Published online May 18, 2015. doi: 10.4254/wjh.v7.i8.1149
Peer-review started: December 23, 2014
First decision: January 20, 2015
Revised: February 10, 2015
Accepted: April 1, 2015
Article in press: April 7, 2015
Published online: May 18, 2015
Processing time: 150 Days and 11.6 Hours
AIM: To define the normal range of liver stiffness (LS) values using transient elastography in living-related liver transplantation candidate donors with normal liver histology.
METHODS: LS was measured using Fibroscan in 50 (16 women, 34 men) healthy potential donors (mean age 28.4 ± 5.9 years) who were being evaluated for liver donation for their relatives at the National Liver Institute, Menoufeya University, Egypt. All potential donors had normal liver tests and were negative for hepatitis B or C virus infection. Abdominal ultrasounds showed normal findings. None of the subjects had diabetes, hypertension, renal impairment, heart disease, or body mass index > 30 kg/m2. All subjects had normal liver histology upon liver biopsy. They all donated the right lobe of their liver with successful outcomes.
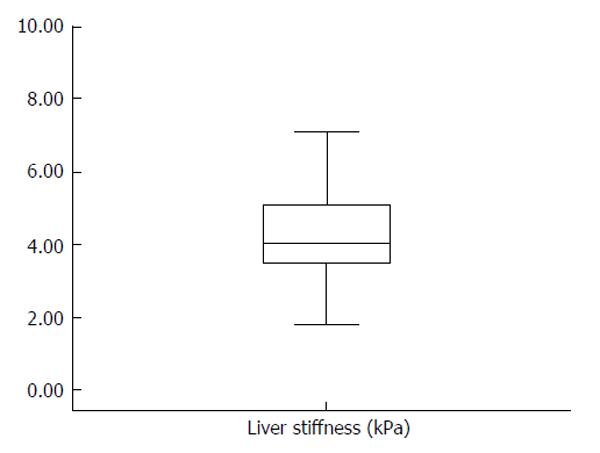
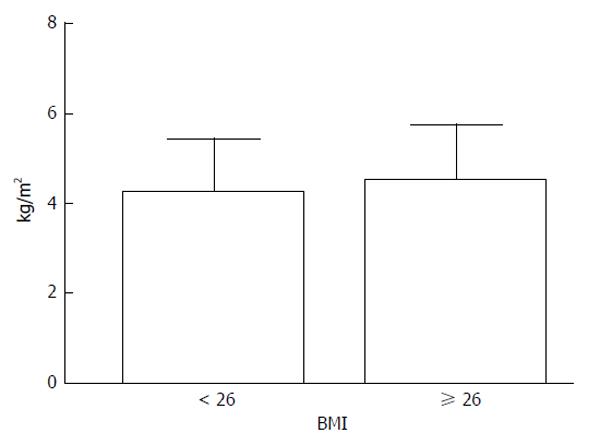
RESULTS: The mean LS was 4.3 ± 1.2 kPa (range: 1.8-7.1 kPa). The 5th and 95th percentiles of normal LS were 2.6 kPa and 6.8 kPa, respectively, with a median of 4 kPa; the interquartile range was 0.6 ± 0.4. LS measurements were not significantly different between men and women (4.4 ± 1.1 kPa vs 3.9 ± 1.3 kPa) and did not correlate with age. However, stiffness values were significantly lower in subjects with a body mass index < 26 kg/m2 compared to those with an index ≥ 26 kg/m2 (4.0 ± 1.1 kPa vs 4.6 ± 1.2 kPa; P <0.05). There were no differences in hospital stay or postoperative bilirubin, albumin,alanine and aspartate transaminases, or creatinine levels (at discharge) between donors with livers stiffness ≤ 4 kPa and those with stiffness > 4 kPa.
CONCLUSION: Healthy donors with normal liver histology have a median LS of 4 kPa. Stiffness values are elevated relative to increase in body mass index.
Core tip: Although some studies have measured liver stiffness by transient elastography in healthy populations, few reports evaluate these with respect to liver biopsy results. This study adds to the knowledge of liver stiffness values in clinically and histologically normal livers of an Arab population, which may form the basis for future clinical practice. The results of this study suggest a new normal level of liver stiffness for this particular population, which differs from other populations reported in the literature.
- Citation: Alsebaey A, Allam N, Alswat K, Waked I. Normal liver stiffness: A study in living donors with normal liver histology. World J Hepatol 2015; 7(8): 1149-1153
- URL: https://www.wjgnet.com/1948-5182/full/v7/i8/1149.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i8.1149
Liver stiffness (LS) measurement (LSM) is a noninvasive method for the evaluation of liver fibrosis, and is used in clinical practice for the diagnosis and follow-up of liver diseases[1,2]. As liver fibrosis may develop slowly in subjects showing persistently normal liver tests, identifying subjects with normal liver histology without fibrosis or undiagnosed histologic changes is of paramount importance in defining the true normal range of LS values. However, most studies to date have focused on LSM in patients with different chronic liver diseases[3-11]. A few European studies have addressed LSM in apparently healthy subjects, though these did not correlate LS with liver histology[12-14]. Hence, the primary aim of this study was to define the normal range of LS values using transient elastography in individuals with normal liver histology as determined by liver biopsy during evaluation as candidate donors for living-related liver transplantation. Furthermore, LS values are examined with respect to age, gender, and body mass index (BMI).
This study involved candidate donors for living-related liver transplantation who passed all stages of evaluation for liver donation for their relatives at the National Liver Institute, Menoufeya University during the period from June 2012 to January 2014. They all had normal liver blood tests and blood pictures, were negative for autoimmune markers and hepatitis B and C virus infection, and had normal abdominal imaging studies. None of the subjects had diabetes, hypertension, renal impairment, heart disease, or BMI > 30 kg/m2. Only the LS of the subjects who had normal histology on liver biopsy were included in analyses.
All subjects provided signed informed consent prior to study enrollment. This study was approved by the Institutional Review Board of the National Liver Institute, Menoufeya University (in 2012), Egypt, and conformed to the ethical guidelines of the 1975 declaration of Helsinki.
LS was measured by transient elastography using a FibroScan machine device (EchoSens, Paris, France) according to a previously described method[15]. The procedure was performed in the morning before obtaining a liver biopsy. The physician performing the procedure was blinded to clinical and biochemical data. The median value of ten successful measurements was recorded as the representative LS value, and is representative of the elastic modulus of the liver[15]. The success rate was calculated as the number of valid measurements divided by the total number of measurements. The interquartile range (IQR) was defined as an index of the intrinsic variability of LSM, corresponding to the interval of LSM results containing 50% of the valid measurements between the 25th and 75th percentiles. The results were considered unreliable if fewer than ten valid readings were obtained, success rate was < 60%, or IQR/LS value was > 30%. LSM failure was recorded when no value was obtained after ten measurements.
Continuous data were compared using the Student’s t-test and categorical data were compared using the Fisher’s exact test. The Mann-Whitney U test was used to compare non-parametric variables. A Pearson’s test was used for correlational analysis. All two-sided P < 0.05 were considered significant. Statistical analyses were performed using SPSS version 17 for Windows (SPSS Inc., Chicago IL, United States). Data are presented as mean ± SD.
A total of 128 healthy subjects underwent liver biopsy for evaluation as potential liver donors for their relatives. Subjects excluded from donation due to histologic changes (n = 20) or with minimal histologic changes (n = 58) that did not prevent donation were not included in this analysis. Fifty individuals between 19 and 42 years of age were finally included in the study. The baseline characteristics of the fifty recruited subjects are shown in Table 1.
| Characteristic | Value |
| Age (yr) | 28.4 ± 5.9 (range: 19-42) |
| Sex (male/female) | 34/16 |
| BMI (kg/m2) | 25.9 ± 2.8 (range: 18-30) |
| Total bilirubin (mg/dL) | 0.6 ± 0.3 |
| ALT (U/L) | 16.8 ± 7.2 |
| AST (U/L) | 18.8 ± 3.9 |
| Albumin (g/dL) | 4.7 ± 0.3 |
| Alkaline phosphatase (U/L) | 76.5 ± 17.7 |
LSM was performed with a 100% success rate. IQR was 0.6 ± 0.4. LS values ranged from 1.8 kPa to 7.1 kPa (Figure 1), with a mean of 4.3 ± 1.2 kPa. The 5th and 95th percentiles of LS were 2.6 kPa and 6.8 kPa, respectively, with a median of 4 kPa.There was no significant difference in LS between men and women (4.4 ± 1.1 kPa vs 3.9 ± 1.3 kPa). Moreover, LS did not correlate with age. Stiffness values were significantly lower in subjects with BMI < 26 kg/m2 than those with BMI ≥ 26 kg/m2 (4 ± 1.07 kPa vs 4.6 ± 1.2 kPa; P < 0.05) (Figure 2).
The donors donated their right liver lobes. The duration of their hospital stay and postoperative bilirubin, albumin, alanine and aspartate transaminase levels, and creatinine results (on discharge) were recorded. Using the median LS value to divide the donors into two groups, there were no significant differences found in any of these measures (Table 2).
| Characteristic | Stiffness < 4 kPa | Stiffness≥4 kPa | P-value |
| (n = 21) | (n = 29) | ||
| Sex (male/female) | 14/7 | 21/8 | 0.58 |
| Age (yr) | 28.70 ± 6.38 | 28.24 ± 5.81 | 0.79 |
| BMI | 25.00 ± 3.34 | 26.42 ± 2.26 | 0.11 |
| Hospital stay (d) | 10.00 ± 2.89 | 10.50 ± 4.40 | 0.80 |
| Bilirubin (mg/dL) | 0.42 ± 0.39 | 0.57 ± 0.46 | 0.60 |
| Albumin (g/dL) | 3.68 ± 0.36 | 3.74 ± 0.22 | 0.75 |
| AST (U/L) | 37.25 ± 27.28 | 39.6 ± 28.99 | 0.90 |
| ALT (U/L) | 57.25 ± 39.43 | 55.6 ± 48.29 | 0.96 |
| Creatinine (mg/dL) | 0.79 ± 0.12 | 0.67 ± 0.09 | 0.05 |
| INR | 1.09 ± 0.14 | 1.04 ± 0.02 | 0.42 |
The possibility of using LSM as a screening tool for liver disease in the general population has been raised[16], but true normal LS values have not been well-identified, especially among various populations. Using the 5th and 95th percentiles from a non-obese population, the present study tentatively estimates a healthy liver stiffness range of 2.6 kPa to 6.8 kPa, with a median stiffness of 4 kPa within an Egyptian population. This is lower than that established by Roulot et al[13] (3.3-7.8 kPa in women and 3.8-8.0 kPa in men). However, in their study, patients with potential liver disease were excluded based only on clinical and laboratory data, and no imaging studies or biopsies were performed. Furthermore, there may have been a selection bias, as their study recruited participants from a free health check, and subjects may have had symptoms that triggered their participation. In contrast, a wider range (2.3-8.8 kPa) was reported in another study conducted in 144 normal Romanian subjects[17]. However, that study comprised a large proportion (about 60%) of subjects that did not receive any laboratory testing or imaging studies, thus their definition of normal was less stringent.
In the present study, normal subjects were selected based on a healthy liver histology, without evidence of fatty liver or fibrosis. Similarly, Kim et al[18] conducted LSM in 12 biopsied healthy donors and reported a lower range of 3.9 kPa to 5.3 kPa. However, their study was in an East-Asian population, with 84.8% of the subjects having a BMI < 25 kg/m2. The present study includes a large proportion (46%) of individuals with a BMI of 27-30 kg/m2, and shows that LS is higher in individuals with a BMI > 26 kg/m2. Importantly, the biopsies did not reveal steatosis, which may influence LSM. Hence, the potential mechanism for the high LS values in healthy subjects with a higher BMI (without histologic changes of steatosis) remains speculative. The increase of LS with BMI was also reported in the study by Roulot et al[13] and Wong et al[19], with higher LS values in subjects with BMI > 30 kg/m2.
Some studies observed higher LS values in healthy men than in women[12,13]. However, consistent with reports by Kim et al[18] and Fung et al[20], this study shows no significant sex effect. However, the lack of significance may be due to the small sample size. There are intrinsic differences between men and women in the density of the extracellular matrix of the liver[21-23], and normal ranges need to be established for each sex in larger studies using the same stringent selection utilized in the present study.
In the current study, age had no significant impact on the LS value. However, the age range is narrow (19-42 years), as older persons are seldom accepted as living liver donors. Sirli et al[17] also found no difference in LS with age within a wider age group (18-69 years), which is consistent with results from other studies in France, South Korea, and India[12,18,24]. On the other hand, a study in a Chinese population demonstrated a decline in median LS in the older age group, from 4.2 kPa in those < 25 years of age to 3.4 kPa for those > 55 years[25].
Although racial differences have not been reported, it is speculated that different cutoff values for normal ranges are needed for various populations[16]. The distribution of body fat varies with race[26-30], and this may affect rates of successful LSM acquisition. This has implications for the normal values used in areas of high ethnic diversity. All previous studies that included biopsies (and reported lower LS values) were performed in the FarEast; Fung et al[20] reported a median LS of 4.6 kPa (all < 7.2 kPa), and Kim et al[18] reported values all < 5.3 kPa. Consequently, the present study is important because it suggests a new normal level of LS for an Arab population, and provides further evidence that normal LS values should be defined for various populations.
Despite having a small sample size, the present study has considerable strengths. The subjects were living-related liver transplantation donors who were extensively evaluated clinically, chemically, radiologically, and histologically, making this the largest reported cohort of histologically normal livers. The healthy condition of the livers in our subjects was further confirmed intraoperatively during and postdonation. Another important aspect to consider is the large range of LS values obtained in studies that did not rely on histology to define normal liver; studies that include liver histology show a narrower range (< 7.2 kPa)[18,20]. A stiff liver is rarely found in the absence of any pathology. Hence, transient elastography may be used to screen the general population and to identify those that require further evaluation. The LS threshold requires further investigation and should take into account the population demographics as well as the likely prevalence of the condition to be screened for.
Liver stiffness (LS) measurement (LSM) is a noninvasive method for the evaluation of liver fibrosis, and is used in clinical practice for the diagnosis and follow-up of liver diseases. Identification of the true normal LS value is an important prerequisite for widespread application of LSM.
Although some studies have investigated LS as measured by transient elastography in healthy populations, few have correlated these values with results of liver biopsy in normal individuals. Therefore, the stiffness of livers with normal histology needs further assessment.
This study adds to the knowledge of LS values in a clinically and histologically normal liver population, which may form the basis for future clinical practice.
Transient elastography may be used in screening the general population and subsequent selection of sub-populations that require further evaluation.
This article presents LS values from healthy livers that were evaluated for living-related liver transplantation in an Arab population. The results are useful in establishing the normal range of LS values in a specific population, which can be used as a reference for further clinical applications.
P- Reviewer: Arai M, Malnick SDH, Savopoulos CG
S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 2. | Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Lédinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Kim BK, Fung J, Yuen MF, Kim SU. Clinical application of liver stiffness measurement using transient elastography in chronic liver disease from longitudinal perspectives. World J Gastroenterol. 2013;19:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1070] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 5. | Scott DR, Levy MT. Liver transient elastography (Fibroscan): a place in the management algorithms of chronic viral hepatitis. Antivir Ther. 2010;15:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 526] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 7. | Hua J, Liu GQ, Bao H, Sheng L, Guo CJ, Li H, Ma X, Shen JL. The role of liver stiffness measurement in the evaluation of liver function and esophageal varices in cirrhotic patients. J Dig Dis. 2015;16:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, Grando-Lemaire V, Trinchet JC, Beaugrand M. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther. 2008;28:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 10. | Kim BK, Kim do Y, Han KH, Park JY, Kim JK, Paik YH, Lee KS, Chon CY, Ahn SH. Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol. 2011;106:1654-1662, 1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Yoshioka K, Hashimoto S, Kawabe N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol Res. 2015;45:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 12. | Corpechot C, El Naggar A, Poupon R. Gender and liver: is the liver stiffness weaker in weaker sex? Hepatology. 2006;44:513-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 14. | Colombo S, Belloli L, Zaccanelli M, Badia E, Jamoletti C, Buonocore M, Del Poggio P. Normal liver stiffness and its determinants in healthy blood donors. Dig Liver Dis. 2011;43:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Cobbold JF, Taylor-Robinson SD. Liver stiffness values in healthy subjects: implications for clinical practice. J Hepatol. 2008;48:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Sirli R, Sporea I, Tudora A, Deleanu A, Popescu A. Transient elastographic evaluation of subjects without known hepatic pathology: does age change the liver stiffness? J Gastrointestin Liver Dis. 2009;18:57-60. [PubMed] |
| 18. | Kim SU, Choi GH, Han WK, Kim BK, Park JY, Kim do Y, Choi JS, Yang SC, Choi EH, Ahn SH. What are ‘true normal’ liver stiffness values using FibroScan?: a prospective study in healthy living liver and kidney donors in South Korea. Liver Int. 2010;30:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Wong GL, Chan HL, Choi PC, Chan AW, Lo AO, Chim AM, Wong VW. Association between anthropometric parameters and measurements of liver stiffness by transient elastography. Clin Gastroenterol Hepatol. 2013;11:295-302.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Fung J, Lai CL, Chan SC, But D, Seto WK, Cheng C, Wong DK, Lo CM, Fan ST, Yuen MF. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol. 2010;105:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Bissell DM. Sex and hepatic fibrosis. Hepatology. 1999;29:988-989. [PubMed] |
| 22. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. [PubMed] |
| 23. | Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, Thabut D, Buffet C, Poynard T. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426-1433. [PubMed] |
| 24. | Kumar M, Sharma P, Garg H, Kumar R, Bhatia V, Sarin SK. Transient elastographic evaluation in adult subjects without overt liver disease: influence of alanine aminotransferase levels. J Gastroenterol Hepatol. 2011;26:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Fung J, Lee CK, Chan M, Seto WK, Wong DK, Lai CL, Yuen MF. Defining normal liver stiffness range in a normal healthy Chinese population without liver disease. PLoS One. 2013;8:e85067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66-74. [PubMed] |
| 27. | Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458-1465. [PubMed] |
| 28. | Eastwood SV, Tillin T, Dehbi HM, Wright A, Forouhi NG, Godsland I, Whincup P, Sattar N, Hughes AD, Chaturvedi N. Ethnic differences in associations between fat deposition and incident diabetes and underlying mechanisms: the SABRE study. Obesity (Silver Spring). 2015;23:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Tillin T, Sattar N, Godsland IF, Hughes AD, Chaturvedi N, Forouhi NG. Ethnicity-specific obesity cut-points in the development of Type 2 diabetes - a prospective study including three ethnic groups in the United Kingdom. Diabet Med. 2015;32:226-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Cameron AJ, Sicree RA, Zimmet PZ, Alberti KG, Tonkin AM, Balkau B, Tuomilehto J, Chitson P, Shaw JE. Cut-points for waist circumference in Europids and South Asians. Obesity (Silver Spring). 2010;18:2039-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |