Published online May 28, 2015. doi: 10.4254/wjh.v7.i9.1287
Peer-review started: September 20, 2014
First decision: November 14, 2014
Revised: February 27, 2015
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: May 28, 2015
Processing time: 246 Days and 21.3 Hours
AIM: To characterize management of telaprevir (TVR)-based triple therapy of hepatitis C virus (HCV) reinfection after liver transplantation (LT).
METHODS: We retrospectively analyzed safety and efficacy of telaprevir - based triple therapy in a single center cohort of 19 patients with HCV genotype (GT) 1 recurrence after LT, with respect to factors possibly predicting sustained viral response (SVR) or non-SVR. All patients were treated with TVR, pegylated (PEG) and ribavirine (RBV) for 12 wk followed by a dual phase with PEG/RBV for 12 wk in 7 patients and for 36 wk in 5 patients.
RESULTS: In total 11/19 (58%) of patients achieved a sustained response. All (11/11) SVR patients showed a rapid viral response at treatment weeks 4 and 11/14 rapid virological response (RVR) patients achieved SVR. Notably, all (7/7) patients who completed 48 wk of therapy and 80% (4/5) patients who completed 24 wk of therapy achieved SVR24. Treatment failure was significantly (P > 0.049) more frequent in GT1a infection (5/7) compared to GT1b (3/12) infection and was associated with emergence of resistance-associated mutations in the NS3 protease domain. Bilirubin level at baseline is also related to SVR (P > 0.030). None of the patients had to discontinue treatment due to side effects.
CONCLUSION: RVR, GT and bilirubin are clearly related to achievement of SVR. Providing a thorough patient selection and monitoring, a full course of TVR-based triple therapy in LT patients is feasible and achieves high SVR rates.
Core tip: Experiences with telaprevir-based triple therapy in 19 patients with hepatitis C virus recurrence after liver transplantation are analysed and described in detail. We observed a exceptionally high sustained viral response rate and analyzed clinicopathological factors which might contribute to predict which patients rather benefit from this therapy and which do not. While the new generation directly acting antivirals start to be available in some countries, many parts of the world will not have the privilege of these therapeutic options for a long time. Therefore we are eager to share our experiences with telaprevir in liver transplantation patients with the international hepatologist community.
- Citation: Herzer K, Papadopoulos-Köhn A, Achterfeld A, Canbay A, Piras-Straub K, Paul A, Walker A, Timm J, Gerken G. Management of telaprevir-based triple therapy for hepatitis C virus recurrence post liver transplant. World J Hepatol 2015; 7(9): 1287-1296
- URL: https://www.wjgnet.com/1948-5182/full/v7/i9/1287.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i9.1287
About 160 million people worldwide are currently affected by a chronic hepatitis C virus (HCV) infection with the deleterious consequences of decompensated cirrhosis and hepatocellular carcinoma (HCC)[1,2]. In western countries, HCV-induced liver cirrhosis and HCC can be therapeutically addressed by liver transplantation (LT). However, reinfection of the liver graft occurs in virtually all patients typically followed by an accelerated course of progressive liver damage. About 3 to 5 years post-LT, 30% of HCV-positive patients develop cirrhosis of the graft with a consecutively unfavorable prognosis[3]. Over the past decade, treatment of HCV-reinfection with pegylated interferon (PEG-IFN) and ribavirin (RBV) was the only treatment option associated with moderate sustained virological response (SVR) rates of only 8%-50% depending on the genotype (GT), defining patients who received liver transplant as a “difficult-to treat” group[4]. In 2011, the first generation of directly acting antivirals (DAAs), the protease inhibitors (PI) telaprevir (TVR) and boceprevir (BOC), were approved for treatment of chronic HCV infection, in combination with PEG-IFN and RBV[5]. The TVR-based triple regimen was reported to achieve SVR rates of up to 75% in treatment naïve GT1 non-LT patients compared to 44% with the previous dual therapy[6]. Unfortunately, in the post-LT setting the triple regimen comprised unforeseen challenges. Severe drug-drug-interactions of the immunosuppressive (IS) agents and the PI which may result in increased toxicity or/ and loss of efficacy of both drugs, potentially resulting in severe allograft rejections[7,8]. Nevertheless, reports from different transplant centers supported the principal feasibility of combining immunosuppressive agents and PIs in the post-LT setting[9,10]. SVR rates of 20%-50% were reported in hard to treat (mostly intensively pretreated) cohorts of patients[11]. In the meantime, new DAA are approved[12]. Thus, a therapeutic hold is observed in the prospect of new treatment options promising no drug-drug interactions and less severe side effects, most importantly because interferon may become dispensable[13]. However, when and whether at all the first generation PIs will be replaced by novel DAAs also depends on economic aspects in a number of countries and potentially the emergence of resistances[14,15].
The aim of our retrospective analysis was to summarize and communicate the rather good experience of our center with treatment course and outcome of a large single center cohort of patients with HCV GT1 reinfection after LT with TVR-based triple therapy. We intend to give a thorough description of our observations and estimations, in order to share our experiences with the community, in particular in those parts of the world were the second generation DAAs are not yet available.
Between April 2012 and January 2013, 19 patients with HCV GT1 recurrence after liver transplantation were selected for treatment with TVR-based triple therapy. For demographic parameters and baseline clinical chemistry (Table 1).
| Demography | ||
| Age, yr | 57 | 41-70 |
| Gender (male/female) | 16/3 | |
| Body mass index (kg/m2) | 26 | 21-33 |
| Race, n/% | 19 | 100 |
| Caucasian, n/% | 18 | 94.74 |
| Hispanic, n/% | 1 | 5.26 |
| Immunosuppressive regimen | ||
| TAC/CSA | 17/2 | |
| MMF (n) | 8 | |
| Steroids (n) | 0 | |
| Baseline clinical parameters | ||
| Duration of therapy | 24 | 4-48 |
| Ishak fibrosis score (grade) | 2 | 0-4 |
| Inflammation (grade) | 1.5 | 0-2 |
| Fibroscan baseline (kPa) | 13.8 | 4.5-46.4 |
| Time from LT to triple therapy (mo) | 22 | 7-295 |
| Baseline clinical chemistry | ||
| Bilirubin total (mg/dL) | 0.8 | 0.3-3.2 |
| GGT (U/L) | 52 | 13-32 |
| GPT (ALAT), U/L | 41 | 21-159 |
| GOT (ASAT), U/L | 52 | 18-88 |
| AP, U/L | 101 | 53-404 |
| Creatinine (mg/dL) | 1.26 | 0.67-1.89 |
| GFR (MDRD) | 57.3 | 23-133 |
| International normalized ratio | 1 | 0.8-1.2 |
| Hämoglobin (g/dL) | 13.5 | 9-16.8 |
| WBC (/μL) | 3.73 | 1.6-8.3 |
| Platelet count (/μL) | 111 | 62-246 |
| Basline viral characteristics | ||
| HCV GT | ||
| 1a, n/% | 7 | 36.8 |
| 1b, n/% | 12 | 63.2 |
| VL (log10 IU/mL) | 1.95 | 0.13-14.9 |
| Recipient IL-28b polymorphism, n/% | ||
| CC | 5 | 26.3 |
| CT | 9 | 47.4 |
| TT | 5 | 26.3 |
| History of any prior PEG-INF/RBV treatment, n/% | 11 | 57.89 |
| History of post-LT PEG-INF/RBV treatment, n/% | 5 | 26.3 |
| HCC prior to LT (n) | 6 | |
| HBV coinfection (n) | 0 |
Patients were considered eligible for TVR-based triple therapy upon clinical and histological evidence of recurrence of HCV GT1 infection. Before PEG-IFN was administered, all patients underwent an allograft biopsy for evaluation of fibrosis stage according to the METAVIR system and for exclusion of graft rejection. Exclusion criteria for antiviral treatment were evidence of biopsy-proven acute rejection during the past 3 mo or any medical contraindication to treatment with PEG-IFN and RBV that would predict the occurrence of complications during IFN administration, such as platelet count lower than 100000/μL or white blood cell count lower than 2000/μL; clinical signs or laboratory values indicating decompensating liver function; renal insufficiency, with a glomerular filtration rate (GFR) lower than 60 mL/min; and anemia, with a hemoglobin level lower than 10 mg/dL at baseline. Whenever possible, treatment with mycophenolate mofetil and corticosteroids was discontinued before the initiation of antiviral treatment[16].
As limited experience exists in the post-LT setting, patients were thoroughly informed of possible interactions and side effects prior to treatment. Serological and clinical data were collected from the patients’ files and retrospectively analyzed. The analysis was conducted in accordance with the Helsinki Declaration of 1975 and approved by the ethics committee of the University Hospital Essen.
Patients were treated with TVR, PEG-IFN and RBV for 12 wk followed by 12 or 36 wk of dual therapy with PEG-IFN/RBV. The RBV dose was administered with 600 mg/d at baseline and only reduced in two cases of acute renal failure. PEG-IFN was initiated with 180 μg/d and reduced in 3 cases due to cytopenia and overall tolerance. TVR was preferred to BOC due to a shorter triple regimen and administered as 1175 mg twice per day. The stopping rule applied was failure to achieve a reduction in HCV viral load (VL) to less than 1000 IU/mL at treatment weeks (TW) 12, a detectable viral load at TW 24 or viral breakthrough (VB) with discontinuation of all antiviral treatment.
For the assessment of efficacy, viral load was monitored in plasma using the ABBOTT Real Time HCV assay (Abbott Molecular, United States; lower limit of detection 12 IU/mL) at baseline and then at week 1, 2, 3, 4, 8, 12, 16, 20, 24, 36 and 48. Genotypes were determined using phylogenetic analyses of the core region. A rapid virological response (RVR) was defined as an undetectable VL at TW 4 of triple therapy. At TW 12, an undetectable viral load was defined as early viral response (EVR). An end of therapy treatment response (EOT) was obtained when the VL remained negative at the time of treatment discontinuation. A SVR24 was defined as negative VL 24 wk after the end of treatment. VB was defined as achieving an undetectable viral load but the subsequent occurrence of a detectable VL over time. In all patients, the whole NS3 region was analyzed by sequencing, and PI resistance mutations were recorded.
The NS3 protease domain was sequenced before therapy and in patients experiencing treatment failure in the first available viremic sample. HCV-RNA was extracted utilizing spin columns and reverse transcribed followed by subsequent amplification of cDNA by nested polymerase chain reaction (PCR). Detailed information on primer sequences and PCR protocols are available upon request. The PCR product was directly sequenced by Sanger technology and obtained sequences were analyzed utilizing the resistance prediction algorithm as implemented in Geno2pheno (HCV) 0.92 (http://hcv.bioinf.mpi-inf.mpg.de).
Immunosuppressive regimen was left unchanged with tacrolimus (TAC) in 17 and cyclosporine A (CyA) in 2 patients. Mycophenolate mofetil (MMF) as comedication in 8 patients was skipped for the period of therapy in order to avoid myelosuppressive effects. Before starting triple therapy, patients were on a stable dose of TAC or CyA with stable therapeutic levels. After initiating TVR, TAC dosage was skipped until start of decline of therapeutic level and then administered as 0.1 mg with once or twice daily dosing as described previously[10]. Trough levels of TAC were checked daily for the first 10 d and twice a week for the next two wk and then once a week during the remaining duration of TVR. Once TVR was stopped, TAC was reinstituted with a goal to achieve pre-TVR doses gradually over a period of 5 d with daily trough level checks. The dose of CyA was reduced by 50% upon start of TVR with control of trough levels as described for TAC. The day after stopping TVR, CyA was reinstituted at the dose before TVR-therapy with controls according to TAC. Trough blood concentrations (TBC) ranged from 5 to 7 ng/mL for TAC and from 50 to 80 ng/mL for CyA[16].
Creatinine clearance was estimated using the MDRD formula. Upon decrease of renal function parameters, renal function was immediately stabilized by intensive daily intravenous fluid application and patients were admonished to increase fluid intake from baseline. Erythropoietin (Epoentin®; Hexal) was administered to support the red blood cell count when hemoglobin (HB) levels dropped to below 10 g/dL. Granulocyte colony stimulating factor (G-CSF) (Neupogen®; Amgen Europe BV) was administered to support the neutrophil count when it fell below 1000/μL despite PEG-IFN dose reduction.
Safety and efficacy data were gathered in short intervals during time of treatment. The modalities of treatment, on-treatment surveillance and follow-up were previously described[10].
Continuous variables were expressed as medians, means and ranges. The Wilcoxon signed-rank-test was used to compare paired groups. A P-value of < 0.05 was considered to be significant. Numeric liver values of small groups (n≤ 30) were compared by Mann-Whitney-U-Test. Categorical variables were analysed by χ2-test with pearson approximation. Statistical analysis were performed using SPSS 19 statistical software (IBM SPSS; Chicago, IL).
Patients with stable blood count, liver and renal function as central inclusion criteria were thoroughly selected for therapy. The median time between LT and treatment was 22 mo (7-295). All patients had histologically proven HCV reinfection of the graft. None of the patients had clinical signs of decompensation. None of the patients was suffering from fibrosing cholestatic hepatitis (FCH). Seven patients were infected with GT1a, 12 patients with GT1b. Seventeen patients received tacrolimus as immunosuppressive regimen and 2 patients received cyclosporine A. Eight patients received MMF as concurrent medication which was stopped at the beginning of triple therapy in order to prevent aggravation of myelosuppressive effects of the antiviral substances. Patients’ characteristics are shown in Table 1.
Eight patients had to discontinue antiviral therapy early: one because of impaired liver function after 4 wk, three because of VB after TW 8 and TW 12. One patient had a partial response with a viral decline of > 2-log10 IU/mL at TW 12 but was stopped when viral load was still detectable at TW 24. One patient had a VB at TW 16 despite RVR and was discontinued. Two patients who had an EVR experienced a VB at TW 14 and TW 24 and were discontinued from therapy. Notably, only one patient had to stop therapy due to side effects, respectably deterioration of liver function, which resolved. All other patients with an unfavourable course of therapy were stopped due to VB or insufficient response (Table 2). Two patients with initial RVR and VB at TW 12 and 16 selected mutations associated with resistance as described below (Table 3).
| Sustained virological response | Treatment failure | |||||||||||||||||||
| Pat. 1 | Pat. 2 | Pat. 3 | Pat. 4 | Pat. 5 | Pat. 6 | Pat. 7 | Pat. 8 | Pat. 9 | Pat. 10 | Pat. 11 | Pat. 12 | Pat. 13 | Pat. 14 | Pat. 15 | Pat. 16 | Pat. 17 | Pat. 18 | Pat. 19 | Pat. 12 | |
| HCV GT | 1b | 1b | 1a | 1b | 1b | 1b | 1b | 1b | 1b | 1a | 1b | 1a | 1a | 1a | 1b | 1a | 1b | 1a | 1b | 1a |
| Basline | 2763000 | 4860000 | 1183000 | 5758000 | 1713000 | 1950000 | 441000 | 14900000 | 1920000 | 456000 | 387700 | 10900000 | 11500000 | 1008000 | 2565000 | 1690000 | 1370000 | 135600 | 580400 | 10900000 |
| TW 4 (RVR) | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | 71 | 37 | 34 | < Q | < Q | 15 | 4668 | < Q |
| TW 12 (EVR) | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | 685 | < Q | < Q | 3322000 | < Q | < Q | 6848 | < Q |
| TW 16 | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | 371 | < Q | < Q | ND | 6260 | ND | ND | < Q |
| TW 24 | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | 164 | 466 | 55400 | ND | ND | ND | ND | < Q |
| TW 48 | < Q | < Q | < Q | < Q | < Q | < Q | < Q | |||||||||||||
| 12 wk pt | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | 457200 | 9704 | 1657 | 1564794 | ND | 966400 | 27 | 660300 | 457200 |
| 24 wk pt | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | < Q | 691400 | 4135000 | 3735000 | ND | 7420000 | 9824 | 3489000 | 691400 | |
| HCV GT | Patient | TW | Outcome | Baseline | End of treatment |
| 1b | Pat.1 | 24 | SVR | Not detected | NA |
| 1b | Pat.2 | 24 | SVR | Not detected | NA |
| 1a | Pat.3 | 24 | SVR | Not detected | NA |
| 1b | Pat.4 | 24 | SVR | Not detected | NA |
| 1b | Pat.5 | 48 | SVR | Not detected | NA |
| 1b | Pat.6 | 48 | SVR | Not detected | NA |
| 1b | Pat.7 | 48 | SVR | Not detected | NA |
| 1b | Pat.8 | 48 | SVR | Not detected | NA |
| 1b | Pat.9 | 48 | SVR | Not detected | NA |
| 1a | Pat.10 | 48 | SVR | Not detected | NA |
| 1b | Pat.11 | 48 | SVR | Not detected | NA |
| 1a | Pat.12 | 24 | Relapse | Not detected | Not done |
| 1a | Pat.13 | 24 | Non response | Not detected | R155K |
| 1a | Pat.14 | 24 | VB | Not detected | V36M, R155K |
| 1b | Pat.15 | 24 | VB | Not detected | - |
| 1a | Pat.16 | 24 | Relapse | V36L | V36L, R155K |
| 1b | Pat.17 | 16 | VB | V36LV | V36L, T54ST |
| 1a | Pat.18 | 4 | Relapse | Not done | R155K |
| 1b | Pat.19 | 8 | VB | Not detected | A156F |
Five of the 12 remaining patients with RVR and undetectable viral load at TW 24 decided to stop therapy at TW 24. Of these, 4 experienced a sustained response with SVR24 while 1 patient relapsed within 12 wk after the end of treatment (Table 2). Seven patients decided to continue treatment for the full course of 48 wk, all of which achieved SVR24 (Table 2). Notably, all patients with sustained clearance of the virus (4 after a 24 wk-course and 7 after a 48 wk-course) were characterized by RVR (Table 2). In brief, we observed an overall RVR4 in 14/19 patients (73.7%), an EVR in 16/19 patients (84.3 %), an EOT response in 12/19 patients (63.1%) and SVR24 in 11/19 patients (57.9%).
Complete sequence information was obtained for the NS3 protease domain from baseline samples and from the first viremic sample of patients with treatment failure. We were unable to amplify the protease domain from one sample after treatment failure. Of the seven remaining patients, six harbored isolates with substitutions known to be associated with resistance to telaprevir. Four patients with genotype 1a infection and subsequent treatment failure selected the R155K substitution, in two cases combined with a substitution V36M/L. Two patients with genotype 1b infection and subsequent treatment failure selected either an A156F subsititution associated with high-level resistance or a combination of the substitutions V36L and T54S. In one patient infected with genotype 1b resistance-associated substitutions were not detectable by bulk sequencing. Two patients with subsequent treatment failure carried the resistance-associated substitution V36M/L already prior to therapy. Notably, resistance-associated substitutions were not detected at baseline in all patients who achieved SVR. Mutation related to PI resistance were detected in eight patients who all experienced a treatment failure, a VB or a non-response (Table 3).
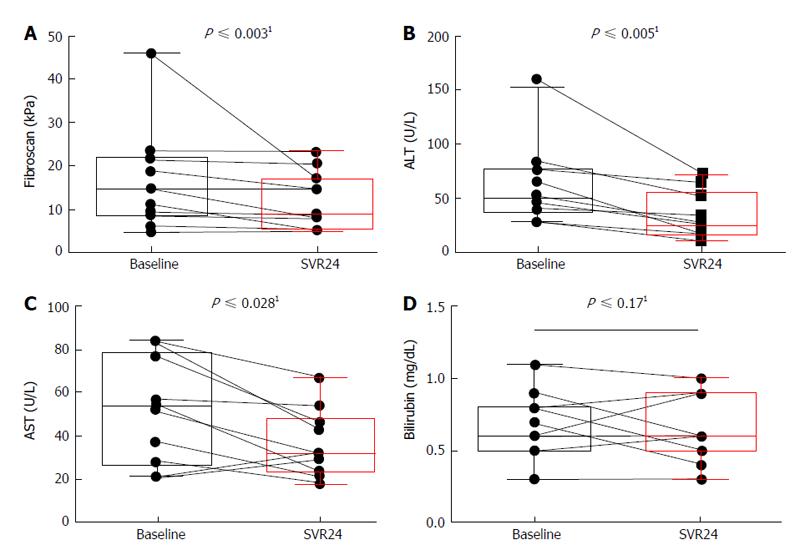
In order to determine whether successful TVR-based triple therapy influences liver parameters, we determined liver stiffness in SVR patients by fibroscan at baseline and at 24 wk post treatment. The fibrosis score improved for all patients significantly (P < 0.003) with 14.6 kPA (4.8-46) at baseline to 8.8 kPa (4.5-23.3) at 24 wk post treatment (pt) (Figure 1A). Concerning liver values, alanine aminotransferases (ALT) improved also significantly (P < 0.005) from 49 U/L (21-159) to 25 U/L (11-73) (Figure 1B). Aspartate aminotransferase (AST) improved significantly (P < 0.028) from 52 IU/L (21-84) to 31.5 IU/L (18-67) (Figure 1C). Bilirubin was not high at baseline in all patients and was stable from 0.6 mg/dL (0.3-1.1) at baseline to 0.6 mg/dL (0.1-0.9) in median 24 wk pt (Figure 1D). Improvement could be observed for all patients described, irrespective of duration of treatment.
With respect to prediction of outcome, we analyzed several clinical and patient characteristics at baseline and compared patients with SVR to patients without SVR (Table 4). Age, body mass index, fibrosis score and time from LT to start of therapy as well as the recipient interleukin-28b polymorphism and previous antiviral treatment did not significantly influence the outcome in our cohort. As well, liver values, platelet count and viral load did not show a significant influence, while however, a lower platelet count and a higher viral load at baseline rather coincides with an unfavorable outcome. However, a low bilirubin at baseline and the HCV GT1b turned out to significantly correlate with SVR.
| SVR | Non SVR | P ≤ | |||
| Patients, n/% | 11 | 60% | 8 | 42% | |
| Age, yr | 53 | 41-70 | 58 | 53-64 | 0.1151 |
| Body mass index (kg/m2) | 26 | 22-30 | 26 | 21-33.1 | 0.5051 |
| Ischak fibrosis score (grade) | |||||
| I-II | 7 | 64% | 6 | 75% | |
| III-IV | 4 | 36% | 2 | 25% | |
| Fibroscan baseline (kPa) | 14.6 | 4.5-23.4 | 11.3 | 5.9-26 | 0.7731 |
| Time from LT to triple therapy (mo) | 22 | 7-156 | 23 | 8-295 | 0.8691 |
| Bilirubin total (mg/dL) | 0.6 | 0.3-1.1 | 1 | 0.4-3.2 | 0.0301 |
| GPT (ALT) (U/L) | 46 | 21-159 | 40 | 24-85 | 0.5631 |
| GOT (AST) (U/L) | 52 | 18-84 | 53.5 | 22-88 | 0.6201 |
| Platelet count (/μL) | 143 | 68-246 | 103.5 | 63-236 | 0.3021 |
| Viral load (log10 IU/mL) | 1.9 | 0.39-14.9 | 4.2 | 0.13-13.70 | 0.4091 |
| HCV GT | 0.0492 | ||||
| 1a, n/% | 2 | 18% | 4 | 50% | |
| 1b, n/% | 9 | 81% | 4 | 50% | |
| Recipient IL-28b polymorphism, n/% | 0.5522 | ||||
| CC | 4 | 36% | 1 | 12.50% | |
| CT | 3 | 27% | 6 | 75% | |
| TT | 4 | 36% | 1 | 12.50% | |
| History of any prior PEG-INF/RBV treatment, n/% | 7 | 63% | 4 | 50% | 0.5522 |
| History of post-LT PEG-INF/RBV treatment, n/% | 2 | 18% | 3 | 37.50% | 0.3452 |
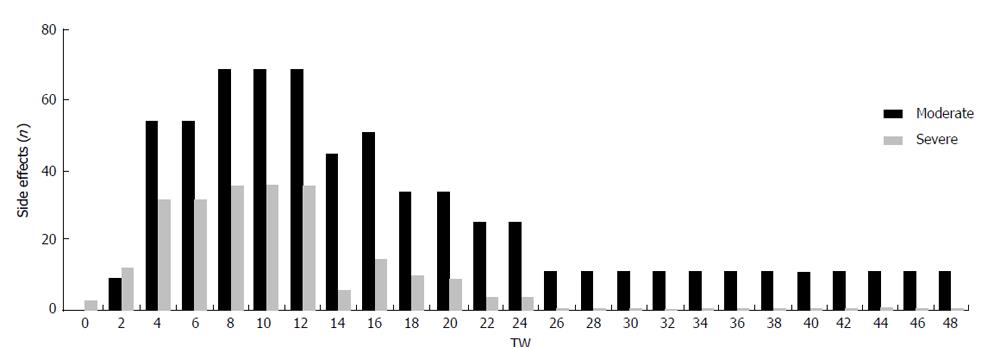
The TVR-based triple therapy has been associated with various and severe adverse events. We tend to differentiate between moderate (treatment not compulsory) and severe (treatment compulsory) side effects that might occur during triple phase (TW 1-12) or during the consecutive dual phase (TW 13-24/48) (Figure 2 and Table 5). The most frequent side effects were changes of the blood count with anemia being the most preponderant, affecting almost all patients. Reduction of the hemoglobin level was observed throughout the whole course of therapy in almost all patients. Therapeutic procedures like blood transfusion and erythropoietin injection were necessary in the majority of cases (n = 8) during the triple therapy phase between TW 6 and 12 while only 2 patients needed further erythropoietin injections after TW 13. The RBV dose was reduced in only 2 patients due to renal dysfunction, however, in order not to impair efficiency of therapy RBV dose was not adjusted due to HB changes.
| Moderate side effects | Severe side effects | |||
| TW 1-12 | TW 13-48 | TW 1-12 | TW 13-48 | |
| Hematological toxicity | 0 | 0 | 0 | 0 |
| Anemia | ≤ 10 g/dL | ≤ 10 g/dL | ≤ 8 g/dL | ≤ 8 g/dL |
| 9 (47.4) | 11 (57.9) | 8 (42.1) | 2 (10.5) | |
| Low WBC (< 1/μL) | < 3.4/μL | < 3.4/μL | < 1/μL | < 1/μL |
| 16 (84.2) | 14 (73.7) | 2 (10.5) | 3 (15.8) | |
| Low PT (< 50/μL ) | < 50/μL | < 50/μL | < 20/μL | < 20/μL |
| 3 (15.7) | 6 (31.6) | 0 | 0 | |
| Renal failure | 8 (42.1) | 0 | 1 (5.2) | 0 |
| Dermatological toxicity | 0 | 0 | 0 | 0 |
| Rash std. I | 7 (36.8) | 0 | 0 | 0 |
| Rash std. II | 0 | 0 | 1 (5.2) | 0 |
| Rash std. III | 0 | 0 | 0 | 0 |
| Anorectal pain | 9 (47.4) | 0 | 10 (52.6) | 0 |
| Pruritus | 4 (21.0) | 0 | 4 (21.0) | 0 |
| Stomatitis | 3 (15.7) | 0 | 1 (5.2) | 0 |
| Loss of appetite | 5 (26.3) | 0 | 0 | 0 |
| Loss off weight > 10% | 6 (31.6) | 0 | 1 (5.2) | 0 |
| Diarrhoe | 5 (26.3) | 0 | 1 (5.2) | 0 |
| Weakness | 10 (52.6) | 7 (36.8) | 0 | 0 |
| Hospitalisation | 0 | 0 | 3 (10.5) | 2 (15.8 ) |
| Hepatic decompensation | 0 | 0 | 1 (5.2) | 0 |
| Edema | 0 | 0 | 3 (15.8) | 0 |
| Diabetes melitus | 0 | 0 | 3 (15.8) | 3 (15.8) |
| Psychiatric disorders | 0 | 4 (21.0) | 0 | 1 (5.2) |
| Medical induced fever | 0 | 9 (47.4) | 0 | 0 |
| Infection | 0 | 0 | 0 | 2 (10.5) |
Two patients required a reduction of the PEG-IFN dose due to neutropenia and received G-CSF for neutropenia weekly from TW 2 ongoing. One more patient received G-CFS after TW 12. All patients were treated early with G-CSF when white blood count dropped below 1000/μL. Two patients developed flue-like symptoms and recovered under symptomatic therapy and prophylactic antibiotic treatment. Six patients developed a low platelet count below 50/μL without the need for treatment with platelet growth factors (Table 5).
No serious dermatological adverse events occurred. Seven patients developed a slight rash between TW 4 and 8 which disappeared after a few days and did not require therapeutic intervention. All patients developed anorectal pain between TW 2 and 10, nine of which requiring treatment. Most patients complained about gastrointestinal side effects like diarrhea and loss of appetite. Interestingly, all of these side effects peaked between TW 6 and TW 12 and disappeared upon discontinuation of TVR (Table 5).
Two patients had to be hospitalized due to diarrhea and weight loss during the triple phase. Symptoms improved considerably after stop of TVR and the patients could be dismissed at TW 14. One patient had to be hospitalized for an acute flare-up of chronic kidney disease at TW 2 and recovered after rehydration. Half of the patients experienced a reduction of their renal function with a nadir of the GFR between TW 8 and 12 which was stabilized by the intense recommendation to increase fluid intake and daily intravenous fluid application in 3 cases in the outpatient clinic. No severe impairment of renal function could be observed after the triple phase (TW 13-48). Median GFR decreased hardly in patients receiving 24 wk of therapy with - 4 mL/min from baseline to end of treatment, median GFR yet increased in patients receiving 48 wk of therapy by 13 mL/min (Table 5). HB levels decreased in line with deteriorating renal function.
In general, adverse events including moderate and severe adverse events were more frequent during the first 12 wk of therapy. After discontinuation of TVR, adverse events declined substantially to moderate disorders during the second half of the 48 wk course (Figure 2). This pattern encourages us to rather apply TVR instead of BOC in LT patients, as the period of intense monitoring and potential complications due to side effects can be shortened to 12 wk. However, we observe IFN-induced psychiatric side effects and depressive disorders as major problem severely compromising the motivation of the patients. Depressive disorder, weakness and loss of appetite together with weight loss were the most preponderant problems between TW 12 and TW 48, most likely related to IFN.
In our cohort, only one patient had to stop therapy because of deteriorating liver function. None of the patients had to stop therapy because of severe side effects and none died. All patients recovered completely from all side effects after discontinuation of treatment. Trough blood concentrations of IS were kept stable using a special dosing regimen as described[16]. Frequent controls of TBC were performed. Thus, none of the patients experienced acute rejection which had been excluded by graft biopsies in all patients after end of treatment.
We report our single center experience with TVR-based triple therapy in a cohort of 19 LT patients with recurrent HCV reinfection, retrospectively analyzing treatment response, SVR rates, adverse events, resistance mutations before and after treatment and clinical and patients characteristics with potential predictive value for SVR.
We observed a substantial rate of sustained viral response in 11 out of 19 patients (58%) in our cohort of 11 pre-treated and 8 treatment-naïve patients. Of note, all (7/7; 100%) of the patients who completed the full course of 48 wk of treatment and 4/5 (80%) of the patients who completed 24 wk of treatment achieved SVR24. While we observe a SVR rate of 58%, from other multi-centric post-LT cohorts, where SVR rates between 20% and 41% were reported[17,18].
Of 8 patients who were discontinued, only one patient had to discontinue because of deterioration of liver function. Seven patients were discontinued due to non-response or viral break through. None of the patients had to discontinue because of adverse events.
RVR4 is considered as a positive predictive factor for SVR[19]. A significant number of our patients (14/19) displayed RVR with non-detectable viral load at treatment-weeks 4, and all patients with SVR achieved RVR. RVR4 has been reported to be an important predictor of SVR[19] and response-guided therapy is well established in non-LT patients[20,21]. This is reflected in our cohort, as all patient achieving SVR24, had a HCV viral load below LLOQ (12 IU/mL) at TW 4. All patients with a detectable HCV viral load at TW 4 had a virological failure later on. These findings underline the exceptional prognostic impact of a rapid viral response in TVR-based triple therapy through all treatment cohorts. These results might suggest a reduction in length of treatment to 24 wk based on prediction by RVR for TVR-based triple therapy after LT. Four out of five patients who decided to stop therapy at TW 4 achieved SVR. Thus, in case of RVR, shortening TVR-based triple therapy to a 24 wk-course in LT patients may be considered to spare IFN-related side effects for the patient and also to increase cost effectiveness.
Clinical parameters (fibrosis score, AST, ALT) improved significantly in all patients achieving SVR. While liver values and platelet count have been described as independent predictors for SVR, these factors differ not with significance between patients with SVR or without SVR in our cohort. However, in our patients a low bilirubin turns out to be favourable together with HCV GT1b.
Notably, there was a significant difference in SVR rates between GT1a and 1b. Although the cohort size is not sufficient to adequately address this question, this reveals a clear trend towards lower SVR rates in patients infected with GT1a. Here, 2 of 7 patients infected with genotype 1a achieved SVR while 9 of 12 patients infected with GT1b were successfully treated. Importantly, treatment-failure in genotype 1a was associated with selection of R155K in all samples tested. This goes in line with previous reports indicating that the barrier to resistance to TVR is lower in GT1a compared to GT1b[22]. Interestingly, two patients, one infected with GT1a and subsequent relapse and one infected with GT1b and subsequent VB, already carried the resistance-associated substitution V36L/M prior to therapy. Resistance-associated substitutions were undetectable in all patients achieving SVR. Although not conclusive, this may suggest that the pre-existence of the resistance-associated substitutions may have contributed to treatment-failure in those two patients. Resistance testing prior to PI treatment in the setting of LT-patients should be considered and the clinical relevance needs further evaluation.
Interactions with IS were a major concern before the treatment of LT patients with PIs. PIs are potent inhibitors of the CYP3A4 enzyme and numerous drug-drug interactions have been described with CNIs[8]. Based on these reports, patients were monitored daily regarding trough blood levels. In addition, we chose a daily low dose application of TAC in order to avoid major variations in trough levels with nephrotoxic potential and risk of rejection as previously described[10]. In our patients, dosage of IS had to be reduced 30-fold for TAC and 2,5 fold for CyA as reported elsewhere[10,16]. Still, our observations confirm that tight monitoring of CNI trough levels is necessary but can be managed. Even daily dosing and trough levels of CNIs are, in our point of view, a hallmark in order to avoid trough peaks with consecutive toxicity. In combination with intensified oral and intravenous fluid supply, stabilization of renal function is a central factor to avoid RBV accumulation with aggravation of anemia. RBV reduction below a certain level should be avoided regarding the consequence of therapeutic efficiency.
Severe adverse events are a major drawback of TVR-triple therapy, being considerably more pronounced in the post-LT group of patients[23]. The most predominant adverse event was anemia in almost all patients, in line with other post-LT experiences. The abundance of erythrocyte concentrates, which were administered to our patients, illustrates this fact impressively. As the baseline hemoglobin level was a predictor for the probability of developing anemia during telaprevir treatment[24], we excluded patients with a HB < 10 g/dL from treatment. It has been reported, that those patients with a complicated course post-LT before onset of treatment (FCH, recirrhosis, signs of decompensation) developed the most severe adverse events, most importantly viral or bacterial infections. We therefore did not consider patients with decompensated cirrhosis for treatment.
Taken together, our results confirm that TVR-based triple therapy represents a considerable alternative for LT patients with HCV GT1 reinfection in terms of effectiveness. Moreover, our data suggest that 100% response rate after completing a 48 wk course and 80% after completing a 24 wk course can be achieved. A RVR at TW 4 can be confirmed as positive predictor for SVR, and also a low bilirubin at baseline and GT1b are related to a beneficial course and outcome of TVR-based triple therapy. However, conclusions have to be drawn with cautiousness as the sample size is reduced due to a limited number of patients who is eligible for this treatment, analysis has been performed retrospectively and controls are missing for possible confounding factors.
The high rate of treatment failure associated with emergence of resistance mutations in GT1a suggests that GT1b should be preferably selected for TVR-based triple therapy. We recommend daily low dose application of IS and eager stabilization of renal function in order to maintain RBV doses of not less than 600 mg/die. However, severe adverse events are frequent during therapy, therefore, careful selection of patients eligible for TVR-based triple therapy is of eminent importance. To this end, stable liver function and stable blood count at baseline and intensive patient monitoring are recommended. In the prospect of IFN-free DAA-based treatment regimens associated with less harmful side effects, post-LT treatment of HCV with the first generation PIs should be avoided in patients with signs of decompensation, FCH or instable blood count. In order to achieve a maximum benefit together with the least risk, patients should be screened for alternative IFN-free therapy options in those countries where next generation DAAs are available.
This manuscript forms part of the thesis of A. P.-K.
Chronic hepatitis C (HCV) infection is a serious health burden world-wide. Previous therapeutic options were inefficient and accompanied by serious side effects. The introduction of new direct acting antivirals (DAAs), starting with Telaprevir in 2011, improved the efficiency of treatment significantly. However, first generation protease inhibitors must still be combined with interferon (IFN), serious side effects are still common and thorough monitoring of the patients during therapy is still necessary. In particular, patients who received liver transplant (LT) due to HCV cirrhosis and who experienced reinfection of their graft, do have a high medical need for an effective HCV therapy.
In 2014, several IFN-free treatment options for HCV were introduced and approved in several countries. Still, real life data are missing for those applications and tremendous costs of the second generation DAAs makes the access difficult for many countries. Patients who received LT due to HCV cirrhosis are appreciated as a special patient population due to additional difficulties concerning the application of HCV therapeutics, like, e.g., drug-drug interactions with immunosuppressants and the risk of graft rejection.
The authors introduce their experience with a large cohort of patients with recurrent HCV-GT1 infection after LT, who received Telaprevir-based triple therapy for 48 wk or 24 wk. They display characteristics for each course of treatment and discuss predictors for potential shortening of treatment duration without limitation of efficacy.
They quite extensive and elaborate description of procedure and experiences will be of interest for centers who might chose to apply a telaprevir-based triple therapy due to lack or availability of next generation DAAs.
TLV, telaprevir: first generation protease inhibitor with direct antiviral efficacy against HCV; 48 wk course of treatment: standard duration of treatment with telaprevir-based triple therapy; predictors of outcome: to define factors that predict the benefit for the patient if undergoing a given treatment with potential severe side effects.
The study is considered to be of high interest to the field of hepatology due to the concise and thorough work up of a large data set with the described treatment modality.
P- Reviewer: Hu R, Teoh AYB, Voutsas V S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Thomas DL. Global control of hepatitis C: where challenge meets opportunity. Nat Med. 2013;19:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 740] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 3. | Terrault N. Liver transplantation in the setting of chronic HCV. Best Pract Res Clin Gastroenterol. 2012;26:531-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Marks KM, Jacobson IM. The first wave: HCV NS3 protease inhibitors telaprevir and boceprevir. Antivir Ther. 2012;17:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Forestier N, Zeuzem S. Telaprevir for the treatment of hepatitis C. Expert Opin Pharmacother. 2012;13:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Werner CR, Egetemeyr DP, Nadalin S, Königsrainer A, Malek NP, Lauer UM, Berg CP. Treatment of recurrent genotype 1 hepatitis C post-liver transplantation: single center experience with telaprevir-based triple therapy. Z Gastroenterol. 2014;52:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Charlton M. Telaprevir, boceprevir, cytochrome P450 and immunosuppressive agents--a potentially lethal cocktail. Hepatology. 2011;54:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Werner CR, Egetemeyr DP, Lauer UM, Nadalin S, Königsrainer A, Malek NP, Berg CP. Telaprevir-based triple therapy in liver transplant patients with hepatitis C virus: a 12-week pilot study providing safety and efficacy data. Liver Transpl. 2012;18:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Herzer K, Papadopoulos-Köhn A, Timm J, Paul A, Jochum C, Gerken G. [HCV reinfection after liver transplantation - management and first experiences with telaprevir-based triple therapy]. Dtsch Med Wochenschr. 2013;138:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Forestier N, Zeuzem S. Triple therapy with telaprevir: results in hepatitis C virus-genotype 1 infected relapsers and non-responders. Liver Int. 2012;32 Suppl 1:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 13. | Feld JJ. The beginning of the end: what is the future of interferon therapy for chronic hepatitis C? Antiviral Res. 2014;105:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Salvatierra K, Fareleski S, Forcada A, López-Labrador FX. Hepatitis C virus resistance to new specifically-targeted antiviral therapy: A public health perspective. World J Virol. 2013;2:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Papadopoulos-Köhn A, Achterfeld A, Paul A, Canbay A, Timm J, Jochum C, Gerken G, Herzer K. Daily Low-dose Tacrolimus Is a Safe and Effective Immunosuppressive Regimen During Telaprevir-based Triple Therapy for Hepatitis C Virus Recurrence After Liver Transplant. Transplantation. 2015;99:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Werner CR, Franz C, Egetemeyr DP, Janke-Maier P, Malek NP, Lauer UM, Berg CP. Efficacy and safety of telaprevir (TVR) triple therapy in a ‚real-life‘ cohort of 102 patients with HCV genotype 1: interim analysis after 24 weeks of treatment. J Viral Hepat. 2014;21:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, Radenne S, Pageaux GP, Si-Ahmed SN, Guillaud O, Antonini TM. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014;60:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Sarrazin C, Schwendy S, Möller B, Dikopoulos N, Buggisch P, Encke J, Teuber G, Goeser T, Thimme R, Klinker H. Improved responses to pegylated interferon alfa-2b and ribavirin by individualizing treatment for 24-72 weeks. Gastroenterology. 2011;141:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 22. | Wyles DL, Gutierrez JA. Importance of HCV genotype 1 subtypes for drug resistance and response to therapy. J Viral Hepat. 2014;21:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, Ryland KL, Yataco ML, Satyanarayana R, Rosser BG, Vargas HE. Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl. 2013;19:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Zeuzem S, DeMasi R, Baldini A, Coate B, Luo D, Mrus J, Witek J. Risk factors predictive of anemia development during telaprevir plus peginterferon/ribavirin therapy in treatment-experienced patients. J Hepatol. 2014;60:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |