Published online May 28, 2015. doi: 10.4254/wjh.v7.i9.1184
Peer-review started: September 6, 2014
First decision: September 28, 2014
Revised: January 18, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: May 28, 2015
Processing time: 257 Days and 1.8 Hours
Intermediate stage, or stage B according to Barcelona Clinic Liver Cancer classification, of hepatocellular carcinoma (HCC) comprises a heterogeneous population with different tumor burden and liver function. This heterogeneity is confirmed by the large variability of treatment choice and disease-relate survival. The aim of this review was to highlight the existing evidences regarding this specific topic. In a multidisciplinary evaluation, patients with large (> 5 cm) solitary HCC should be firstly considered for liver resection (LR). When LR is unfeasible, locoregional treatments are evaluable therapeutic options, being transarterial chemoembolization (TACE), the most used procedure. Percutaneous ablation can be an evaluable treatment for large HCC. However, the efficacy of all ablative procedures decrease as tumor size increases over 3 cm. In clinical practice, a combination treatment strategy [TACE or transarterial radioembolization (TARE)-plus percutaneous ablation] is “a priori” preferred in a relevant percentage of these patients. On the other hands, sorafenib is the treatment of choice in patients who are unsuitable to surgery and/or with a contraindication to locoregional treatments. In multifocal HCC, TACE is the first-line treatment. The role of TARE is still undefined. Surgery may have also a role in the treatment of multifocal HCC in selected cases (patients with up to three nodules, multifocal HCC involving 2-3 adjacent liver segments). In some patients with bilobar disease the combination of LR and ablative treatment may be a valuable option. The choice of the best treatment in the patient with intermediate stage HCC should be “patient-tailored” and made by a multidisciplinary team.
Core tip: Intermediate stage, or stage B according to Barcelona Clinic Liver Cancer classification, of hepatocellular carcinoma (HCC) comprises a heterogeneous population with different tumor burden and liver function. This heterogeneity is confirmed by the large variability in treatment and survival, the choice of the best treatment in the patient with intermediate stage HCC is a difficult task. A multisciplinary evaluation of each intermediate stage HCC patient is recommended for planning the best therapeutic strategy and this review was aimed to discuss about the existing evidences regarding this topic. Due to the heterogeneity of intermediate HCC, the use of different therapies (combination treatment) is likely the best choice in most of the cases offering the opportunity of a treatment tailored to the single patient.
- Citation: Di Costanzo GG, Tortora R. Intermediate hepatocellular carcinoma: How to choose the best treatment modality? World J Hepatol 2015; 7(9): 1184-1191
- URL: https://www.wjgnet.com/1948-5182/full/v7/i9/1184.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i9.1184
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the leading cause of death among cirrhotic patients[1,2]. The management of this cancer represents a challenge for physicians being complicated by the coexistence in the same patient of two severe diseases, HCC and cirrhosis. Therefore, in the last two decades several staging and prognostic systems have been proposed to better define the prognosis and the treatment strategy[3-9]. The Barcelona Clinic Liver Cancer (BCLC) classification was first published in 1999 by Llovet et al[6] and is actually the most widely used staging system. The BCLC classification takes into account cancer characteristics (number and size of nodules, macrovascular invasion, and extrahepatic metastasis), cirrhosis related variables (liver function and portal hypertension), and general health status of the patients (performance status). Using these parameters, five distinct HCC stages each associated with different prognosis and specific treatment recommendations are identified. In Western countries, between 20% and 30% of the HCC population at their first observation falls into the stage B and many patients progress to this stage during follow-up. Intermediate stage or stage B, according to BCLC, of HCC includes all Child-Pugh A or B patients, with a performance status 0, and with a single nodule > 5 cm, or multiple nodules > 3 in number or at least one of these > 3 cm, without macrovascular invasion and extrahepatic metastases. According to these criteria, the intermediate stage comprises a heterogeneous population with different tumor burden and liver function. This heterogeneity is confirmed by the large variability in survival among control patients of randomized controlled trials on transarterial chemoembolization (TACE), with a 1-year survival rate ranging from 3% and 75% (median 49.6%-test for heterogeneity, P < 0.0001)[10]. The unique treatment recommended by BCLC group for stage B patients is TACE with a wide range of expected survival, from 14 to 45 mo[11]. Therefore TACE is effective only in a proportion of intermediate patients, the others might likely benefit from other treatments. Due to this heterogeneity, diverse therapies, single or combined, are offered to intermediate patients in field practice. Unfortunately, guidelines to define the best therapeutic approach in the single patient are lacking. The main problem is to distinguish between stage B patients with expected better survival who could have the largest benefit from an aggressive therapeutic approach, and those with poor prognosis in whom treatment should be modulated to offer the best quality and duration of life to the patient. In the attempt to solve this issue, a panel of European experts in 2012 discussed about unresolved questions in the management of stage B patients and proposed a sub-classification into four stages to facilitate treatment decisions[12]. This sub-classification was based on Child-Pugh score, up-to-seven criteria, ECOG (Eastern Cooperative Oncology Group PS performance status), and portal vein thrombosis. The need for a sub-classification of intermediate patients has been claimed also by Asian experts[13] who recently proposed a modification of the European sub-classification using alpha-feto protein to re-classify patients into three modified stages[14]. Further studies are needed before these sub-classifications can be implemented in field practice.
Actually, a multisciplinary evaluation of each intermediate stage HCC patient is recommended for planning the best therapeutic strategy[15,16] and the aim of this review is to discuss about the existing evidences regarding this topic.
According to the BCLC classification, patients with intermediate HCC are unsuitable for liver resection (LR). However, in the last decades advances in surgical technique, preoperative preparation, and postoperative care have expanded LR indications. Nowadays, peri-operative mortality after LR has decreased from 15% to less than 5% in referral centers. To prevent the occurrence of postoperative liver failure, two selection protocols have been proposed based on extimated resection volume and: (1) bilirubin serum level and indocyanine green retention rate at 15 min[17]; and (2) MELD score and serum sodium level[18]. Laparoscopic video-assisted LR is increasingly used as an alternative to the classical open procedure for reducing the risk of postoperative liver deterioration[19]. However, this technique is performed only in few centers and in a restricted proportion of patients due to the stringent selection criteria. In patients with huge cancer masses and poor remnant liver volume after LR, pre-operative percutaneous transhepatic portal vein embolization has been used to increase the size of non-tumorous liver[20,21]. In cirrhotic patients, this procedure may cause severe complications in up to 20% of cases and its use should be carefully evaluated[22].
According to European and American guidelines, TACE is the first line treatment for BCLC B stage patients, but a large variability exists in the protocols, schedule, and indications among centers[23,24]. TACE can be performed with chemotherapeutic agents emulsified with lipiodol followed by embolic agents (conventional transarterial chemoembolization or c-TACE) or with embolic microspheres preloaded with chemotherapeutic agents [Drug Eluting-Beds-TACE (DEB-TACE)]. Main contraindications to TACE are shown in the Table 1. TACE can be scheduled at fixed intervals or “on demands”. Prospective comparative studies between the two schedules are lacking, but this last option is likely more effective reducing the exposure of patients to the toxic effects of the treatment and increasing the compliance. When c-TACE is used, radiological assessment of tumor response must be done with magnetic resonance imaging because computed tomography evaluation is hindered by artifacts caused by lipiodol retention. It is not established how many times TACE can be repeated, but the treatment should be shifted from TACE to sorafenib (stage migration strategy)[11] in patients who have not experienced at least a partial response (according to mRECIST criteria)[25] after two TACE cycles. Furthermore, TACE should be discontinued when a deterioration of the performance status or of the liver function occurr.
| Liver failure |
| Refractory ascites |
| Encephalopathy |
| Bilirubin level > 3 mg/dL |
| Renal failure |
| Creatinine > 2 mg/dL or creatinine clearance < 30 mL/min |
| Coagulopathy |
| Platelet count < 50 × 109/L |
| Prothrombin time < 50% or prolonged > 4 s |
| Portal hypertension |
| Variceal bleeding within past 3 mo |
| Varices at high risk of bleeding |
| Circulatory impairment |
| Main portal venous thrombosis |
| Severely reduced portal flow or hepatofugal blood flow |
| Untreatable arteriovenous fistula |
| Hepatic artery thrombosis |
| Severe atheromatosis |
Transarterial radioembolization (TARE) is a novel treatment using hepatic intra-arterial infusion of radioactive substances such as β-emitting yttrium-90 integral to the glass matrix of microspheres or Iodine-131-labeled lipiodol. Published series showed comparable median survival and toxic effects among patients treated with TACE and TARE, and therefore no defined selection criteria to choose between these techniques have been established so far[11,26-30]. Further studies are needed to evaluate the utility of TARE and its role in the treatment strategy of BCLC B stage patients. However, a widespread use of TARE is limited by its high costs.
Thermal ablation using radiofrequency (RFA), microwave (MWA), or laser (LA), is the most widely employed locoregional treatment for HCC. It achieves a complete ablation rate > 90% in nodules ≤ 3 cm[31-33]. Due to the improvement in devices and techniques, percutaneous ablation has been demonstrated effective also for the treatment of large HCC[34-37]. In these cases, overlapping ablative technique with multiple electrode insertions or simultaneous use of multiple applicators are required to ablate the tumor[38]. This last technique may be more effective because the simultaneous activation of multiple electrodes has a synergistic effect increasing the ablation volume and reducing the procedural time.
The combination treatment strategy, using both transarterial and percutaneous procedures, offers the opportunity of a treatment tailored to the single patient. The occlusion of the hepatic arterial flow supplying the tumor with TACE would theoretically increase the ablation volume after RFA/MWA/LA by reducing the heat loss due to blood flow[39]. Furthermore, the alternate use of intravascular and percutaneous approach allows to increase the time interval between TACE procedures reducing the risk of liver failure caused by cumulating toxic effects. Several studies have evaluated the efficacy of combined locoregional treatments[40-49]. Metanalysis of observational and randomized controlled studies comparing single and combined locoregional treatments showed significant better survival in patients who underwent to combined treatment[50-55].
The combination of TACE and sorafenib has been evaluated in some studies[56-61]. The rationale of sorafenib use is to block vascular endothelial growth factor (VEGF) receptors for counterbalancing the increase in VEGF induced by post-TACE ischemia which facilitates tumor growth and metastasis[62]. It is still unclear if sorafenib potentiate the therapeutic effects of TACE[63]. However, a recent metanalysis including both randomized and retrospective trials showed that TACE-sorafenib combination increased the risk of adverse reactions, but was associated with better overall survival and longer time-to-progression[64].
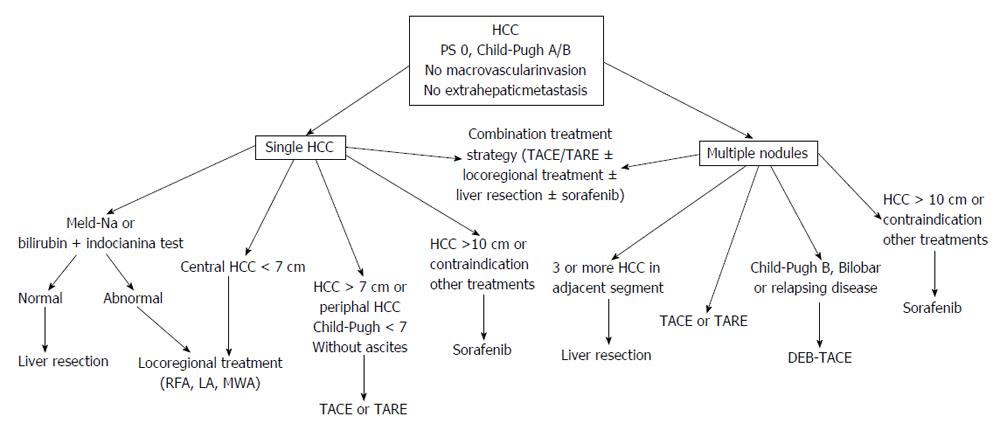
In the setting of a multidisciplinary evaluation, patients with large (> 5 cm) solitary HCC should be firstly considered for LR[65-68]. Radical LR can be considered a valuable option in patients with: (1) peripherically located HCC, < 30% of tissue destroyed as evaluated at imaging, or > 50% compensatory hepatic hypertrophy[65]; (2) no or mild portal hypertension; and (3) no history of liver decompensation.
When LR is unfeasible, locoregional treatments are evaluable therapeutic options and the most used is TACE. Best candidates to TACE are patients with well preserved liver function (Child-Pugh score ≤ 7) and without ascites. Complete HCC necrosis after TACE is seldom observed and local recurrence rates within one year are as high as 60%[69]. Up to now, there are no studies designed to define the maximum tumor size that can be treated. In the two RCTs showing survival benefit of TACE compared to best supportive care, the mean size of HCC was 5-7 cm (range 4-14 cm)[70,71]. In patients with large solitary tumor, TARE may be preferred because there are some evidences of a higher rate of response after TARE as compared to TACE[28].
Percutaneous ablation can be an evaluable treatment for large HCC. However, the efficacy of all ablative procedures decrease as size increases over 3 cm and the probability of obtaining the complete ablation of nodules larger than 7 cm is very low[35,72]. Candidates for percutaneous local ablation are patients with centrally located HCC having a diameter no more than 7 cm, in whom a complete response rate > 80% has been reported[72,73]. In patients with residual peripherical cancer tissue after ablation, the use of TACE increases the rate of complete tumor response[74]. In practice field, a combination treatment strategy (combination of TACE or TARE with percutaneous ablation) is “a priori” preferred in a relevant percentage of these patients.
In patients who are unsuitable to surgery and with contraindication to locoregional treatments or with huge HCC masses (> 10 cm) sorafenib is the treatment of choice. A subanalysis of the SHARP trial has shown that in BCLC B patients sorafenib as compared to placebo increased the median overall survival (14.5 mo vs 11.4 mo, HR = 0.72) and the time-to-progression (6.9 mo vs 4.4 mo, HR = 0.47)[75] (Figure 1).
Most of BCLC B stage patients are affected by multifocal HCC. In these cases, TACE is the first-line treatment. Best candidates are patients with few nodules having a small size: no more than 5 nodules with a size up to 5 cm is likely a good proposal[12]. According to a multicenter European trial, DEB-TACE is more effective than c-TACE in patients classified as Child-Pugh B, and with bi-lobar or relapsing disease, but differences in survival between patients treated with these techniques have not been demonstrated up to now[76-79]. The role of TARE in the management of BCLC B stage patients with multifocal disease is still undefined. However, in some case TARE might be teoretically more safe than TACE as in patients with portal thrombosis because of only minimal embolic effect of microspheres[80]. In field practice, the combined use of transarterial and percutaneous treatment for multifocal HCC is used by many centers and in the position paper of the AISF (Associazione Italiana per lo Studio del Fegato) this approach is recommended as “particularly evaluable”[66]. The use in the same patient of combined locoregional treatments and sorafenib might be theoretically useful, but due to high costs it should be evaluated by a multidisciplinary team.
Surgery may have also a role in the treatment of multifocal HCC in well selected cases[81]. In fact, LR may be a valuable treatment in patients with up to three nodules and multifocal HCC involving 2-3 adjacent liver segments. In some patients with bilobar disease the combination of LR and ablative treatment may be a valuable option. TACE before surgical resection should not be recommended because this strategy offers no benefit[82] (Figure 1).
In selected BCLC B stage patients treatment can be performed with the aim of reducing the tumor burden within Milan criteria. This is the downstaging strategy and patients who have been successfully treated can undergo to liver transplantation with good results[83-85]. The most used treatment for downstaging is TACE[86]. After downstaging treatment, a waiting period of at least 3-6 mo before performing liver transplantation is recommended[87]. During this time, patients should be carefully monitored for tumor response with imaging. The rationale of this strategy is to evaluate tumor biology and risk of recurrence after transplant. In fact, about a third of these patients can be affected by HCC with aggressive biology that can progress during the waiting period and they are not good candidates for transplantation due to the high risk of recurrence[88,89]. Other factors that can indicate a high risk of posttransplant recurrence are AFP serum level above a threshold of 400-1000 ng/mL[80,81,90,91] and poor HCC differentiation at histology[92]. The use in combination with locoregional treatments of systemic targeted therapy with sorafenib may theoretically further increase the rate of tumor control and reduce the recurrences, but appropriately designed studies are needed to confirm it[93].
The choice of the best treatment in the patient with intermediate stage HCC is a difficult task. It should be made by a multidisciplinary team. Due to the heterogeneity of intermediate HCCs, the use of different therapies (combination treatment) is likely the best choice in most of the cases offering the opportunity of a treatment tailored to the single patient.
P- Reviewer: Chetty R, Kabir A S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | World Health Organization. International Agency for Research in Cancer. Globocan 2008: Cancer incidence and mortality worldwide; Available from: http://globocan.iarc.fr. |
| 2. | Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 444] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 3. | Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4:3S-6S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2873] [Article Influence: 110.5] [Reference Citation Analysis (1)] |
| 7. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Leung TW, Tang AM, Zee B, Yu SC, Lai PB, Lau WY, Johnson PJ. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 10. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 11. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4518] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 12. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 13. | Park JW, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver Int. 2013;33:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Wang JH, Kee KM, Lin CY, Hung CH, Chen CH, Lee CM, Lu SN. Validation and modification of a proposed substaging system for patients with intermediate hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Livraghi T, Brambilla G, Carnaghi C, Tommasini MA, Torzilli G. Is it time to reconsider the BCLC/AASLD therapeutic flow-chart? J Surg Oncol. 2010;102:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Piscaglia F, Bolondi L. The intermediate hepatocellular carcinoma stage: Should treatment be expanded? Dig Liver Dis. 2010;42 Suppl 3:S258-S263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22. [PubMed] |
| 18. | Cescon M, Cucchetti A, Grazi GL, Ferrero A, Viganò L, Ercolani G, Zanello M, Ravaioli M, Capussotti L, Pinna AD. Indication of the extent of hepatectomy for hepatocellular carcinoma on cirrhosis by a simple algorithm based on preoperative variables. Arch Surg. 2009;144:57-63; discussion 63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Croome KP, Yamashita MH. Laparoscopic vs open hepatic resection for benign and malignant tumors: An updated meta-analysis. Arch Surg. 2010;145:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [PubMed] |
| 21. | Haghighi KS, Glenn D, Gruenberger T, Morris DL. Extending the limits for curative liver resections by portal vein embolization. Int Surg. 2009;94:43-47. [PubMed] |
| 22. | Ribero D, Curley SA, Imamura H, Madoff DC, Nagorney DM, Ng KK, Donadon M, Vilgrain V, Torzilli G, Roh M. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol. 2008;15:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [PubMed] |
| 24. | Bargellini I, Florio F, Golfieri R, Grosso M, Lauretti DL, Cioni R. Trends in utilization of transarterial treatments for hepatocellular carcinoma: results of a survey by the Italian Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2014;37:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3301] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 26. | Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechêne A, Abdella H, Mueller S, Barakat E, Lauenstein T, Bockisch A. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 29. | Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 30. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 31. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 634] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 32. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 33. | Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694-1702. [PubMed] |
| 34. | Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 286] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [PubMed] |
| 36. | Strickland AD, Clegg PJ, Cronin NJ, Swift B, Festing M, West KP, Robertson GS, Lloyd DM. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2002;89:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Brace CL, Sampson LA, Hinshaw JL, Sandhu N, Lee FT. Radiofrequency ablation: simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol. 2009;20:118-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am J Roentgenol. 2001;177:777-782. [PubMed] |
| 39. | Tsochatzis EA, Fatourou EM, Triantos CK, Burroughs AK. Transarterial therapies for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Tanaka K, Okazaki H, Nakamura S, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, Carrai M, Maltinti G, Capria A, Conte PF. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516-1524. [PubMed] |
| 43. | Akamatsu M, Yoshida H, Obi S, Sato S, Koike Y, Fujishima T, Tateishi R, Imamura M, Hamamura K, Teratani T. Evaluation of transcatheter arterial embolization prior to percutaneous tumor ablation in patients with hepatocellular carcinoma: a randomized controlled trial. Liver Int. 2004;24:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J Gastroenterol. 2005;11:6104-6109. [PubMed] |
| 45. | Liao M, Huang J, Zhang T, Wu H. Transarterial chemoembolization in combination with local therapies for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e68453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Kamada K, Kitamoto M, Aikata H, Kawakami Y, Kono H, Imamura M, Nakanishi T, Chayama K. Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 48. | Tanaka K, Nakamura S, Numata K, Kondo M, Morita K, Kitamura T, Saito S, Kiba T, Okazaki H, Sekihara H. The long term efficacy of combined transcatheter arterial embolization and percutaneous ethanol injection in the treatment of patients with large hepatocellular carcinoma and cirrhosis. Cancer. 1998;82:78-85. [PubMed] |
| 49. | Dettmer A, Kirchhoff TD, Gebel M, Zender L, Malek NP, Panning B, Chavan A, Rosenthal H, Kubicka S, Krusche S. Combination of repeated single-session percutaneous ethanol injection and transarterial chemoembolisation compared to repeated single-session percutaneous ethanol injection in patients with non-resectable hepatocellular carcinoma. World J Gastroenterol. 2006;12:3707-3715. [PubMed] |
| 50. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Yu D, Meyer T, Patch DW, Burroughs AK. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treat Rev. 2006;32:594-606. [PubMed] |
| 51. | Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2012;57:3026-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Wang N, Guan Q, Wang K, Zhu B, Yuan W, Zhao P, Wang X, Zhao Y. TACE combined with PEI versus TACE alone in the treatment of HCC: a meta-analysis. Med Oncol. 2011;28:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: a meta-analysis. Liver Int. 2010;30:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872-3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 56. | Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He CY, Li RJ, Wu KC, Xia JL, Fan DM. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis. 2013;14:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 458] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 58. | Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Muhammad A, Dhamija M, Vidyarthi G, Amodeo D, Boyd W, Miladinovic B, Kumar A. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol. 2013;5:364-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 63. | Weintraub JL, Salem R. Treatment of hepatocellular carcinoma combining sorafenib and transarterial locoregional therapy: state of the science. J Vasc Interv Radiol. 2013;24:1123-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Zhang L, Hu P, Chen X, Bie P. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e100305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Zhang ZM, Guo JX, Zhang ZC, Jiang N, Zhang ZY, Pan LJ. Therapeutic options for intermediate-advanced hepatocellular carcinoma. World J Gastroenterol. 2011;17:1685-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Bolondi L, Cillo U, Colombo M, Craxì A, Farinati F, Giannini EG, Golfieri R, Levrero M, Pinna AD, Piscaglia F. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45:712-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 67. | Régimbeau JM, Farges O, Shen BY, Sauvanet A, Belghiti J. Is surgery for large hepatocellular carcinoma justified? J Hepatol. 1999;31:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57:1258-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 70. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 71. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 72. | Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, Liu GJ, Liang JY, Lau WY. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 74. | Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678. [PubMed] |
| 75. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 653] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 76. | Xie F, Zang J, Guo X, Xu F, Shen R, Yan L, Yang J, He J. Comparison of transcatheter arterial chemoembolization and microsphere embolization for treatment of unresectable hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:455-462. [PubMed] |
| 77. | Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 78. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 79. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 44.0] [Reference Citation Analysis (1)] |
| 80. | Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71-81. [PubMed] |
| 81. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [PubMed] |
| 82. | Yamasaki S, Hasegawa H, Kinoshita H, Furukawa M, Imaoka S, Takasaki K, Kakumoto Y, Saitsu H, Yamada R, Oosaki Y. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206-211. [PubMed] |
| 83. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 84. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 85. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 86. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 87. | Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, Roberts J, Reich DJ, Schwartz ME, Mieles L. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 88. | Millonig G, Graziadei IW, Freund MC, Jaschke W, Stadlmann S, Ladurner R, Margreiter R, Vogel W. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 89. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 90. | Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 91. | Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 92. | Decaens T, Roudot-Thoraval F, Badran H, Wolf P, Durand F, Adam R, Boillot O, Vanlemmens C, Gugenheim J, Dharancy S. Impact of tumour differentiation to select patients before liver transplantation for hepatocellular carcinoma. Liver Int. 2011;31:792-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Gutierrez JA, Gish RG. Efficacy of combination treatment modalities for intermediate and advanced hepatocellular carcinoma: intra-arterial therapies, sorafenib and novel small molecules. Transl Cancer Res. 2013;2:460-471. |