Published online May 28, 2015. doi: 10.4254/wjh.v7.i9.1272
Peer-review started: August 30, 2014
First decision: November 27, 2014
Revised: December 19, 2014
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 28, 2015
Processing time: 265 Days and 14.4 Hours
Chronic hepatitis B (CHB) remains a significant unmet medical need, with 240 million chronically infected persons worldwide. It can be controlled effectively with either nucleoside/nucleotide-based or interferon-based therapies. However, most patients receiving these therapies will relapse after treatment withdrawal. During recent years, the advances in molecular biology and immunology have enabled a better understanding of the viral-host interaction and inspired new treatment approaches to achieve either elimination of the virus from the liver or durable immune control of the infection. This review aims to provide a brief overview on the potential new therapies that may overcome the challenge of persistent CHB infection in the near future.
Core tip: Current hepatitis B treatments can only control the disease, but they rarely lead to substantial rates of hepatitis B surface antigen loss and seroconversion. Several new therapeutic approaches are being developed so as to attain the elusive goal of successful functional cure of chronic hepatitis B infection.
- Citation: Phyo WW, Soh AYS, Lim SG, Lee GH. Search for a cure for chronic hepatitis B infection: How close are we? World J Hepatol 2015; 7(9): 1272-1281
- URL: https://www.wjgnet.com/1948-5182/full/v7/i9/1272.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i9.1272
Chronic hepatitis B (CHB) remains a global health challenge. More than 240 million persons have been infected with CHB virus. CHB has become one of the most common causes of liver cirrhosis and hepatocellular carcinoma, and has led to more than 780000 deaths per year[1,2]. At this point, there is no cure for CHB, even though potent drugs are available to control the virus and prevent complications. In the last few years, several new drug classes targeting the various stages of hepatitis B replication cycle are under investigation. Whether and which of these agents could become clinically useful therapies are still unclear[3]. The review will discuss these new investigational approaches for the treatment of hepatitis B virus (HBV) infection, and their respective stages of development in clinical trials.
The Food and Drug Administration-approved chronic hepatitis B treatment of choice is either an immunomodulators [standard and pegylated interferon (Peg-IFN)] or nucleoside/nucleotide analogues (lamivudine, adefovir, telbivudine, entecavir and tenofovir). The latter class is less expensive and orally available. These drugs have minimal side-effects comparing to interferon and can be used for decompensated cirrhosis and after liver transplantation[4]. However, they have to be taken on a long-term basis in general, and result in hepatitis B e antigen (HBeAg) seroconversion rate of 20%-25% and HBV antigen (HBsAg) loss of 1% or less[5]. This is because they are designed to be reversible transcriptase inhibitors, a crucial step in viral replication, but not to eliminate the HBV minichromosomes [HBV covalently closed circular DNA (cccDNA), Figure 1] persisting in the nucleus of the hepatocytes[6]. There is a significant risk of HBV reactivation and sometimes HBV flare after withdrawal of the antiviral agents. Drug resistance may evolve after long-term therapy[3]. In contrast, the interferon-alfa therapy is of finite duration and results in 30%-40% HBeAg seroconversion, 5%-10% HBsAg seroclearance, with no risk of drug resistance. However, its systemic adverse effects limit its usefulness, especially in patients with decompensated liver cirrhosis[4].
Modifications to existing therapies are being investigated, such as combining nucleoside/nucleotide analogues with interferon, switching treatments, and extending Peg-IFN. Twenty-five HBeAg negative patients were given an extended (96-wk) course of Peg-IFN plus adefovir in a study by Cao et al[7]. All patients achieved HBV DNA of less than 500 copies/mL, and HBsAg seroconversion rates were 12%, 28%, and 32% at weeks 48, 96 and 120 respectively. HBeAg positive patients who had been on entecavir for 9 to 36 mo, with HBV DNA of < 1000 copies/mL and HBeAg < 100 PEIU/mL, randomized to switch to Peg-IFN or to continue entecavir for 48 wk in OSST trial. The trial showed that switching to Peg-IFN led to a higher HBeAg seroconversion at week 48 (14.9% vs 6.1%, P = 0.0467). Among Peg-IFN-treated patients with HBeAg loss and HBsAg < 1500 IU/mL at randomization, as high as 22.2% achieved HBsAg loss[8]. Larger studies for the optimal combination regimen are ongoing, but HBsAg loss much higher than 30% is unlikely using current modalities available.
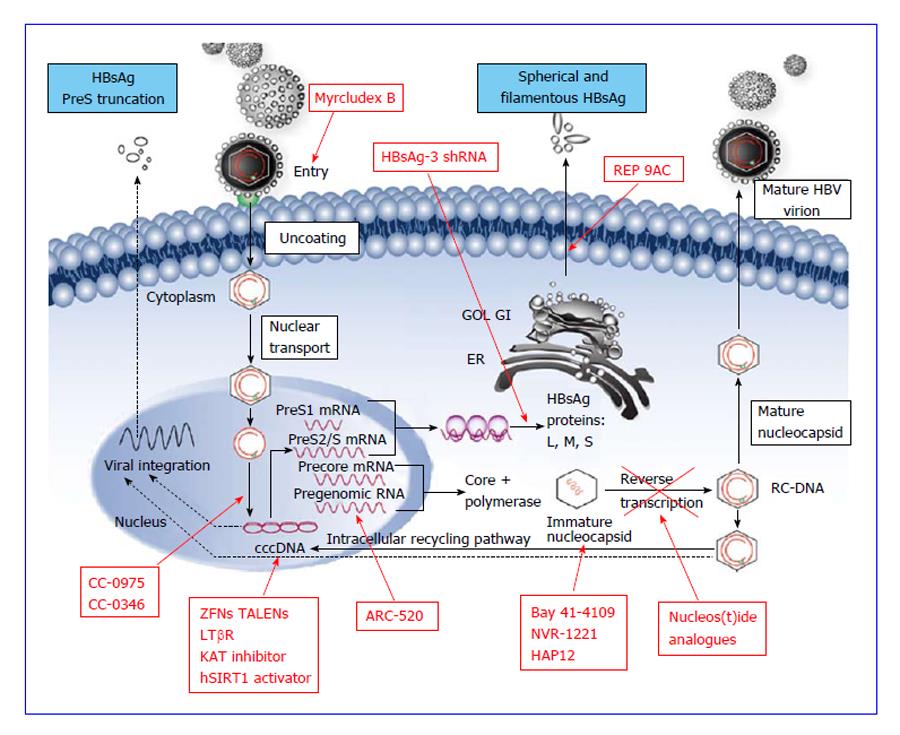
Understanding the HBV life cycle is essential before attempting to discuss the mechanisms of the new targets. HBV, belonging to hepadnaviridae family, is 42 nm in diameter comprising of approximately 3.2 kb double-stranded relaxed coiled DNA (rcDNA) formed by the reverse transcription of pregenomic RNA (pgRNA)[9]. HBV is hepatotrophic and hepatocytes are the only cells that support HBV replication in the human body[10]. Availability of the cell lines susceptible to HBV infection has led to the better understanding of the early stages of its life cycle, starting from viral attachment, entry and translocation of rcDNA into nucleus to form cccDNA, as well as the later stages viral replication, including transcription of viral RNA, reverse transcription to form daughter DNA, assembly of viral particles and secretion out of the cells[11] (Figure 1).
HBV virion enters the hepatocyte via endocytosis by its N-terminal region of large (L) envelope (preS1) binding to the sodium taurocholate cotransporting polypeptides (NTCP) receptor on the plasma membrane of hepatocyte[12]. A peptide derived from this preS1 region is a possible therapeutic target to inhibit viral entry by binding to its receptors[13]. After uncoating and releasing into the cytoplasm, nucleocapsid containing rcDNA is transported to the nucleus to form cccDNA[3]. This formation is mediated by the viral polymerase that completes the incomplete plus-strand of viral rcDNA after which the polymerase is removed by cellular enzymes, leading to the formation of cccDNA by covalent ligation of both DNA strand[14]. cccDNA is also known as episomal minichromosome and is crucial for the persistence of the virus in the host hepatocytes and cause chronic infection[15]. It acts as the sole DNA template for the formation of 4 groups of viral RNA, namely precore mRNA (pre-C); pgRNA; mRNA coding for surface (S), middle (M) and large (L) envelope proteins; and mRNA coding for X protein[11]. Pre-C mRNA is processed into HBeAg that can be detected in the circulation with commercial assays, which reflects infectivity of the HBV infection. The pgRNA serves as a template for viral DNA by reverse transcription, DNA polymerase and viral capsid protein. pgRNA, together with core protein and DNA polymerase, are self-assembled and encapsidated[3]. Inside the nucleocapsid, pgRNA is reverse transcribed into rcDNA, enveloped and is either secreted out of the hepatocyte[16] or shunted back into the nucleus to replenish the HBV cccDNA pool[17].
PreS1 region of viral L protein is required to bind to cell surface receptor for viral entry. The functional receptor is the heparan sulphate proteoglycans, specifically NTCP on the surface of hepatocytes[13,19]. With this knowledge, the scientists discovered that a synthetic myristoylated lipopeptide derived from HBV envelop protein[13], myrcludex-B, reversibly inhibits HBV entry into the naïve hepatocyte[19]. Six weeks of subcutaneous treatment of Myrcludex B to humanized mice infected with HBV reduced amplification of existing intrahepatic cccDNA as well as the spread of infection[20,21], without interfering with viral replication[19]. The drug has also been tested in chronic HBV infected subjects showing good tolerability and lack of serious side-effects with doses up to 5 mg intravenous and 0.8 mg subcutaneous[22]. Moreover, this entry inhibitor could be used as a treatment option for infected patient and high risk neonates. The application could potentially be extended clinically to post-liver transplantation and post-immunosuppression therapy to prevent HBV reactivation or flare[19]. In order to achieve the optimal outcome, the entry inhibitor was suggested to be used together with existing antivirals[20].
Persistence of HBV cccDNA in patients after successful long-term viral suppression by antiviral agents suggests that the key to HBV eradication in established CHB infection lies in the elimination of the reservoir of HBV minichromosomes from the hepatocyte[23]. Efforts in this area are still in early pre-clinical phases. This can be achieved by inhibiting cccDNA synthesis and maintenance, which include inhibition of its establishment, silencing its activity by transcription inhibitors, direct deactivation of cccDNA using engineered nucleases and activation of host innate immune response.
Blocking of HBV cccDNA formation: Recently, two novel compounds were reported that block conversion of relaxed circular HBV DNA into cccDNA at micromolar concentrations. Broadly known as distributed sulphonamide (DSS) compounds, they have phosphodiesterase or protease inhibitor activity, and can inhibit the conversion to cccDNA from rcDNA in human and duck hepatocytes through direct inhibition of deproteinization of rcDNA. The compounds were identified through a cell-based high throughput screen and neither the mechanism nor the target for these compounds is currently known. Among DSS, CCC-0975 and CCC-0346 were found to be the most potent in duck hepatocytes. Further studies are needed to improve their potency in order to obtain optimal benefits[24].
Promoting HBV cccDNA loss: One of the most exciting finding in 2014 in the field of HBV was the work by Lucifora et al[25], which described how IFN-α can induce specific degradation of the nuclear HBV cccDNA without detectable hepatotoxicity. Similar effect can also be achieved by activating the lymphotoxin-β receptor (LTβR), a receptor engaged by members of the TNF cytokine superfamily, which up-regulated APOBEC3A and APOBEC3B cytidine deaminases in HBV-infected cells. This appeared to be mediated through HBV core protein and its interaction with nuclear cccDNA, resulting in cytidine deamination, apurinic/apyrimidinic site formation. Extensive nucleotide changes [guanine (G) to adenine (A)] in cccDNA, a transition that was a hallmark of cytidine deamination, eventually led to cccDNA degradation that prevented HBV reactivation. Remarkably, this deamination did not occur in cellular DNA, which indicates that viral clearance might involve cccDNA-specific mechanisms[26].
Although there were some questions raised on the technical aspects of the study, the major concern with regards to the clinical application of this discovery was safety issue. LTβR agonists had been known to trigger apoptosis, hepatocellular proliferation, inflammation, and hepatocellular carcinoma. Thus safety considerations are likely to preclude regulatory approval in its current form[27].
Scientists have also developed engineered site-specific mutagenic nucleases as a powerful laboratory technique, and are working to apply these methods to the treatment of human diseases. This type of technology includes the zinc-finger nucleases (ZFNs), the transcription activator-like effector nucleases (TALENs) and the CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-associated caspase-9)[28,29]. Potentially it may be used to strategically disrupt the targeted gene related to viruses such as human immunodeficiency virus (HIV) and HBV, with the aim of mutating the intra-nuclear viral DNA reservoir, making it no longer competent for replication[30].
In HBV infection, the target gene is the HBV cccDNA[29]. For viral hepatitis B, these cleavage enzymes are delivered through viral vectors to reach the target HBV minichromosomes inside the hepatocyte nucleus[31]. ZFNs are derived from consecutive alteration of the amino acids on the zinc-finger protein domains[32]. Three days after cotransfection of ZFN pair with a target cccDNA plasmid, 26% of target remained linear while 10% was cleaved and misjoined tail-to-tail, resulting in loss of function in both groups. It was also found that the simultaneous use of multiple pairs of ZFNs not only inactivate the target DNA but also circumvent the viral resistance if combined with existing antivirals[32].
Alternative to ZFNs are TALENs, which can also specifically disrupt or cleave the target episomal DNA and inactivate it. Bloom et al[29] successfully engineered mutagenic TALENs that target four HBV-specific sites within the viral genome. TALENs targeting sequences in the S or C open-reading frames (ORFs) can efficiently disrupt the sequences at the intended sites and suppressed HBV replication. The S TALEN caused targeted mutation in about one third of cccDNA molecules following triple transfection of the TALEN-expression plasmids into HepG2.2.15 cell lines that contained stably integrated copies of HBV genome. Markers of viral replication were also inhibited in vivo in a murine hydrodynamic injection model of HBV replication. HBV target sites within S and C ORFs of the injected HBV DNA were mutated without evidence of toxicity. Efficacy in vivo indicated these engineered nucleases have potential for use in treatment of chronic HBV infection[29]. Other groups of scientists were trying to develop the best possible mathematical model for how to achieve the best outcome with the effective minimal doses of nucleases while taking all parameters, such as vector delivery, intracellular pharmacodynamics and resistance into consideration, which may eventually bring these DNA-editing technology to clinical applications[31].
HBV cccDNA transcription inhibitor: Small molecules had been identified that can hinder cccDNA transcription to pgRNA and HBV replication by alteration of epigenetic regulation. By controlling the acetylation or methylation of the histone proteins surrounding the cccDNA, the transcription of the HBV genome can be inhibited. These small molecules include Class I, II and III histone deacetylase inhibitors; p300 and P300/CBP-associated factor histone acetyltransferases inhibitors; hSirt1 activators; JMJD3 histone demethylase inhibitors[33]. All of these are still in the preclinical phase of development. Due to the wide ranging effect of these target enzymes on the expression multiple genes, the safety profiles of these compounds still requires extensive evaluation before clinical use.
As a proof-of-concept, liver regeneration was tested in uPA/SCID chimeric mice, which showed potent reduction of cccDNA levels and viral replication, when there is rapid regeneration of cccDNA-free hepatocytes. However, this regeneration technology requires further investigations in combination with existing antiviral agents, to have a possible chance of curing of chronic HBV infection[34].
RNA interference is carried out using complementary double stranded RNA to silence the homologous gene in post-transcriptional mRNA level[35]. In mammalian cells, the silencing is induced by the small interfering RNA (siRNA). In HBV-infected cells, siRNAs had been used to produce sequence-specific and dose-dependent knockdown of HBV surface or polymerase region of viral mRNA. siRNA functions by binding to complementary HBV mRNA and pgRNA, which will be degraded, resulting in no translation/reverse transcription[36]. Animal studies conducted by biotechnology company had demonstrated long-term suppression of HBV RNA, HBsAg, HBeAg and HBV DNA can be achieved by co-injection of ARC-520 (an anti-HBV siRNA) with DPC delivery vehicle into chimpanzees. This was followed by a phase 1 study, showing that all studied doses were safe and well-tolerable by normal volunteers and this result had led to an ongoing phase 2a clinical trial of ARC-520 in combination with entecavir in Hong Kong. This study will evaluate not only the safety and pharmacodynamics of the drug but also its efficacy on the levels of HBsAg, HBeAg and HBV DNA quantity, when given in combination with entecavir[37]. Meanwhile, some researchers suggested that the use of combination of siRNAs may achieve a stronger inhibition on HBV replication and antigen expression in HepG2.2.15 cell line and in mice models[38,39].
Like lamivudine and tenofovir, a number of drugs designed to target the reverse transcriptase of HIV are also potential new active therapies for HBV.
Besifovir (LB80380): Besifovir (LB80380), a potent acyclic nucleotide phosphonate, has demonstrated excellent preclinical safety profile, being effective against both wild-type and YMDD mutant HBV. In a randomized placebo-controlled Phase I/II study of besifovir, 29 HBeAg positive patients were treated for 4 wk, and led to maximum HBV DNA reduction of up to 4 log10 copies/mL respectively[40].
A Phase IIb randomized trial of besifovir vs entecavir in chronic hepatitis B patients was carried out[41]. One hundred and fourteen patients were randomized to receive besifovir, at 90 mg or 150 mg daily or Entecavir 0.5 mg daily. Mean log10 HBV DNA changes from baseline were -5.84, -5.91 and -6.18 for HBeAg-positive patients, and -4.65, -4.55, and -4.67 respectively for HBeAg-negative patients (P > 0.05). Of ninety-four point one percent of patients on besifovir had lowering of serum L-carnitine, which returned to normal with carnitine supplement.
MIV-210: 2,3-dideoxy-3-fluoroguanosine (FLG), a fluorinated guanosine analogue, was initially developed for treatment of HIV-infected patients, and is currently being investigate for HBV therapy. In 2006, Jacquard et al[42] studied its mode of inhibition and found that it inhibits the priming of the reverse transcription and also a competitive inhibitor of deoxyguanosine triphosphate incorporation resulting in termination of the DNA chain. It is a more potent inhibitor of wild-type DHBV minus-strand DNA synthesis than lamivudine. In Huh7 cells transfected with HBV, the inhibition of wild-type, lamivudine-resistant, adefovir-resistant and adefovir-plus-lamivudine-resistant HBV mutants by FLG were similar.
In woodchucks infected with woodchuck hepatitis virus (WHV), oral administration of MIV-210 at 20 or 60 mg/kg per day led to a rapid virological response, reducing WHV DNA levels by 4.76 log10 and 5.72 log10 respectively[43]. A daily dose of 10 mg/kg was found to decrease serum WHV load by 400-fold after 4 wk of treatment, and a dose at 5 mg/kg per day was sufficient to maintain this antiviral effect. MIV-210 at 20 or 60 g/kg per day led to a 2.0 log10 drop in hepatic content of WHV cccDNA. MIV-210 is currently undergoing Phase II trial in South Korea by Daewoong.
Tenofovir alafenamide (GS-7340): Tenofovir alafenamide (TAF, or GS-7340), a new oral prodrug of tenofovir, was found to be more stable in plasma compared to tenofovir disoproxil fumarate (TDF). TAF achieved higher levels of tenofovir diphosphate in target cells at lower doses than TDF[44]. In an open-label phase 1b trial, patients were randomized to TAF 8 mg, 25 mg, 40 mg, 120 mg, and TDF 300 g for 2 d. Most adverse reactions were mild to moderate, with the most common being headache, nausea, vomiting and fatigue. There was also minimal decline in creatinine clearance with TAF compared to TDF. At doses of 8 mg to 120 mg of TAF, there were no differences in viral decline[44]. Compared to TDF, TAF does not interact with OAT1 or OAT3 (renal transporters) and hence is unlikely to accumulate in renal proximal tubules[45].
Multiple studies performed on HIV patients had shown that both TAF and TDF were well tolerated, and adverse events were mild to moderate in severity and self-limiting[46,47]. Patients on the TAF regimen had smaller reductions in estimated creatinine clearance, less renal tubular proteinuria and smaller changes in bone mineral density for hip and spine. However, they also had higher increases in HDL, LDL and total cholesterol. Phase 2 studies of TAF on anti-HBV are currently in progress.
The nucleocapsid of HBV is composed of hundreds of core proteins[48] and its structure is important for HBV DNA synthesis and virion assembly[49,50]. Heteroaryldihydropyrimidines (HAPs) are inhibitors of nucleocapsid formation or assembly of core particles. This is achieved by misdirecting the process, without primarily affecting the core protein level and viral transcription level. Nonetheless, diminished core proteins level was observed as a consequence of its inhibitory effect on the capsid assembly and viral replication[51].
Bay 41-4109, a member of HAPs is a highly potent non-nucleoside antiviral whose inhibitory effect on the viral replication could be detected within 5 d of drug administration in mice model, while at the same time controlling the spread of infection. Hepatotoxicity was detected only at doses over 100 mg/kg per day[52]. Unexpectedly, studies revealed its effects on the epigenetic state of host genes, which controlled the host innate immunity[53]. However, the drug action ceased after its withdrawal tested[54]. Despite its reversibility, Bay 41-4109 is still a potential novel therapeutic which could be useful for patients infected with resistant HBV strains or those who failed standard therapy[52].
Another member of the HAP with intriguing property is HAP12 which binds to core proteins on the nuclear minichromosome, causing structural changes and formation of core protein that does not support cccDNA transcription and further production of pgRNA. Therefore, HAP12 is also another potential therapeutic agent that can suppress cccDNA function in addition to its effective inhibition of capsid proteins[55].
Alternative to HAPs is a direct-acting agent against HBV core protein, known as NVR-1221, which is currently in phase Ia trial with the drug test tested on approximately 40 healthy volunteers[56]. Other similar alternatives with inhibitory effects on capsid proteins are 2-amino-N-(2,6-dichloropyridine-3-yl) acetamide derivatives[50], sulphamoylbenzamide derivatives[57] and sulfanilamide derivatives[49], with the first derivatives shown to have synergistic inhibitory effect on HBV load if used together with lamivudine[50].
The release of HBsAg is independent of virion release and the antigen itself is postulated to be involved in suppressing the host innate immunity allowing the virus to cause persistent infection in human liver. REP 9AC, a nucleic acid polymer compound[58], is a potent HBsAg secretory inhibitor, which can eliminate HBsAg from the human circulation as early as 7 d of administration in a small study. This was followed by reactivation of the suppressed innate immune system of the host to produce anti-HBs antibody by weeks 15 and achieved sustained virological response after stopping the administration[59]. HBV DNA remained undetectable if add on immunotherapy was given. More work is being carried out to better understand the optimal duration of treatment to sustain its effects, tolerability and the best route of administration[58].
Host immune response can be broadly divided into the innate and adaptive immunity. The adaptive HBV-specific, T cell-mediated immune responses are widely regarded as the most important elements against HBV. As an essential part of host innate immune system, Toll-like receptors are membrane-bound receptors involved in recognition of pathogens thereby activating the expression of several genes that contribute to antiviral immune responses[60]. Their importance in chronic HBV infection is increasingly recognized in recent years.
Toll-like receptor (TLR)-7, expressed on the dendritic cells and B lymphocytes[61], is able to recognize nucleic-acid like structures of viruses[60] and the stimulation of their respective receptors enhance dendritic cells to produce interferon alpha and other cytokines to further activate natural killer and cytotoxic T lymphocytes[61]. It is also found that TLR-7 and a number of other receptors are suppressed in chronic HBV infected patients leading to immune dysfunction against the infection[62], especially in the presence of HBsAg[63]. GS-9620, a potent oral agent containing TLR-7 agonist action, was shown to have ability to reduce HBsAg as well as HBV DNA in both serum and liver with even short-term usage in woodchucks and chimpanzees. The immune responses triggered by TLR-7 eliminate HBsAg, HBeAg and hepatitis B core antigen (HBcAg) positive hepatocytes and inhibit viral replication directly[61]. The drug also has high bioavailability and tolerability among healthy volunteers without serious side-effects[64]. The combination of GS-9620 and nucleoside analogues may be able to treat HBV infection effectively without significant systemic side-effects associated with interferon-based therapy[61]. An ongoing research has recently been conducted on the safety and pharmacodynamics of GS-9620 in chronic hepatitis B patients[65].
GS-4774 is a yeast-based vaccine expressing a recombinant X, large S and core antigens of HBV. Its action includes activation of dendritic cells after phagocytosis, stimulation of CD4+ and CD8+ T cells and reduction of regulatory T cells level. Phase 1 studies of GS-4774 in normal healthy adults provided satisfactory results showing high tolerability while achieving satisfactory immune responses to the administered recombinant antigens and peptides. Further study to further evaluate its usage in chronic hepatitis B patients is being conducted recently[66].
DV-601 is another therapeutic vaccine, containing recombinant HBV surface and core antigens, whose action is to trigger immune responses and to produce antibodies relating to HBV-specific cytotoxic T lymphocyte and B cell. The vaccine was given intramuscularly to chronic HBV infected patients, 8 HBeAg positive treatment-naïve and 6 HBeAg negative entecavir-treated subjects. The vaccine was found to be well-tolerable with only mild adverse effects that resolved without treatment. All patients were found to achieve the desired lymphoproliferative response and HBsAg, HBeAg and viral load were found to be reduced satisfactorily. Anti-HBs antibody and anti-HBe antibody were detected in those receiving the higher dose of DV-601[67].
A combination therapeutic vaccine containing HBsAg and HBcAg (known as NASVAC) was developed at CIGB, Cuba. NASVAC is delivered through nasal spray and in phase I trial was found to be safe and tolerable. All subjects developed anti-HBc antibody at days 30 after immunization, with 75% developing anti-HBs antibody of more than or equals 10 IU/L at a maximum at days 90[68]. The phase III trial to evaluate the therapeutic effects on lowering HBV DNA and other clinically important parameters is presently being conducted.
Synthetic HBV core antigen vaccine, firstly invented in 2012 by Inovia researchers aimed at reducing the risk of liver cancer, was reported to be a highly potent immune-therapeutic vaccine. This vaccine was designed to eliminate the HBV infected hepatocytes, without causing liver damage, through strong HBcAg-specific killer T-cell and antibody responses whose action will add on to existing host T-cells responses in the liver[69].
Most of the new antiviral agents discussed above remains in the early preclinical phase (laboratory and animal study) (summarized in Table 1). A few of these, driven by established biotechnology and pharmaceutical companies, had made it to phase-I/IIA clinical trials. Gaining momentum and safety profiles from the HIV trials, the newer nucleoside analogues are likely to be the first group of drugs to obtain formal approval for clinical use. Being in the same class as the existing therapy, they are unlikely to be game changers, but they may add to the arsenal of reverse transcriptase inhibitors to tackle the drug-resistant HBV strains that may emerge with long-term use of current agents.
| Therapeutic agents | Mechanism | Manufacturer | Status |
| Viral entry inhibitors | |||
| Myrcludex B | Entry inhibition | Myr_GmbH, Germany | Phase IIA, Russia |
| cccDNA Transcription inhibitors | |||
| CC-0975, CC-0346 (DSS) | Induces deproteinization of rcDNA | Preclinical | |
| LTβR | Induces cccDNA cytidine deamination | Preclinical | |
| Small molecules | cccDNA transcription inhibitor | Preclinical | |
| DNA editing technology | |||
| ZFNs | cccDNA inactivation | Preclinical | |
| TALENs | cccDNA inactivation | Preclinical | |
| RNAi gene silencer | |||
| ARC 520 | RNAi gene silencer | Arrowhead Research Pasadena, CA | Phase IIA |
| New nucleoside analogues | |||
| Besifovir (LB80380) | Inhibits viral DNA polymerase | LG Life Sciences, South Korea | Phase II |
| MIV-210 | Inhibits viral DNA polymerase | Medivir/Daewoong, South Korea | Phase II |
| Tenofovir alafenamide (GS-7340) | Prodrug of tenofovir | Gilead Foster City, CA | Phase III |
| Interruption of HBV capsid assembly | |||
| Heteroaryldihydropyrimidine (Bay 41-4109) | Inhibits viral nucleocapsid | AiCuris, Germany | PhaseI |
| Heteroaryldihydropyrimidine (HAP12) | Inhibits capsid assembly | Preclinical | |
| NVR-1221 | Capsid inhibitor | Novira Therapeutics, Doylestown, PA | Phase IA |
| HBsAg release inhibitor | |||
| REP 9AC | HBsAg release inhibitor | REPLICor Inc. Montreal, Quebec | Phase I |
| HBsAg-3 shRNA | HBsAg expression inhibitor | Preclinical | |
| Immunomodulator | |||
| GS-9620 | TLR-7 agonist | Gilead Foster City, United States | Phase I |
| CYT 107 (interleukin-7) | Immunomodulator | Cytheris, Paris, France | Phase I/IIA |
| Therapeutic vaccination | |||
| GS-4774 | HBV X, surface and core antigens | Gilead Sciences with Globe Immune Louisville, CO | Phase II |
| DV601 | HBV surface and core antigens | Dynavax, Berkeley, CA | Phase IB |
| NASVAC | HBV surface and core antigens | CIGB, Cuba | Phase I |
| HBV core Ag vaccine | T-cell mediated therapeutic vaccine | Emergent Europe, United Kingdom | Phase I |
However for the new classes of anti-HBV compounds that truly aims to cure CHB, the development is still in their infancy and major breakthrough is required to deliver the compound to the target (infected hepatocyte nucleus for the cccDNA inhibitors) or overcome established immune tolerance (new immunomodulators), at acceptable safety profiles.
We discussed the new anti-HBV compounds and new targets that can be broadly divided into two main categories: (1) therapies that target the virus either directly or by targeting host factors required for viral replication; and (2) therapies targeting the host innate or adaptive immune response.
To achieve the goal of curing CHB, any new approaches based on targeting the virus, will need to establish that target inhibition ultimately translates into significantly increased cccDNA depletion and HBsAg loss. For example, simply blocking viral replication, even by a mechanism other than nucleoside/nucleotides, is unlikely to cure infection unless the cccDNA reservoir is removed. Directly targeting cccDNA fundamentally tackles the issue of viral persistence but such new technologies as sequence-targeting nucleases and modulation of epigenetic regulators face challenges including drug delivery to target cells and risk of off-target effects. Approaches such as blocking antigen secretion, directly targeting viral RNA and blocking the HBV entry receptor are more technically feasible in the near term, but likely will not clear the cccDNA reservoir on their own. Our growing understanding of the immune defects in CHB is enabling the development of new immunotherapy. TLR-7 agonist represents one of such promising immunotherapeutic approaches. There remains the concern of safety in activating the immune system.
Although many potential new approaches to treat CHB have been identified, therapeutic translation has been challenging and relatively few drug candidates have emerged and entered clinical trials. If the success story of developing effective therapies leading to the ability to cure nearly all hepatitis C infection is any guide, the search for a functional cure for CHB is definitely not an illusive dream, and it is likely to come in the form of combination therapies of current effective antiviral agents with one or more new anti-HBV agents that either eliminate HBV cccDNA or overcoming specific immune pathways that will result in immunoclearance of the virus.
P- Reviewer: Elalfy H, Li GL S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | World Health Organization. Hepatitis B. Available from: http://www.who.int/mediacentre/factsheets/fs204/en. |
| 2. | Uhl P, Fricker G, Haberkorn U, Mier W. Current status in the therapy of liver diseases. Int J Mol Sci. 2014;15:7500-7512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Grimm D, Thimme R, Blum HE. HBV life cycle and novel drug targets. Hepatol Int. 2011;5:644-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Perrillo RP. Overview of treatment of hepatitis B: key approaches and clinical challenges. Semin Liver Dis. 2004;24 Suppl 1:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Int. 2009;3:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Spangenberg HC, Thimme R, Blum HE. Tracking cccDNA in chronic HBV infection. Hepatology. 2004;39:1736-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cao ZH, Ma LN, Zhang HW, Liu YL, Chen XY. Extended treatment with peginterferon α-2a in combination with lamivudine or adefovir for 96 weeks yields high rates of HBeAg and HBsAg seroconversion. J Dig Dis. 2013;14:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, Tang H, Sheng J, Zhao M. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol. 2014;61:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 9. | Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1113] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 10. | Jilbert AR, Freiman JS, Gowans EJ, Holmes M, Cossart YE, Burrell CJ. Duck hepatitis B virus DNA in liver, spleen, and pancreas: analysis by in situ and Southern blot hybridization. Virology. 1987;158:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Block TM, Guo H, Guo JT. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11:685-706, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Schmitt S, Glebe D, Alving K, Tolle TK, Linder M, Geyer H, Linder D, Peter-Katalinic J, Gerlich WH, Geyer R. Analysis of the pre-S2 N- and O-linked glycans of the M surface protein from human hepatitis B virus. J Biol Chem. 1999;274:11945-11957. [PubMed] |
| 13. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1599] [Article Influence: 123.0] [Reference Citation Analysis (1)] |
| 14. | Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. 2008;134:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Newbold JE, Xin H, Tencza M, Sherman G, Dean J, Bowden S, Locarnini S. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69:3350-3357. [PubMed] |
| 16. | Perlman DH, Berg EA, O’connor PB, Costello CE, Hu J. Reverse transcription-associated dephosphorylation of hepadnavirus nucleocapsids. Proc Natl Acad Sci USA. 2005;102:9020-9025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Wu TT, Coates L, Aldrich CE, Summers J, Mason WS. In hepatocytes infected with duck hepatitis B virus, the template for viral RNA synthesis is amplified by an intracellular pathway. Virology. 1990;175:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, Kao JH, Jia JD, Wedemeyer H. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Allweiss L, Volz T, Giersch K, Petersen J, Lohse AW, Lutgehetmann M, Dandri M. Proliferation of hepatitis B virus infected human hepatocytes in humanized mice treated with the entry inhibitor Myrcludex-B induces strong cccDNA reduction and maintenance of cccDNA-free hepatocytes. Z Gasteroenterol. 2014;52:5-36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Haefeli WE, BlankA , Mikus G, Mier W, Alexandrov A, Urban S. Successful first administration of Myrcludex B, a first-in-class HEPATITIS B and D Virus entry inhibitor, in humans. Hepatology. 2012;56 Suppl:369. |
| 23. | Petersen J, Lutgehetmann M, Volz T and Dandri M. What is the Role of cccDNA in Chronic HBV Infection? Impact on HBV Therapy. Hepatology Rev. 2007;4:9-13. |
| 24. | Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277-4288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Lucifora J, Reisinger F, Xia Y, Bester R, Zhang K, Sprinzl M, Protzer U, Heikenwalder M. Lymphotoxin beta receptor activation leads to degradation of HBV cccDNA from infected hepatocytes. J Hepatol. 2013;58 Suppl 1:S27. [DOI] [Full Text] |
| 26. | Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 738] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 27. | Xia Y, Lucifora J, Reisinger F, Heikenwalder M, Protzer U. Virology. Response to Comment on “Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA”. Science. 2014;344:1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Mao R, Nie H, Cai D, Zhang J, Liu H, Yan R, Cuconati A, Block TM, Guo JT, Guo H. Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog. 2013;9:e1003494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 29. | Bloom K, Ely A, Mussolino C, Cathomen T, Arbuthnot P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol Ther. 2013;21:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Manjunath N, Yi G, Dang Y, Shankar P. Newer gene editing technologies toward HIV gene therapy. Viruses. 2013;5:2748-2766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Schiffer JT, Swan DA, Stone D, Jerome KR. Predictors of hepatitis B cure using gene therapy to deliver DNA cleavage enzymes: a mathematical modeling approach. PLoS Comput Biol. 2013;9:e1003131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Cradick TJ, Keck K, Bradshaw S, Jamieson AC, McCaffrey AP. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol Ther. 2010;18:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, Raimondo G, Levrero M. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc Natl Acad Sci USA. 2009;106:19975-19979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 34. | Allweiss L, Volz T, Giersch K, Lohse AW, Petersen J, Lutgehetmann M, Dandri M. Proliferation of hepatitis B virus infected human hepatocytes induces suppression of viral replication and rapid cccDNA decrease in humanized mice. J Hepatol. 2013;58:S45-S61. [DOI] [Full Text] |
| 35. | Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 541] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 36. | Giladi H, Ketzinel-Gilad M, Rivkin L, Felig Y, Nussbaum O, Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol Ther. 2003;8:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Li GQ, Yu DM, Lu J, Chen SL, Zhao JY, Wang YC. Study of the efficacy of combination therapy of SiRNAs in HepG2.2.15 cells. Hepatogastroenterology. 2011;58:570-574. [PubMed] |
| 39. | Li G, Fu L, Jiang J, Ping Y, Huang Y, Wang Y. siRNA combinations mediate greater suppression of hepatitis B virus replication in mice. Cell Biochem Biophys. 2014;69:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Yuen MF, Kim J, Kim CR, Ngai V, Yuen JC, Min C, Kang HM, Shin BS, Yoo SD, Lai CL. A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir Ther. 2006;11:977-983. [PubMed] |
| 41. | Lai CL, Ahn SH, Lee KS, Um SH, Cho M, Yoon SK, Lee JW, Park NH, Kweon YO, Sohn JH. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut. 2014;63:996-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Jacquard AC, Brunelle MN, Pichoud C, Durantel D, Carrouée-Durantel S, Trepo C, Zoulim F. In vitro characterization of the anti-hepatitis B virus activity and cross-resistance profile of 2’,3’-dideoxy-3’-fluoroguanosine. Antimicrob Agents Chemother. 2006;50:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Michalak TI, Zhang H, Churchill ND, Larsson T, Johansson NG, Oberg B. Profound antiviral effect of oral administration of MIV-210 on chronic hepadnaviral infection in a woodchuck model of hepatitis B. Antimicrob Agents Chemother. 2009;53:3803-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD, Flaherty JF, Lawson E, Zhao S, Subramanian M. Twenty-eight day safety and efficacy of tenofovir alafenamide (TAF) fumarate in chronic hepatis B (CHB) patients. 64th Annual Meeting of the American Association for the Study of Liver Diseases. 2013; Available from: http://www.natap.org/2013/AASLD/AASLD_91.htm. |
| 45. | Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, Zhong L, Ramanathan S, Rhee MS, Fordyce MW. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 47. | Sax PE, Zolopa A, Brar I, Elion R, Ortiz R, Post F, Wang H, Callebaut C, Martin H, Fordyce MW. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2014;67:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 48. | Zlotnick A, Mukhopadhyay S. Virus assembly, allostery and antivirals. Trends Microbiol. 2011;19:14-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 49. | Cho MH, Song JS, Kim HJ, Park SG, Jung G. Structure-based design and biochemical evaluation of sulfanilamide derivatives as hepatitis B virus capsid assembly inhibitors. J Enzyme Inhib Med Chem. 2013;28:916-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Cho MH, Jeong H, Kim YS, Kim J-W, Jung G. 2-Amino-N-(2,6-dichloropyridin-3-yl)acetamide derivatives as a novel class of HBV capsid assembly inhibitor. J Viral Hepat. 2014;12:843-852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Stray SJ, Zlotnick A. BAY 41-4109 has multiple effects on Hepatitis B virus capsid assembly. J Mol Recognit. 2006;19:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Brezillon N, Brunelle MN, Massinet H, Giang E, Lamant C, DaSilva L, Berissi S, Belghiti J, Hannoun L, Puerstinger G. Antiviral activity of Bay 41-4109 on hepatitis B virus in humanized Alb-uPA/SCID mice. PLoS One. 2011;6:e25096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Gruffaz M, Testoni B, Luangsay S, Ait-Goughoulte M, Petit M-A, Ma H, Klumpp K, Javanbakht H, Durantel D, Zoulim F. Hepatitis B core (HBC) protein is a key and very early negative regulator of the interferon response. J Hepatol. 2013;58 Suppl 1:S155-S156. [DOI] [Full Text] |
| 54. | Deres K, Schröder CH, Paessens A, Goldmann S, Hacker HJ, Weber O, Krämer T, Niewöhner U, Pleiss U, Stoltefuss J. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science. 2003;299:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 418] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 55. | Belloni L, Li L, Palumbo GA, Chirapu SR, Calvo L, Finn M, Zlonick A, Levrero M. HAPs Hepatitis B virus (HBV) capsid inhibitors affect core protein interaction with the minichromosome and target cccDNA function. J Hepatol. 2013;58:S153. [DOI] [Full Text] |
| 57. | Campagna MR, Liu F, Mao R, Mills C, Cai D, Guo F, Zhao X, Ye H, Cuconati A, Guo H. Sulfamoylbenzamide derivatives inhibit the assembly of hepatitis B virus nucleocapsids. J Virol. 2013;87:6931-6942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 58. | REPLICor presents clinical efficacy and toxicology results in patients with chronic hepatitis B with short term exposure to immunotherapy after REP 9AC’ induced clearance of serum HBsAg. The 15th annual TIDES meeting; 2013 May 14; Boston, USA. Available from: http://replicor.com/replicor-presents-clinical-efficacy-toxicology-results-patients-chronic-hepatitis-b-short-term-exposure-immunotherapy-rep-9ac-induced-clearance-serum-hbsag/. |
| 59. | Al-Mahtab M, Bazinet M, Vaillant A, Sheikh B. REP 9AC is a potent HBsAg release inhibitor which clears serum HBsAg and elicits SVRS in patients with chronic hepatitis B. J Hepatol. 2011;54 Suppl 1:S34. |
| 60. | Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2255] [Cited by in RCA: 2376] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 61. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508-1517, 1517.e1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 62. | Momeni M, Zainodini N, Bidaki R, Hassanshahi G, Daneshvar H, Khaleghinia M, Ebrahim M, Karimi-Googheri M, Askari A, Arababadi MK. Decreased expression of toll like receptor signaling molecules in chronic HBV infected patients. Hum Immunol. 2014;75:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 63. | Jiang M, Broering R, Trippler M, Poggenpohl L, Fiedler M, Gerken G, Lu M, Schlaak JF. Toll-like receptor-mediated immune responses are attenuated in the presence of high levels of hepatitis B virus surface antigen. J Viral Hepat. 2014;21:860-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 64. | Lopatin U, Wolfgang G, Tumas D, Frey CR, Ohmstede C, Hesselgesser J, Kearney B, Moorehead L, Subramanian GM, McHutchison JG. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral Toll-like receptor 7 agonist. Antivir Ther. 2013;18:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Gane EJ, Sicard E, Gruener D, Lau D, Visvanathan K, Gordon SC, Roberts SK, Kim S, Cheng W, Coffin CS. Safety and Pharmacodynamics of Oral TLR-7 agonist GS-9620 in patients with Chronic Hepatitis B. 2013; Available from: http://liverlearning.aasld.org/aasld/2013/thelivermeeting/35900/doctor.edward.gane.safety.and.pharmacodynamics.of.oral.tlr-7.agonist.gs-9620.html. |
| 66. | Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, Subramanian GM, McHutchison JG. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32:4925-4931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Spellman M, Martin JT. Treatment of chronic hepatitis B infection with DV-601, a therapeutic vaccine. J Hepatol. 2011;54 Suppl:S302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Betancourt AA, Delgado CA, Estévez ZC, Martínez JC, Ríos GV, Aureoles-Roselló SR, Zaldívar RA, Guzmán MA, Baile NF, Reyes PA. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis. 2007;11:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Foundation HB. HBV Drug Watch: Compounds in Development for Chronic Hepatitis B. Available from: http://www.hepb.org/professionals/hbf_drug_watch.htm. |