectively interpreted as without evidence of residual or recurrent tumor. Patients were identified through our electronic medical record.
While HCC is a radiologic diagnosis, subcentimeter lesions cannot be designated as “definite” HCC by either the American Association for the Study of Liver Diseases or the LI-RADS criteria in recognition of the fact that early tumors may not demonstrate hypervascularity and technical limitations preclude adequate assessment of lesions below this threshold[1,5]. Therefore, we considered foci of HCC identified on explant significant for the purpose of our study only if it measured ≥ 1.0 cm in maximal diameter.
Foci of viable tumor detected histologically on explant, distinct from a previously treated lesion were not included in our analysis. These foci were treated as incidental findings as our current analysis regards the MRI findings in lesions previously identified as HCC subsequently undergoing treatment.
All MRI data sets were reviewed on a workstation equipped with image review software (iSite, version 3.6; Philips, Andover, MA). Retrospective image interpretations were performed independently by two body MRI specialists, each with more than 10 years of experience. The study coordinator, a radiology resident, prepared the exams for review by correlating the lesions described in the explant pathology report with the treated lesions on the MRI, marking the lesions to be evaluated with an arrow. Exams were reviewed in random order by the interpreting radiologists, who were blinded to all other patient history, including pathology and other imaging results.
Each liver lesion was assessed by the interpreting radiologist for the presence or absence of: Arterial-phase nodular enhancement, arterial-phase non-nodular enhancement, gradual enhancement, partial or complete T1 signal hypointensity, partial or complete T2 signal hyperintensity, lipid as determined by comparison of in-phase and opposed-phase images, and restricted diffusion if DWI was performed. Findings were recorded in prepared data sheets.
All explanted livers were received as surgical resection specimens. Each explant was serially sectioned in contiguous slices at 5 mm intervals, and processed for routine Hematoxylin and Eosin stains. These slides were prospectively reviewed for the presence of viable HCC and the pathology report produced was used to retrospectively correlate the histologic findings with the pretransplant MRI.
Statistical review of the study was performed by a biomedical statistician. Statistical software (SAS version 9.4; SAS Institute, Cary, NC) was used for all data analysis. Fisher’s exact test was first performed to identify significant imaging features in a bivariate analysis followed by a step-wise logistic regression if more than one variable was significant. The significance threshold was set at a P-value of 0.05 and any variable with P > 0.05 was removed from the model and determined to be insignificant. The agreement level between readers was measured by using k coefficient. We defined k values for level of agreement as follows: 0.81-0.99, almost perfect agreement; 0.61-0.80, substantial agreement; 0.41-0.60, moderate agreement; 0.21-0.40, fair agreement; and 0.01-0.20, slight agreement[6].
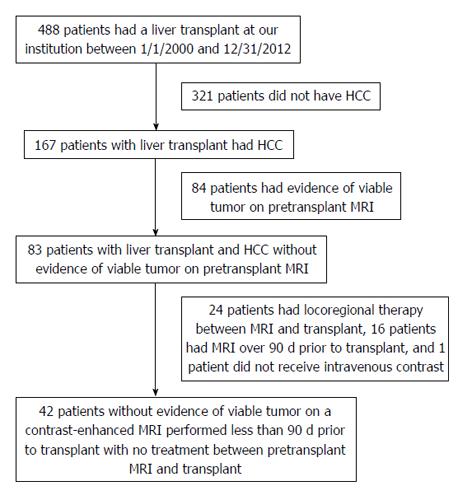
A search of our electronic medical record showed that 488 patients had a liver transplant at our center between 1/1/2000 and 12/31/2012, of which 167 (34.2%) had HCC, all of whom were treated with locoregional therapy prior to transplant. Of these patients, 84 (50.3%) had findings suspicious for recurrent or residual HCC on the pre-transplant MRI, 24 (14.4%) underwent locoregional treatment between the pre-transplant MRI and transplant, 16 (9.6%) had the pre-transplant MRI over 90 d before transplant, and 1 (0.6%) did not receive intravenous contrast and were excluded from our study. Patient accrual details are presented in Figure 3. The final cohort of 42 patients (mean age, 59 years; age range, 46-73 years) included 34 men (mean age, 59 years; age range, 46-73 years) and 8 women (mean age, 59 years; age range, 53-70 years). Patients had cirrhosis secondary to hepatitis C (n = 29), hepatitis C and alcohol abuse (n = 5), alcohol abuse (n = 4), nonalcoholic steatohepatitis (n = 1), or an unknown cause (n = 3). MRI was performed an average of 40 d before transplant (range, 1-89 d).
Prior to transplant 33 (79%) patients were treated with transarterial chemoembolization (TACE) only, 3 (7%) were treated with radiofrequency ablation only, 2 (5%) were treated with radioactive embolization only, 1 (2%) was treated with bland transarterial embolization only, and 3 (7%) were treated with TACE and radiofrequency ablation.
The 42 patients who met our inclusion criteria included 18 (43%) who had no viable tumor, 10 (24%) who had viable tumor that was considered clinically insignificant, and 14 (33%) who had at least one focus of clinically-significant viable tumor on explant pathology. Two patients had two foci of clinically-significant viable tumor. The explant Pathology report mentioned a single lesion in 27 patients (64%), 2 lesions in 9 patients (21%), 3 lesions in 3 patients (7%), 4 lesions in 2 patients (5%), and 5 lesions in 1 patient (3%) for a total of 65 treated lesions. Sixteen treated lesions (25%) had clinically significant viable tumor (mean size, 1.5 cm; range, 1.0-3.5 cm), 13 treated lesions (20%) had a focus of tumor < 1.0 cm (mean size, 0.5 cm; range, 0.1-0.9 cm), and 36 (55%) treated lesions had no viable tumor.
Of the 42 patients, 15 received gadoextate disodium (Eovist) (36%), 13 received gadopentate dimeglumine (Magnevist) (31%), 8 received gadobutrol (Gadavist) (19%), and 6 received gadobenate dimeglumine (MultiHance) (14%). DWI was only performed in 19 patients (45%) as DWI was not included as a part of our routine MRI exam of the abdomen until 2011.
For reader #1 “arterial-phase non-nodular enhancement” and “partial or complete T1 signal hypointensity” were significant predictors of viable tumor. For reader #2 “partial or complete T2 signal hyperintensity” was the sole significant predictor of viable tumor.
There was fair agreement for arterial-phase nodular enhancement and non-nodular enhancement (k = 0.37, 0.23 respectively); slight agreement for gradual enhancement, partial or complete T1 signal hypointensity, and partial or complete T2 signal hyperintensity (k = 0.07, 0.10, 0.15 respectively); and no agreement for the presence of lipid (k = -0.03). The P-values and kappa values are presented in Table 1.
| P-values | Kappa values | ||
| Reader #1 | Reader #2 | ||
| Arterial-phase nodular enhancement | 0.44 | 0.14 | 0.37 (fair agreement) |
| Arterial-phase non-nodular enhancement | 0.25 | 0.008b | 0.23 (fair agreement) |
| Gradual enhancement | 0.47 | 0.15 | 0.10 (slight agreement) |
| Partial or complete T1 signal hypointensity | 0.21 | 0.001b | 0.15 (slight agreement) |
| Partial or complete T2 signal hyperintensity | 0.047a | 0.47 | 0.07 (slight agreement) |
| Lipid | 0.56 | 0.44 | -0.03 (no agreement) |
| Restricted diffusion | N/A1 | N/A1 | N/A1 |
There was a single post-transplant recurrence in the 18 patients without viable tumor (mean length of followup, 4.9 years; range, 1.1-9.0 years). There was a single recurrence in the 10 patients with clinically insignificant cancer (mean length of followup, 5.2 years; range, 2.6-13.4 years). There was a single recurrence in the 14 patients with clinically significant viable tumor (mean length of followup, 3.4 years; range, 0.2-7.1 years). One patient with only 0.2 years of followup died from a stroke.
Retrospective review of true negative and false negative MR exams did not identify any subtle findings that can reliably and reproducibly indicate the presence of viable HCC in studies that were prospectively interpreted as negative. T1 and T2 signal intensity are highly variable after locoregional therapy and are not reliable indicators of viable tumor. Delayed enhancement is often seen after treatment and indicates fibrosis. Arterial-phase enhancement is associated with viable tumor but may be subtle or absent and has a reported sensitivity of as low as 82%[2]. In other words, some patients who do not have evidence of HCC on MRI will have viable tumor on explant pathology.
A limitation of this study was the low level of agreement between readers. This can be partially explained due to the low number of “positive” imaging features. When there is a low base rate a small number of discordant findings will have a disproportionately large effect on Cohen’s kappa coefficient[7]. Agreement regarding enhancement characteristics is only slight to fair because any case that demonstrated obvious enhancement was prospectively read as suspicious for viable HCC and excluded from our study. The only cases that remained were those that demonstrated subtle enhancement. Agreement for signal intensity on T1- and T2-weighted images is only slight due to the inherent difficulty in classifying a highly heterogeneous area. That being said the low kappa value limits the value and reliability of any imaging finding that was positively associated with viable HCC. Therefore, we do not propose that signal intensity on unenhanced T1-weighted or T2-weighted images is predictive of viable tumor. It is possible that non-nodular arterial-phase enhancement is predictive of viable tumor but this finding was not reliable enough in our study for clinical use.
Another limitation was the inconsistency of the explant pathology reports. Some pathology reports measured the size of the viable component or state the percentage of necrosis within the measured treated lesion, whereas, other reports used subjective terminology such as “partially necrotic” or “largely necrotic” which made exact measurement of the viable component difficult. Another problem was that some of the treated lesions demonstrated partial diffuse necrosis and contained only microscopic islands of tumor. These lesions are considered viable by histology but impossible to identify by imaging.
Noting the limitations above it is clear that occasionally treated lesions without evidence of viable HCC by MRI can contain foci of viable tumor. This supports the utilization of regular short-term imaging surveillance even when prior MRIs have been negative and is clinically important for patients being considered for liver transplantation. For example, a decision to delay transplant to allow treatment of underlying chronic viral hepatitis C should be made with caution, without over-reliance on a sense of security suggested by surveillance imaging with no definite evidence of viable HCC. The possibility of a false negative MRI exam needs to be considered when managing patients after locoregional therapy.
Unresectable hepatocellular carcinoma (HCC) is often treated with locoregional therapy to decrease disease burden and as a bridge to transplant. After locoregional therapy magnetic resonance imaging (MRI) interpretation can be more difficult due to a number of factors. Several imaging findings have been shown to correlate with the presence of viable HCC in this setting including diffusion restriction and arterial-phase enhancement.
Liver imaging reporting and data system (LI-RADS) was not developed to be applied to treated lesions and as such theses lesions are designated “LR-treated”. Further investigation into the imaging characteristics of treated lesions could lead to the development of a version of LI-RADS that can be applied to these lesions.
In this study, the authors demonstrate that treated lesions can harbor foci of viable HCC but demonstrate no MRI findings.
The research supports the utilization of regular short-term imaging surveillance in patients with HCC treated with locoregional therapy even when prior MRIs have been negative due to the possibility of a false negative exam.
Diffusion-weighted imaging: MRI sequence that measures random Brownian motion of water molecules within a voxel of tissue; LI-RADS: Set of terminology developed by the American College of Radiology to standardize the reporting of imaging findings of liver lesions; Locoregional therapy: Transarterial and/or local ablative therapy.
This is an interesting manuscript providing information for an easily neglected field. It is thus of value to be considered for publication.
P- Reviewer: Kayaalp C, Zhang Q S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 2. | Goshima S, Kanematsu M, Kondo H, Yokoyama R, Tsuge Y, Shiratori Y, Onozuka M, Moriyama N. Evaluating local hepatocellular carcinoma recurrence post-transcatheter arterial chemoembolization: is diffusion-weighted MRI reliable as an indicator? J Magn Reson Imaging. 2008;27:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Mannelli L, Kim S, Hajdu CH, Babb JS, Clark TW, Taouli B. Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol. 2009;193:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 446] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |