Published online May 8, 2015. doi: 10.4254/wjh.v7.i7.1007
Peer-review started: December 17, 2014
First decision: January 8, 2015
Revised: January 26, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: May 8, 2015
Processing time: 147 Days and 22.7 Hours
The clinical manifestations of hyperammonemia are usually easily identifiable to the clinician when associated with liver disease and lead to prompt diagnosis and treatment. However, hyperammonemia-induced encephalopathy is rare in adults in the absence of overt liver disease, thus diagnosis is often delayed or missed leading to potentially life threatening complications. Without proper treatment, such patients can decompensate rapidly with poor outcomes including seizures, coma, and death. Early assessment of plasma ammonia levels in patients with normal hepatic function and characteristic symptoms of encephalopathy can lead to early intervention while investigating the underlying etiology. We describe a patient who presented with a 2-year progression of waxing and waning acute mental status changes after a Roux-en-Y gastric bypass surgery. He was found to have elevated ammonia level as well as orotic aciduria; results consistent with a urea cycle disorder. After consulting neurology as well as toxicology, he ultimately improved after dietary protein restriction, sodium benzoate and lactulose therapy. While rare, clinicians should have a high index of suspicion for late onset urea cycle disorders in symptomatic patients presenting with encephalopathy secondary to hyperammonemia.
Core tip: Encephalopathy secondary to hyperammonemia in the absence of hepatic dysfunction presents a diagnostic dilemma to many clinicians. As such, early and accurate diagnosis can be easily missed, leading to increased morbidity and mortality. We describe a case of adult onset urea cycle disorder presenting with encephalopathy after gastric bypass surgery. Although this challenging diagnosis is rare, treatment is inexpensive and readily available. Thus early recognition and intervention can prevent the rapid decline that may occur if the diagnosis is unrecognized.
- Citation: Kromas ML, Mousa OY, John S. Hyperammonemia-induced encephalopathy: A rare devastating complication of bariatric surgery. World J Hepatol 2015; 7(7): 1007-1011
- URL: https://www.wjgnet.com/1948-5182/full/v7/i7/1007.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i7.1007
Obesity is a prevalent and challenging issue in healthcare, affecting more than 60% of the United States population and contributing to a myriad of other comorbidities. Gastric bypass surgery (GBS) has been offered as an effective treatment for morbid obesity with reported success rates of weight loss up to 60%-70% of excess body weight[1,2]. However, GBS can be complicated by nutritional derangements and rare neurological manifestations, including encephalopathy[3,4]. Carnitine deficiency has been implicated in hyperammonemia-induced encephalopathy in the setting of valproic acid use as well as GBS[5-8]. Case reports have also unmasked ornithine transcarbamylase (OTC) deficiency in the setting of GBS[9]. Our case presents hyperammonemic encephalopathy following GBS related to an underlying late onset urea cycle disorder (UCD).
The disposal of nitrogen in the body is based on ammonia conversion to urea. Complete or partial enzyme deficiencies in the process of ammonia conversion leads to UCD. Classical presentation of such disorders occurs in neonates who can develop neurological manifestations and fail to thrive. Interestingly, some patients with similar manifestations presented in adulthood. The mechanism was either related to partially deficient enzymes of the urea cycle (UC) or enzyme mutation that is provoked by a certain stressor.
A 56-year-old male patient was hospitalized with worsening neurological symptoms including tremors, confusion, ataxia, and labile emotions. He reported his recurrent neurologic symptoms that progressed over a 2-year period to his primary care physician. His symptoms started mildly within 2 mo of a Roux-en-Y GBS, which the patient underwent for morbid obesity without comorbidities. He was otherwise healthy with no previous substance abuse or exposure to toxins. Of note he had lost 20 pounds prior to the surgery with proper nutritional guidance and 108 pounds post-operatively. He sought multiple medical subspecialties for advice and was diagnosed with Parkinson’s disease. He was treated with levodopa and he followed with a neurologist without improvement. His disabling symptoms continued to progress, mandating referral to the emergency room. On physical examination he had tremors, ataxia, poor concentration and impaired memory. He developed general weakness that made him wheelchair-bound and dependent. Laboratory investigation and appropriate imaging studies reflected a UCD (Table 1). We managed our patient conservatively through the administration of lactulose and sodium benzoate as well as dietary modification, by providing foods low in protein. The symptoms of our patient improved gradually during his hospitalization. He specifically had resolution of confusion, ataxia, and tremors over a course of 1 wk following treatment initiation.
| Laboratory investigation/imaging | Values |
| BMP | Within normal limits (Cr 1.0 mg/dL) |
| CBC | Within normal limits (platelets 158 K/μL) |
| Iron panel | Within normal limits |
| Hepatic panel | AST 30 U/L |
| ALT 9 U/L | |
| Alkaline phosphatase 52 U/L | |
| Total Bilirubin 0.9 mg/dL | |
| Urine orotic acid | 1.4 mmol/mol of creatinine |
| Ammonia level | 155 mcg/dL |
| Carnitine level, zinc, manganese, vitamin B12, vitamin A, vitamin D | Within normal limits |
| Abdominal ultrasound | No evidence of liver disease or cirrhosis |
| MRI brain | No acute or chronic intracranial abnormalities |
Ammonia is a usual component of the body fluids, which exists mainly as ammonium ion. Excess ammonia from the products of protein catabolism enters the UC in the liver for conversion into urea, prior to renal excretion[10,11]. Abnormalities in this process can lead to hyperammonemia, which increases the entry of ammonia to the brain and leads to neurological disorders. This can be due to impaired hepatic function and portal hypertension, where an excess nitrogen load over saturates the capacity of liver metabolism and bypasses it via portosystemic shunting[12]. Other causes of hyperammonemia include congenital UCD, Reye syndrome, as well as encephalopathies of metabolic or toxic natures. Hyperammonemia can be toxic with signs and symptoms that include: episodic irritability, vomiting, ataxia, mental retardation, and lethargy that can progress to alteration of consciousness and coma[13,14].
Both acute and chronic hyperammonemia alters the brain neurotransmitter system. Acute hyperammonemia causes accumulation of glutamate extracellularly in the brain, which activates the N-methyl D-aspartate receptor, causing seizures. Chronic hyperammonemia leads to an increase in inhibitory neurotransmission via down regulation of glutamate receptors and increased GABAergic tone, causing deterioration of cognitive function and coma. Most cases of hyperammonemia in the pediatric population are due to enzyme defects in the UC. This includes deficiencies of N-acetylglutamate synthetase, carbamoyl phosphate synthetase I, ornithine transcarbamoylase, argininosuccinic acid synthetase, argininosucciniclyase and arginase. Adults with partially deficient enzymes can have disease manifestations during stressful medical conditions such as postpartum stress, short bowel disease, parenteral nutrition with high nitrogen consumption, heart-lung transplantation, and gastrointestinal bleeding as discussed in previous reports[15-18]. Previous reports suggested that encephalopathy secondary to hyperammonemia in the setting of UCD can be unmasked by GBS[19,20]. Furthermore, the rapid weight loss that occurs in these patient’s results in protein catabolism and a large nitrogen load, which can further contribute to the symptoms. The mechanism by which gastric bypass disturbs the function of UC and the disposal of ammonia in such patient populations is not fully understood. Table 2 shows the different etiologies of hyperammonemia of non-hepatic origin.
| Age | Category | Examples |
| Adults | Stressful events | - |
| Partial enzyme deficiency | - | |
| Infection | Urinary tract infections (urease-producing organism, such as proteus mirabilis) | |
| Pediatrics | Medications | Valproate |
| Topiramate | ||
| Salicylates such as aspirin | ||
| Acetazolamide | ||
| Zonisamide | ||
| High-dose chemotherapy (5-fluorouracil) | ||
| Infection | Urinary tract infections (urease-producing organism, such as proteus mirabilis) | |
| Organic acidemias | Isovalericacidemia, propionic acidemia, methylmalonicacidemia, glutaricacidemia type II, multiple carboxylase deficiency, beta-ketothiolase deficiency | |
| Congenital lactic acidosis | Pyruvate dehydrogenase deficiency | |
| Pyruvate carboxylase deficiency | ||
| Mitochondrial disorders | ||
| Fatty acid oxidation defects | Acyl CoA dehydrogenase deficiency | |
| Systemic carnitine deficiency | ||
| Dibasic aminoacid transport defects | Lysinuric protein intolerance | |
| Hyperammonemia-hyperornithinemia-homocitrullinuria | ||
| Miscellaneous | Transient hyperammonemia of the newborn asphyxia | |
| Reye syndrome | ||
| Lactic acidosis |
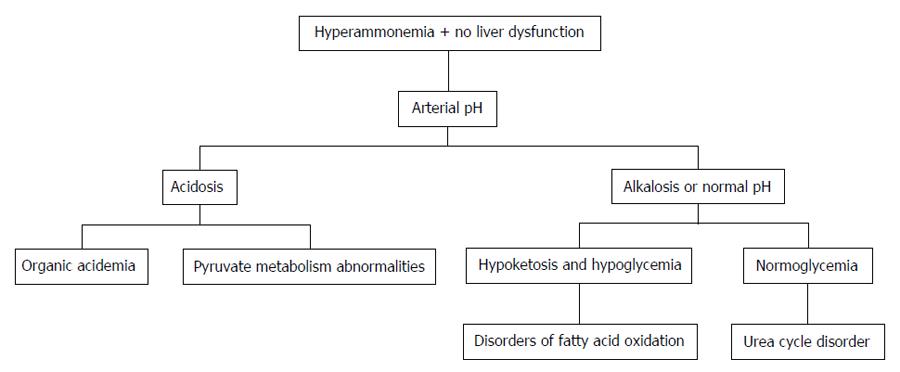
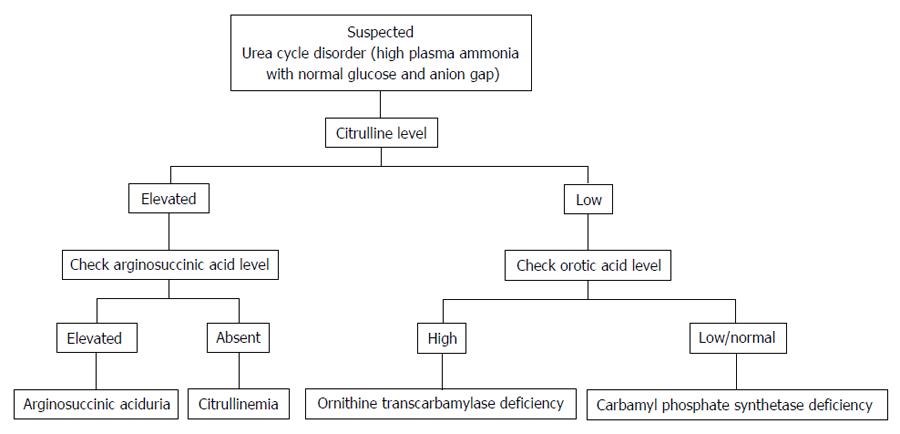
Distinguishing features of UCD in neonates comprises critically elevated ammonia levels (> 1000 mmol/L), whereas other etiologies seldom present with ammonia levels greater than 200-300 mmol/L. In addition, UCD is suggested by normal blood glucose and anion gap, as well as respiratory alkalosis from central hyperventilation. The initial workup of a UCD should include glucose, electrolytes, amino acids, serum ammonia, lactate, urine organic acids, orotic acid and arterial blood gases looking for arterial pH and carbon dioxide. OTC gene mutations cannot be revealed by DNA analysis in up to 20% of patients with OTC deficiency. Therefore other lab measures may give an indication as to the etiology of hyperammonemia without a history of liver disease[21]. The workup to identify the etiology of hyperammonemia of non-hepatic origin is outlined in Figure 1 and the workup to identify the UCD specifically is further outlined in Figure 2.
The management of hyperammonemia includes prevention of seizures and cerebral edema, medical therapy to remove excess ammonia and dietary protein restriction. The medications used for treatment assist in offloading the UC by converting nitrogen to non-urea products that are easily excreted. These include sodium benzoate and arginine, followed by phenylacetate and phenylbutyrate. Our patient’s cognitive impairment resolved after dietary protein withdrawal and implementation of medical therapy that included sodium benzoate and lactulose. He showed significant improvement over a period of one week following initiation of medical therapy.
A major proportion of the United States population is obese resulting in very high rates of annual bariatric surgeries. Even though it is rare for hyperammonemic encephalopathy to complicate GBS, it is yet more unusual for the etiology to be adult onset UCD. Such non-specific neurological presentation is a true diagnostic challenge especially when liver disease is absent, thus delaying the management plan, thus increasing associated morbidity and mortality.
Early diagnosis is crucial to prevent complications. Conservative management should include dietary modification with protein restriction. Patient satisfaction following GBS can be improved by educating these patients about the potential complications. Raising awareness among internists of this possible complication of GBS is also important and having a high index of suspicion for unmasked UCD post-GBS can prevent the debilitating consequences of unrecognized and untreated disease, improve its’ outcomes as well as have a great impact on utilization of health care resources.
A 56-year-old male presents with progressive tremors, confusion, ataxia and emotional lability.
The authors’ patient was not oriented to place or time, demonstrated tremor and ataxia.
Manganese toxicity, Parkinson’s disease, hepatic encephalopathy.
Basic metabolic panel, hepatic function panel, complete blood count, iron panel, carnitine level, zinc level, manganese, vitamin B12, vitamin A, and vitamin D levels were within normal limits. Ammonia level 155 mcg/dL and urine orotic acid 1.4 mmol/mol of creatinine.
Imaging of the abdomen was unremarkable.
The authors administered lactulose, sodium benzoate and modified his diet.
The physiology of adult onset urea cycle disorder is poorly understood and several case reports of similar occurrences have been cited in the case report presented by the authors.
All terms used in this case report are common and do not require further explanation.
Hyperammonemia encephalopathy in the absence of overt liver disease identified on imaging and blood work, can be the result of non-hepatic etiology and should be thoroughly evaluated as described in this case report.
The authors have described the rare complication of non-hepatic hyperammonemia-induced encephalopathy in the setting of gastric bypass surgery that has not been well described in the literature. Furthermore, the article highlights the appropriate workup, differential diagnosis, and treatment for this clinical scenario.
P- Reviewer: Chiang TA, Galvao FFH, Zhong YS S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Sugerman HJ, Kellum JM, Engle KM, Wolfe L, Starkey JV, Birkenhauer R, Fletcher P, Sawyer MJ. Gastric bypass for treating severe obesity. Am J Clin Nutr. 1992;55:560S-566S. [PubMed] |
| 2. | Benotti PN, Forse RA. The role of gastric surgery in the multidisciplinary management of severe obesity. Am J Surg. 1995;169:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Koffman BM, Greenfield LJ, Ali II, Pirzada NA. Neurologic complications after surgery for obesity. Muscle Nerve. 2006;33:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE, Dyck PJ. A controlled study of peripheral neuropathy after bariatric surgery. Neurology. 2004;63:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Hamer HM, Knake S, Schomburg U, Rosenow F. Valproate-induced hyperammonemic encephalopathy in the presence of topiramate. Neurology. 2000;54:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Verrotti A, Greco R, Morgese G, Chiarelli F. Carnitine deficiency and hyperammonemia in children receiving valproic acid with and without other anticonvulsant drugs. Int J Clin Lab Res. 1999;29:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Lokrantz CM, Eriksson B, Rosén I, Asztely F. Hyperammonemic encephalopathy induced by a combination of valproate and pivmecillinam. Acta Neurol Scand. 2004;109:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ohtani Y, Endo F, Matsuda I. Carnitine deficiency and hyperammonemia associated with valproic acid therapy. J Pediatr. 1982;101:782-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 178] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Hu WT, Kantarci OH, Merritt JL, McGrann P, Dyck PJ, Lucchinetti CF, Tippmann-Peikert M. Ornithine transcarbamylase deficiency presenting as encephalopathy during adulthood following bariatric surgery. Arch Neurol. 2007;64:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Wakabayashi Y, Iwashima A, Yamada E, Yamada R. Enzymological evidence for the indispensability of small intestine in the synthesis of arginine from glutamate. II. N-acetylglutamate synthase. Arch Biochem Biophys. 1991;291:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol. 1981;241:E473-E480. [PubMed] |
| 12. | Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 257] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Butterworth RF. Effects of hyperammonaemia on brain function. J Inherit Metab Dis. 1998;21 Suppl 1:6-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Cooper AJ. Role of glutamine in cerebral nitrogen metabolism and ammonia neurotoxicity. Ment Retard Dev Disabil Res Rev. 2001;7:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Summar ML, Barr F, Dawling S, Smith W, Lee B, Singh RH, Rhead WJ, Sniderman King L, Christman BW. Unmasked adult-onset urea cycle disorders in the critical care setting. Crit Care Clin. 2005;21:S1-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Panlaqui OM, Tran K, Johns A, McGill J, White H. Acute hyperammonemic encephalopathy in adult onset ornithine transcarbamylase deficiency. Intensive Care Med. 2008;34:1922-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Schultz RE, Salo MK. Under recognition of late onset ornithine transcarbamylase deficiency. Arch Dis Child. 2000;82:390-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Yoshino M, Nishiyori J, Yamashita F, Kumashiro R, Abe H, Tanikawa K, Ohno T, Nakao K, Kaku N, Fukushima H. Ornithine transcarbamylase deficiency in male adolescence and adulthood. Enzyme. 1990;43:160-168. [PubMed] |
| 19. | Fenves A, Boland CR, Lepe R, Rivera-Torres P, Spechler SJ. Fatal hyperammonemic encephalopathy after gastric bypass surgery. Am J Med. 2008;121:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012;49:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Yamaguchi S, Brailey LL, Morizono H, Bale AE, Tuchman M. Mutations and polymorphisms in the human ornithine transcarbamylase (OTC) gene. Hum Mutat. 2006;27:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |