Published online May 18, 2015. doi: 10.4254/wjh.v7.i8.1020
Peer-review started: September 5, 2014
First decision: November 27, 2014
Revised: January 15, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 18, 2015
Processing time: 256 Days and 16.5 Hours
Hepatocellular carcinoma (HCC) is the sixth most prevalent malignancy worldwide and is a rising cause of cancer related mortality. Risk factors for HCC are well documented and effective surveillance and early diagnosis allow for curative therapies. The majority of HCC appears to be caused by cirrhosis from chronic hepatitis B and hepatitis C virus. Preventive strategies include vaccination programs and anti-viral treatments. Surveillance with ultrasonography detects early stage disease and improves survival rates. Many treatment options exist for individuals with HCC and are determined by stage of presentation. Liver transplantation is offered to patients who are within the Milan criteria and are not candidates for hepatic resection. In patients with advanced stage disease, sorafenib shows some survival benefit.
Core tip: Hepatocellular carcinoma (HCC) is a rising cause of cancer related mortality and viral causes of cirrhosis appear to be a major cause. Surveillance helps to detect early stage disease and treatment options are determined by stage of presentation. Three potentially curative options are radiofrequency ablation, liver transplantation and tumor resection. Emerging therapies such as drug-eluting beads-transarterial chemoembolization or sorafenib will continue to advance treatment options in HCC. The following will provide a concise review of HCC from prevention to treatment.
- Citation: Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol 2015; 7(8): 1020-1029
- URL: https://www.wjgnet.com/1948-5182/full/v7/i8/1020.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i8.1020
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and is the leading cause of mortality in patients with cirrhosis[1]. An estimated half million new cases are diagnosed each year world-wide with disease burden highest in developing countries (85% of all cases)[2,3]. The average age of diagnosis is 65 years with a shift in the last decade toward diagnosis at an earlier age[4]. This trend is especially seen in developing countries and has implications for treatment. Rates of HCC are two to four times higher in men compared to women[5]. Over the past 20 years there has been a 3 fold increase in the number of new HCC cases in the United States (estimated 33190 in 2014)[2,6,7]. The rising incidence of HCC in Western countries appears to correlate with the increasing prevalence of hepatitis C virus (HCV). Currently, the incidence of HCC continues to rise and the 5 year survival rate remains low[7]. Monotherapy agents targeting HCV have made curative therapy in chronic infection possible and may eventually translate into lower rates of HCC. One may presume that despite the high cost of the monotherapy agents, there will be a profound impact on the downstream costs and related complications from chronic HCV and HCC.
Risk factors for HCC are well documented and effective surveillance with early diagnosis allows for curative measures.
Cirrhosis is the most important risk factor for developing HCC and is present in 80% to 90% of individuals[8]. The annual incidence of liver cancer in patients with cirrhosis is 1% to 6 %[8]. Although there exists wide regional variations in distribution and etiology of HCC, chronic hepatitis B virus (HBV) and HCV infection represent the majority of HCC cases worldwide[9]. The highest incidence of HBV is in eastern Asia and sub-Saharan Africa where it accounts for the majority of cases (greater than 50%)[10]. Viral load, duration of infection and rate of replication are related to the incidence of HCC[11,12]. Further, a risk association between HBV and HCC is present in endemic areas where the pattern of transmission is from mother to newborn. Several mechanisms for HBV progression to HCC are proposed. Viral integration into liver cells may cause chromosomal instability and alteration of normal cellular replication resulting in HCC[13,14]. Further, inflammatory and/or necrotic changes from HBV may alter hepatocyte genetic expression or directly induce malignancy[15].
On the other hand, HCC cases in North America, Europe and Japan are highest among HCV infected patients. Annual incidence of HCC is 1% to 4% in patients with HCV related cirrhosis[16,17]. Compared to HCV negative patients, individuals with chronic HCV infection have a 17 times higher risk of developing HCC. In the United States, it is estimated that the incidence of HCV will continue to rise in the following decades[18,19]. It is hypothesized that the primary mechanism for HCC in HCV patients is inflammatory hepatocyte damage from oxidative stress, promoting cirrhosis[20].
Alcohol related liver disease and non-alcoholic fatty liver disease increase the risk of HCC alone or in combination with HBV/HCV. Further, obesity and diabetes are independent risk factors for the development of HCC[21-23]. In patients with chronic viral hepatitis, obesity may synergistically increase the risk of HCC by 100 fold[24]. It has also been elicited that patients with a higher BMI often have a higher rate of mortality[25]. In addition, the number of metabolic syndrome components in a given patient appears to correlate with an increased risk of HCC[26]. As rates of patients diagnosed with metabolic syndrome rise around the world, even a small contribution to the development of HCC would have a devastating impact.
Finally, a number of less common risk factors for HCC include hereditary hemochromatosis, autoimmune hepatitis, glycogen storage diseases, primary biliary cirrhosis, alpha1-antitrypsin deficiency, and Wilson’s disease.
Studies for preventive strategies have centered on viral causes of HCC and minimal data exists on risk reduction for other etiologies. Although vaccination and anti-viral treatment remain the primary means of prevention, counseling patients on dietary modifications, weight loss and tobacco/alcohol cessation remain important steps to address.
The HBV vaccine is effective at preventing HCC and vaccination programs have lowered rates of related malignancy[27]. Over a 10-year period, the Taiwan universal vaccination program reduced the annual incidence of HCC from 0.70 to 0.36 per 100000 children. Thus, one would suspect that initiation of universal vaccination programs in children would have an overall reduction in HCC disease burden in adults. For adults with chronic HBV infection, vaccinations have no role in preventing HCC. Rather, one must focus on anti-viral treatment. Treatment with interferon alpha (IFN-α) reduced the risk of HCC by 6.4% in a meta-analysis of seven studies[28]. Further analysis revealed that the protective effects of IFN-α were limited to patients with cirrhosis[29]. Other treatment options include nucleoside/nucleotide analog treatments and most published data is on lamivudine or adefovir. Treatment with these agents appear to effectively suppress viral replication and decrease the risk of developing HCC[30-32].
Antiviral treatment for HCV may also reduce the risk of HCC. In several studies, treatment by IFN with sustained viral response correlated with a decreased risk of HCC compared to non-responders or no treatment[33,34]. Newer treatment options for HCV with improved viral response rates may effectively reduce progression to HCC.
Practice guidelines recommend standardized surveillance programs for HCC with decision analysis models showing that surveillance improves survival and is cost effective if the annual rate of HCC exceeds 1.5% in a given population[35,36]. Diagnosis at an early stage of HCC confers a survival benefit compared to patients diagnosed with advanced disease[37]. Curative treatment options such as liver transplantation available in early stage disease likely contribute to this survival benefit.
Hepatic ultrasound and alpha-fetoprotein (AFP) have historically played a prominent role in HCC surveillance. A randomized controlled trial of 18861 participants assessed the effect of screening on HCC mortality. All study participants had HBV and were divided into 2 groups: patients who underwent screening with ultrasound every 6 mo and AFP compared with no surveillance. Surveillance was associated with a 37% reduction in HCC mortality, despite sub-optimal adherence to surveillance (< 60%)[38].
For over 40 years AFP has been used in the detection of HCC with variable sensitivity (39% to 65%), specificity (76% to 94%) and positive predictive value (9% to 50%)[39-43]. Results from several studies have challenged the utility of AFP in screening. A randomized controlled trial of 5581 HBV patients showed that AFP bi-annual screening improved detection rates of HCC but earlier detection did not translate to decreased mortality[44]. Concurrent AFP and ultrasound testing increased false positive rates and led to unnecessary diagnostic testing. Further, data suggest that for lesions less than 2 cm in diameter, AFP will rarely be elevated[41,45,46]. An inherent disadvantage of AFP is that it can be elevated in chronic hepatitis even without HCC, resulting in low specificity. Current AASLD guidelines do not recommend AFP for screening or diagnostic purposes. Research into novel biomarkers for early HCC detection continue. As more sensitive assays such as AFP-L3 are developed, the role of serology for surveillance maybe re-analyzed[47].
The ideal modality for HCC screening remains an area of controversy. Although the recommended method of surveillance is liver ultrasonography, diagnosis by this modality remains operator and equipment dependent (sensitivity of 65% and specificity of 90%)[45]. Older studies have shown ultrasonography to be equivalent to computed tomography (CT) in detecting hepatic lesions[48,49]. But more recently, research into CT and magnetic resonance imaging (MRI) for HCC screening have yielded promising results in lesions greater than 2 cm[50]. Prospective trials are needed before CT or MRI can replace ultrasonography as the primary screening method for HCC. Specifically cost effectiveness, cumulative radiation exposure and mortality benefit will need to be addressed.
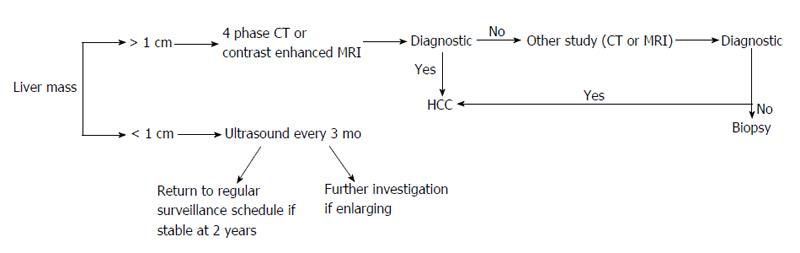
The 6 mo interval length for screening is based on tumor doubling time and is not dictated by risk factors for HCC. A shorter 3 mo interval increased small nodule detection without affecting survival rates[51], while longer periods between screening (12 mo) showed an increased rate of advanced tumors[52]. Once a lesion has been detected, the size of the lesion determines the next step. Hepatic nodules less than 1 cm should be followed with repeat ultrasonography every 3 mo. If the lesion is stable over 2 years then a return to routine 6 mo surveillance is acceptable[53]. Liver lesions exceeding 1 cm warrant further evaluation as described below.
Definitive diagnosis via non-invasive testing includes four-phase multidetector CT (unenhanced, arterial, venous and delayed) or dynamic contrast enhanced MRI. The presence of arterial hyper-enhancement with a venous or delayed phase washout of contrast medium, confirms a diagnosis of HCC[35]. While MRI provides superior contrast resolution compared to CT, metallic implants, respiratory artifact, significant ascites, cost and availability all limit its use. Patients with atypical features for HCC either on CT or MRI should undergo the other imaging modality or lesion biopsy. Individuals with discordant CT/MRI findings or hepatic lesions without cirrhosis should also receive a liver biopsy. The imaging modalities above are valid for patients with cirrhosis or chronic HBV without cirrhosis. Contrast enhanced ultrasonography should not be used for diagnostic purposes as it lacks specificity for HCC[16]. Unfortunately, biopsies also carry a high false negative rate (up to 30%) - attributed to inadequate sampling[54]. Despite a negative biopsy, surveillance of the lesion at 3 to 6 mo intervals for changes characteristic for HCC or for lesion enlargement should be completed[16]. Lesions less than 1 cm are difficult to assess even with the combination of imaging and biopsy (Figure 1).
Several treatment options exist for patients with HCC and can be categorized as curative or palliative. The three potentially curative options are radiofrequency ablation, liver transplantation, or tumor resection. Given the heterogeneity of HCC and complexity of treatment options patients are optimally managed by a multi-disciplinary team. The best therapy is determined based on the stage of presentation. The barcelona clinic liver cancer staging system, developed in 1999, is a common means to assess prognosis and select appropriate therapy for HCC[55]. In general, surgical resection or liver transplantation is the first line treatment option for early stage HCC; whereas asymptomatic patients with intermediate stage disease benefit from chemoembolization. Patients with end stage HCC or extensive extrahepatic disease often have a less than 3 mo rate of survival. In these individuals, pain and symptom control to improve quality of life should be the primary focus[35].
Other staging systems such as Cancer of Liver Italian Program, Okuda stage, French staging system have been validated to a lesser extent. Biomarkers such as vascular endothelial growth factors may have prognostic value in the future[56].
Surgical resection is the therapy of choice in early stage HCC without cirrhosis or in the absence of portal hypertension. Selection criteria have been refined over the years and include individuals with a tumor size less than 3 cm in diameter, normal bilirubin and absence of portal hypertension. In patients without cirrhosis, a 60% to 75% five year survival rate can be achieved[57,58]. Hepatic function evaluated by Model for End Stage Liver Disease (MELD) or Child-Pugh correlate with survival following resection. As expected, patients in Child-Pugh A classification have an improved survival rate following resection compared with those in class B or C[59,60]. In the United States only 5% of individuals will qualify for resection, while in Asia younger age of presentation allows 40% of patients to qualify for surgical resection[61]. Laproscopic liver resection accounts for 10%-20% of procedures in the United States and minimize postoperative morbidity compared to open resection. Patients with multiple intra-hepatic tumors are not ideal candidates for resection as this often represents intrahepatic metastasis[62]. Although technically feasible in some patients, multiple hepatic lesion resection must be reviewed on a case by case basis[63,64]. Further, vascular invasion significantly reduces the five year survival rate from around 50% to 10%[65]. Individuals with a MELD score greater than 9 have a high mortality rate after resection and alternative therapies should be considered[66].
Unfortunately, hepatic resection does not alter the course of underlying cirrhosis. At 2 years, 43% to 65% of patients will have a recurrent tumor and by 5 years post-resection 70% will have recurrent HCC[67,68]. Pre-operative predictors of recurrent free survival include: Child-Pugh class, hepatic function, degree of fibrosis, total serum bilirubin, platelet count, portal hypertension, micro/macroscopic vascular invasion and tumor burden (number and size)[69]. A case by case selection for patients with cirrhosis is essential to limit complications and mortality. Operative mortality ranges from 4% to 4.7% for resection with the majority of deaths likely in patients with underlying cirrhosis and large tumor burden[70]. As newer treatment options for HCV are developed, treatment of underlying cirrhosis after resection may alter/delay the development of recurrent HCC.
Liver transplantation offers a potential cure of HCC as it treats the malignancy and the underlying cirrhosis. Given the scarcity of livers available for transplantation, one must carefully select patients to optimize outcomes.
Patients with HCC complicated by cirrhosis and/or portal hypertension should be evaluated for liver transplantation as it carries the lowest rate of tumor recurrence. Traditionally 3 scoring criteria are utilized to determine eligibility [Milan Criteria, University of California San Francisco (UCSF)] and prioritize patients for transplant MELD. The Milan Criteria considers patients eligible for liver transplantation if they present with a single nodule less than 5 cm in diameter or 3 nodules with each less than 3 cm, without evidence of distant metastasis or vascular invasion. With the initial trial showing a 4 year survival rate of 75% and results verified in further studies, organ allocation societies including united network for organ sharing have adopted this criteria[71-73]. Recurrent free survival for patients meeting Milan criteria is 90% with a 4 year overall survival rate of 85%[71]. In contrast patients exceeding criteria parameters have a respective 59% and 50% rate of survival[71]. The UCSF criteria proposed in 2001 expands the eligibility requirements set forth by the Milan criteria to include more patients with HCC. This criteria included individuals with a single tumor less than 6.5 cm or those with 3 nodules less than 4.5 cm (total diameter of no more than 8 cm). Experience with the UCSF criteria has shown similar survival rates compared to the Milan criteria[74,75]. Unfortunately, the paucity of organs available for transplant remains a major obstacle.
Liver allocation is prioritized by the MELD score. All HCC patients have an adjusted MELD score of 22 with increases at each 3 mo interval. Prioritized allocation with MELD score adjustment has increased the number of HCC patients undergoing liver transplantation.
Chemical (ethanol, acetic acid) or thermal ablation [radiofrequency ablation (RFA), microwave, laser, cryoablation] are also used to treat HCC. Historically, percutaneous ethanol injection (PEI) had been used to induce cellular dehydration/necrosis in small HCC tumors. RFA has largely replaced PEI as studies have shown higher rates of complete response with fewer number of treatment sessions[76-78]. RFA is superior to PEI in large and small lesions, although the benefit of using RFA is more pronounced in tumors larger than 2 cm in diameter[79]. Combination RFA and PEI for high risk lesions is an area of ongoing research with promising results[80].
In cases of early stage HCC where surgical resection or liver transplantation are not feasible, RFA is a minimally invasive approach to local ablation. Therapeutic effects are a result of thermal tumor necrosis, parenchymal and protein destruction[81]. Overall complication rates for RFA are low and are minimized when performed by an experienced physician[82]. Efficacy of RFA is limited by tumor size and location, with a less than fifty percent rate of ablation in tumors larger than 5 cm[83]. RFA is also discouraged in large lesions as the risk of side effects may outweigh benefits[81]. Further, therapy near large vessels may not achieve adequate temperature for coagulative necrosis[84]. Tumors adjacent to intestine or large bile ducts may also preclude RFA.
Rate of recurrence for RFA is higher compared to surgical resection. For large and small tumors, RFA was associated with a significantly lower survival rate compared to surgical resection[85,86]. Thus investigating RFA as a bridge to surgical intervention is logically area of research. Several retrospective studies have shown that pre-transplant RFA delays tumor progression and extends time on the liver transplant list[87-90]. As a major limitation remains the number of organs available for transplant it remains unclear whether the extended time on the liver transplantation list will translate into improved clinical outcomes. Currently guidelines from AASLD support the use of RFA as a bridge to liver transplantation (level II evidence), although the exact role of bridging therapies has not been defined[35].
Blood supply to HCC tumors are mainly from the hepatic artery. Transarterial chemoembolization (TACE) is the selective occlusion of the blood supply to the tumor with synergistic local distribution of chemotherapy and radioactive substances. The hypervascularity of HCC allows for this targeted therapy, minimizing side effects. The choice of chemotherapeutic agent is not standardized and may include agents such as doxorubicin, cisplatin or epirubicin.
For patients who are not candidates for liver transplantation or resection with tumors too large for local ablation, TACE is effective salvage therapy. Other criteria for treatment include: preserved liver function and no evidence of extrahepatic metastasis or vascular invasion. Approximately 35%-40% of patients will achieve a 25% decrease in tumor size with response rates as high as 60% when surrogate markers for response are utilized[91-93]. A meta-analysis of six randomized controlled trials showed that patients who underwent TACE had a 2-year improved survival rate compared to those who only had supportive therapy[93]. Interestingly, a meta-analysis of nine trials did not show a significant difference in survival based on chemotherapeutic agent used in TACE treatments[94]. Growing literature supports the efficacy of TACE for HCC down-staging and bridging. The first study to use TACE prior to liver transplantation was published in 1997 and showed successful down-staging of tumors greater than 3 cm with a significant improvement in 5-year survival compared to no TACE[95]. More recent studies show that 22% to 70% of patients were successfully downstaged with a 2-year post-transplant survival rate of 81%, and among advanced stage HCC (III/IV) patients a median survival of 20 mo[96-101]. Based on response to therapy, repeat TACE treatments can be scheduled. More intense therapies may be associated with increased risk of acute hepatic decompensation and should be weighed against the potential gains from therapy[91]. Transarterial radioembolization (TARE), a method of delivering internal radiation to the neoplasm using Yttrium 90, represents an alternative to TACE in intermediate stage HCC[102]. This modality of treatment is indicated in patients with portal vein thrombosis where conventional TACE is contraindicated. Survival and response rates for TARE were comparable to TACE while a low side effect profile allows for treatment to be completed in the outpatient setting[103,104].
Novel modalities such as drug-eluting beads-TACE (DEB-TACE) are being investigated in the non-transplant and as neo-adjuvant therapy in patients awaiting transplant. The drug-eluting beads appear to enhance medication delivery and reduce side effects by gradually releasing chemotherapy agents. The PRECISION trial compared non-transplant HCC patients who received DEB-TACE vs TACE. Sub-group analysis revealed a significantly lower hepatic/cardiac toxicity profile in the DEB-TACE group[105]. A small retrospective analysis in transplant patients also showed that DEB-TACE had improved rates of response with minimal adverse effects compared to embolization alone[106].
Systemic therapies for the management of patients with HCC continue to be researched. Cytologic agents such as tamoxifen, doxorubicin, everolimus and thalidomide have shown marginal success. Targeted molecular therapies such as bevacizumab, brivanib, erlotinib may be alternatives to conventional cytologic agents. To date, sorafenib is the only systemic therapy effective for treating advanced stage HCC. Sorafenib is an oral tyrosine kinase inhibitor with anti-angiogenic activity, and now is the standard of care in treating individuals with advanced stage HCC and Child’s A cirrhosis[107,108]. Patients with minimal tumor related symptoms, vascular invasion and extrahepatic spread are considered ideal for treatment. Clinical experience has shown significant delay in tumor proliferation and angiogenesis with sorafenib therapy. Those with decompensated cirrhosis or those with a less than 3 mo life expectancy should not receive sorafenib. Adverse events include diarrhea, hand foot skin reaction, and fatigue and dose reduction achieves tolerance in most patients.
The Sorafenib HCC Assessment Randomized Protocol was a multi-center double-blinded controlled phase III trial that demonstrated a 31% decrease in risk of death with a median 3 mo delay in radiologic progression of disease in patients prescribed sorafenib[108]. Further, the Global Investigation of Therapeutic Decisions in HCC which included a heterogeneous population of unresectable HCC patients showed that sorafenib was generally well tolerated in the clinical setting[109]. The role of sorafenib in treating early stage HCC and as neo-adjuvant therapy prior to liver transplantation is evolving. In pre-transplant patients, sorafenib combined with TACE may inhibit angiogenesis and induce tumor necrosis[110]. Other targeted molecular therapies beyond sorafenib continue to be researched and may represent second line agents for patients that fail or are unable to tolerate sorafenib.
HCC is a common cause of malignancy world-wide. Emphasis should be placed on surveillance and early diagnosis. Treatment of HCC has changed significantly over the past few decades with curative options such as liver transplantation, hepatic resection and radiofrequency ablation now available. Further, novel therapies such as DEB-TACE or sorafenib will continue to be areas of research. Despite these advances, there remains much to be learned about HCC. Research into effective prevention and factors that may mitigate malignant transformation should be further explored.
P- Reviewer: Colagrande S S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | American Cancer Society: Cancer Facts and Figures 2014. Atlanta, Ga: American Cancer Society, 2014. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. |
| 3. | World Health Organization IAfRoC. GLOBOCAN 2012. Available from: http://globocan.iarc.fr/Default.aspx. |
| 4. | Rosenblatt KA, Weiss NS, Schwartz SM. Liver cancer in Asian migrants to the United States and their descendants. Cancer Causes Control. 1996;7:345-350. [PubMed] |
| 5. | Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations), National Cancer Institute. 2012; Available from: http://seer.cancer.gov/csr/1975_2009_pops09/. |
| 6. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 7. | Surveillance , Epidemiology , and End Results Program. SEER - Stat database: incidence - SEER 9 Regs research data. Bethesda, MD: National Cancer Institute. Available from: http://www.seer.cancer.gov/. |
| 8. | Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47-53. [PubMed] |
| 9. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4265] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 10. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 11. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2365] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 12. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 915] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 13. | Brechot C, Pourcel C, Louise A, Rain B, Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980;286:533-535. [PubMed] |
| 14. | Bréchot C. Hepatitis B virus (HBV) and hepatocellular carcinoma. HBV DNA status and its implications. J Hepatol. 1987;4:269-279. [PubMed] |
| 15. | Rossner MT. Review: hepatitis B virus X-gene product: a promiscuous transcriptional activator. J Med Virol. 1992;36:101-117. [PubMed] |
| 16. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 17. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [PubMed] |
| 18. | Tanaka Y, Kurbanov F, Mano S, Orito E, Vargas V, Esteban JI, Yuen MF, Lai CL, Kramvis A, Kew MC. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology. 2006;130:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 666] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 20. | Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297-306. [PubMed] |
| 21. | Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 22. | Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, McGlynn KA. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 23. | Lagiou P, Kuper H, Stuver SO, Tzonou A, Trichopoulos D, Adami HO. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:1096-1099. [PubMed] |
| 24. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 25. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 26. | Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, Izzo F, La Vecchia C, Boffetta P, Montella M. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1196] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 28. | Cammà C, Giunta M, Andreone P, Craxì A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol. 2001;34:593-602. [PubMed] |
| 29. | Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 30. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 31. | Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295-1303. [PubMed] |
| 32. | Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Miyake Y, Iwasaki Y, Yamamoto K. Meta-analysis: reduced incidence of hepatocellular carcinoma in patients not responding to interferon therapy of chronic hepatitis C. Int J Cancer. 2010;127:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [PubMed] |
| 35. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 36. | Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 38. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 945] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 39. | Alpert ME, Uriel J, de Nechaud B. Alpha-1 fetoglobulin in the diagnosis of human hepatoma. N Engl J Med. 1968;278:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 170] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432-438. [PubMed] |
| 41. | Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [PubMed] |
| 42. | Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553-559. [PubMed] |
| 44. | Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 45. | Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 573] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 46. | Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108-110. [PubMed] |
| 47. | Wu CS, Lee TY, Chou RH, Yen CJ, Huang WC, Wu CY, Yu YL. Development of a highly sensitive glycan microarray for quantifying AFP-L3 for early prediction of hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014;9:e99959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336. [PubMed] |
| 50. | Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, Lu DS. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 51. | Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, Roulot D, Mallat A, Hillaire S, Cales P. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 52. | Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnù L, Farinati F, Zoli M. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 53. | Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1145] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 54. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (1)] |
| 55. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 56. | Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009;100:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 59. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 60. | Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka Y, Regimbeau JM, Nagorney DM, Do KA, Ellis LM. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722-730; discussion 730-731. [PubMed] |
| 61. | Bismuth HMP. Hepatobiliary surgery. J Hepatol. 2000;32:208-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Ng IO, Guan XY, Poon RT, Fan ST, Lee JM. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol. 2003;199:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, Cicorelli A, Tumino E, Federici G, Cioni R. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 719] [Article Influence: 39.9] [Reference Citation Analysis (1)] |
| 65. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 66. | Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207-1215; discussion 1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 67. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 68. | Marín-Hargreaves G, Azoulay D, Bismuth H. Hepatocellular carcinoma: surgical indications and results. Crit Rev Oncol Hematol. 2003;47:13-27. [PubMed] |
| 69. | Chen WT, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Wu CW. Recurrent hepatocellular carcinoma after hepatic resection: prognostic factors and long-term outcome. Eur J Surg Oncol. 2004;30:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Kianmanesh R, Regimbeau JM, Belghiti J. Selective approach to major hepatic resection for hepatocellular carcinoma in chronic liver disease. Surg Oncol Clin N Am. 2003;12:51-63. [PubMed] |
| 71. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5311] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 72. | Dutkowski P, De Rougemont O, Müllhaupt B, Clavien PA. Current and future trends in liver transplantation in Europe. Gastroenterology. 2010;138:802-9.e1-802-9.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, Magee JC, Lok AS, Fontana RJ, Marrero JA. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 74. | Leung JY, Zhu AX, Gordon FD, Pratt DS, Mithoefer A, Garrigan K, Terella A, Hertl M, Cosimi AB, Chung RT. Liver transplantation outcomes for early-stage hepatocellular carcinoma: results of a multicenter study. Liver Transpl. 2004;10:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Fernández JA, Robles R, Marin C, Sánchez-Bueno F, Ramirez P, Pons JA, Garre MC, Pérez D, Parrilla A, Navalón JC. Can we expand the indications for liver transplantation among hepatocellular carcinoma patients with increased tumor size? Transplant Proc. 2003;35:1818-1820. [PubMed] |
| 76. | Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 77. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [PubMed] |
| 78. | Shen A, Zhang H, Tang C, Chen Y, Wang Y, Zhang C, Wu Z. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol. 2013;28:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 79. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 80. | Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol. 2008;190:W187-W195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6:14-23. [PubMed] |
| 82. | Koda M283 patients. 346 treated nodules in 13 , Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, Shibata H, Shimoe T, Matsuda T, Toshikuni N, Fujioka S, Ohmoto K, Nakamura S, Kariyama K, Aikata H, Kobayashi Y, Tsutsui A. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: An analysis of 16. Hepatol Res. 2012;42:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422-426; discussion 427. [PubMed] |
| 84. | Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 85. | Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, Yang J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 86. | Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, Nitta H, Ishiko T, Takamori H, Baba H. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, Rudich S, McClure LA, Arenas J. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, Covino M, Ravaioli M, Fagiuoli S, Gasbarrini G. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 89. | Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 90. | DuBay DA, Sandroussi C, Kachura JR, Ho CS, Beecroft JR, Vollmer CM, Ghanekar A, Guba M, Cattral MS, McGilvray ID. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford). 2011;13:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 92. | Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 391] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 93. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 94. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 95. | Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688-701; discussion 701-703. [PubMed] |
| 96. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 97. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 98. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 99. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 100. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [PubMed] |
| 101. | De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 102. | Andreana L, Isgrò G, Marelli L, Davies N, Yu D, Navalkissoor S, Burroughs AK. Treatment of hepatocellular carcinoma (HCC) by intra-arterial infusion of radio-emitter compounds: trans-arterial radio-embolisation of HCC. Cancer Treat Rev. 2012;38:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 104. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 105. | Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562-W570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 106. | Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 107. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4650] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 108. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 109. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 110. | Takada Y, Ueda M, Ito T, Sakamoto S, Haga H, Maetani Y, Ogawa K, Kasahara M, Oike F, Egawa H. Living donor liver transplantation as a second-line therapeutic strategy for patients with hepatocellular carcinoma. Liver Transpl. 2006;12:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |