Published online Apr 28, 2015. doi: 10.4254/wjh.v7.i6.846
Peer-review started: May 20, 2014
First decision: July 27, 2014
Revised: November 26, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: April 28, 2015
Processing time: 346 Days and 1.1 Hours
Non-alcoholic fatty liver disease (NAFLD) is now the most frequent chronic liver disease that occurs across all age groups and is recognized to occur in 14%-30% of the general population, representing a serious and growing clinical problem due to the growing prevalence of obesity and overweight. Histologically, it resembles alcoholic liver injury but occurs in patients who deny significant alcohol consumption. NAFLD encompasses a spectrum of conditions, ranging from benign hepatocellular steatosis to inflammatory nonalcoholic steatohepatitis, fibrosis, and cirrhosis. The majority of hepatocellular lipids are stored as triglycerides, but other lipid metabolites, such as free fatty acids, cholesterol, and phospholipids, may also be present and play a role in disease progression. NAFLD is associated with obesity and insulin resistance and is considered the hepatic manifestation of the metabolic syndrome, a combination of medical conditions including type 2 diabetes mellitus, hypertension, hyperlipidemia, and visceral adiposity. Confirmation of the diagnosis of NAFLD can usually be achieved by imaging studies; however, staging the disease requires a liver biopsy. Current treatment relies on weight loss and exercise, although various insulin-sensitizing agents, antioxidants and medications appear promising. The aim of this review is to highlight the current information regarding epidemiology, diagnosis, and management of NAFLD as well as new information about pathogenesis, diagnosis and management of this disease.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a serious and growing clinical problem due to the growing prevalence of obesity and overweight. Histologically, it resembles alcoholic liver injury but occurs in patients who deny significant alcohol consumption. NAFLD encompasses a spectrum of conditions, ranging from benign hepatocellular steatosis to inflammatory nonalcoholic steatohepatitis, fibrosis, and cirrhosis. The majority of hepatocellular lipids are stored as triglycerides, but other lipid metabolites, such as free fatty acids, cholesterol, and phospholipids, may also be present and play a role in disease progression. NAFLD is associated with obesity and insulin resistance and is considered the hepatic manifestation of the metabolic syndrome, a combination of medical conditions including type 2 diabetes mellitus, hypertension, hyperlipidemia, and visceral adiposity. Confirmation of the diagnosis of NAFLD can usually be achieved by imaging studies; however, staging the disease requires a liver biopsy. Current treatment relies on weight loss and exercise, although various insulin-sensitizing agents, antioxidants and medications appear promising. The aim of this review is to highlight the current information regarding epidemiology, diagnosis, and management of NAFLD as well as new information about pathogenesis, diagnosis and management of this disease.
- Citation: Abd El-Kader SM, El-Den Ashmawy EMS. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol 2015; 7(6): 846-858
- URL: https://www.wjgnet.com/1948-5182/full/v7/i6/846.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i6.846
Non-alcoholic fatty liver disease (NAFLD) is a common clinicopathological condition characterized by significant lipid deposition in the hepatocytes of the liver parenchyma and persistent abnormalities in liver enzyme. The rising prevalence of NAFLD is related to the epidemic of obesity[1]. Although the histologic picture resembles that of alcohol-induced liver injury, NAFLD occurs in patients who do not abuse alcohol[2]. NAFLD comprises a wide spectrum of liver damage, ranging from simple macrovesicular steatosis to steatohepatitis, advanced fibrosis, and cirrhosis[3]. NAFLD is now increasingly being recognized as a cause of end-stage liver disease and is associated with increased rates of hepatocellular carcinoma (HCC), liver transplantation, and death[4-6]. Significant research endeavors are being directed toward understanding the pathogenesis of NAFLD and designing therapeutic strategies. This article provides a clinical overview of NAFLD, focusing on its epidemiology, etiology, pathogenesis, diagnosis, natural history and treatment.
NAFLD is defined as either excessive fat accumulation in the liver with more than 5% of hepatocytes containing visible intracellular triglycerides or steatosis affecting at least 5% of the liver volume or weight in patients consuming less than 30 g (three units) of alcohol per day for men and less than 20 g (two units) of alcohol per day for women. One unit of alcohol (10 g) is defined as one glass of beer (25 cL), one glass of wine (20 cL) or one glass of whisky (3 cL)[7,8].
Globally, NAFLD is the most common form of chronic liver disease among adults and children[9,10]. However, the prevalence of non-alcoholic steatohepatitis (NASH) in the general population is not known. Depending on the cutoff values used to define the upper limit of normal for aminotransferase levels, the estimated prevalence of NAFLD in the general United States population ranges from 5.4% to 24%, but these values may be underestimations because aminotransferase levels have limited sensitivity for steatosis[11,12]. Histologic estimates of NAFLD prevalence via preoperative or intraoperative liver biopsy, mainly obtained from individuals evaluated as donors for living-donor liver transplantation, are 33% to 88%[13-15]. In children, NAFLD prevalence has been estimated to be 9.6%; of great concern, 2% to 8% of children with NAFLD progress to cirrhosis[16,17].
Obesity is the most important risk factor for NAFLD; the prevalence of NAFLD is 4.6 times greater in the obese population, and up to 74% of obese individuals have fatty livers[18]. Among morbidly obese patients undergoing bariatric surgery for weight loss, 84% to 96% have NAFLD and 2% to 12% have severe fibrosis or cirrhosis[19-22]. NAFLD is also strongly associated with hepatic and adipose tissue insulin resistance and metabolic syndrome[23]. Although NAFLD is clearly linked to obesity and metabolic syndrome, it may occur in up to 29% of lean patients lacking associative risk factors[24]. The prevalence of NAFLD is estimated to be at least twice as common among individuals who meet criteria for metabolic syndrome[25]. Among individuals with NAFLD, it is estimated that over 90% have some features of metabolic syndrome[26]. Diabetes is reported in 33% to 50% of patients with NAFLD, whereas insulin resistance may occur in as many as 75%[27].
Other factors that influence the development of NAFLD include age, sex, race, and ethnicity[28-30]. The prevalence of NAFLD increases with age in both adults and children[31]. NAFLD is more common among men than women younger than the age of 50; however, higher prevalence rates are seen in women older than the age of 50, perhaps related to hormonal changes occurring after menopause[11]. The prevalence of NAFLD across the globe varies but in some populations, half of all people may be affected. Among the Asian population, the prevalence of NAFLD diagnosed by ultrasound varies between 5% and 40%[32].
NAFLD encompasses a group of conditions, ranging from benign hepatocellular steatosis to inflammatory NASH, fibrosis, and cirrhosis[9]. The causes may be divided into two main categories: (1) acquired or congenital metabolic abnormalities; and (2) toxins and drugs[26]. Potential causes of NAFLD are listed in Table 1.
| Acquired metabolic disorders |
| Diabetes mellitus |
| Dyslipidemia |
| Kwashiorkor and marasmus |
| Obesity |
| Starvation |
| Cytotoxic and cytostatic drugs |
| L-asparaginase |
| Azacitidine |
| Azaserine |
| Bleomycin |
| Methotrexate |
| Puromycin |
| Tetracycline |
| Other drugs and toxins |
| Amiodarone |
| 4,4'-diethylaminoethoxyhexestrol |
| Dichlorethylene |
| Ethionine |
| Ethyl bromide |
| Estrogens |
| Glucocorticoids |
| Highly active antiretroviral therapy |
| Hydrazine |
| Hypoglycin |
| Orotate |
| Perhexilene maleate |
| Safrole |
| Tamoxifen |
| Metals |
| Antimony |
| Barium salts |
| Chromates |
| Phosphorus |
| Rare earths of low atomic number |
| Thallium compounds |
| Uranium compounds |
| Inborn errors of metabolism |
| Abetalipoproteinemia |
| Familial hepatosteatosis |
| Galactosemia |
| Glycogen storage disease |
| Hereditary fructose intolerance |
| Homocystinuria |
| Systemic carnitine deficiency |
| Tyrosinemia |
| Weber-Christian syndrome |
| Wilson disease |
| Surgical procedures |
| Biliopancreatic diversion |
| Extensive small bowel resection |
| Gastric bypass |
| Jejunoileal bypass |
| Miscellaneous conditions |
| Industrial exposure to petrochemicals |
| Inflammatory bowel disease |
| Partial lipodystrophy |
| Jejunal diverticulosis with bacterial overgrowth |
| Severe anemia |
| Total parenteral nutrition |
Obesity is often associated with NAFLD as the degree of steatosis was found to be correlated with body mass index (BMI)[33,34]; however, there is a significant correlation between the degree of steatosis, waist-to-hip ratio and the risk of metabolic syndrome[35-37]. Also, there is a strong correlation between glucose intolerance, type 2 diabetes mellitus (T2DM) and NAFLD[38]. Moreover, Diabetes mellitus may be an independent predictor of advanced liver cirrhosis, HCC and NAFLD[24,39-41].
The pathogenesis of NAFLD is fully understood, however, no single pathogenic mechanism has been identified[42]. Currently, the development of NASH is considered to be through a ‘‘two hit’’ process[43]. The first “hit” includes accumulation of fat in liver cells, which is usually associated with insulin resistance, central obesity along with triglyceride accumulation inside the liver, and fatty acid metabolism dysregulation that leads to steatosis. The second “hit” causes hepatocyte inflammation and necrosis, which lead to cirrhosis and fibrosis in some patients with NAFLD[43].
Oxidative stress has a principal role in the pathogenesis of NAFLD as levels of lipid peroxide are increased in both NASH and hepatic steatosis[44].
Oxidative stress plays a key role in the second “hit”, which also involves lipid peroxidation in steatotic hepatocytes. Induction of hepatic CYP2E1 promotes oxidative stress and lipid peroxidation[45] and mitochondrial dysfunction leads to reactive oxygen species formation[46]. Moreover, immune responses to lipid peroxidation products may share in NAFLD progression[47,48].
Adaptive and innate immune dysfunction along with inflammatory pathways is involved in the development of NAFLD[49]. Neutrophils, kupffer cells (KCs), natural killer (NK) cells and dendritic cells play an important role in the pathogenesis of NASH.
KCs are activated in acute or chronic liver disease, and this activation increases the pro-inflammatory cytokines, e.g., interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and IL-1b, which activate T cells and induce hepatocytes apoptosis[50]. Also, activation of neutrophils increases the release of pro-inflammatory cytokines and leads to oxidative damage to hepatic cells[51,52]. Moreover, NK cells are abundant in liver tissue and have an anti-fibrotic effect in the liver[53], and reduction of NK cell activity and levels may increase susceptibility to liver cirrhosis among obese subjects. Therefore, NK cells have a role in the development of liver injury and fibrosis and contribute to NASH and NAFLD development[54].
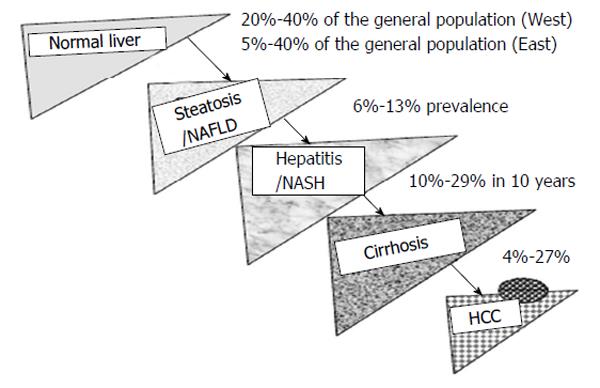
NAFLD is the most common liver disorder worldwide, affecting 20%-40% of population in Western countries and 5%-40% of the general population across the Asia-Pacific region[55,56]. The prevalence of NAFLD, including NASH, is rising in parallel with the obesity, T2DM, and metabolic syndrome[57]. A certain proportion of NASH patients progress to cirrhosis and HCC[58]. Previous studies showed that 10%-29% of NASH patients may have liver cirrhosis within 10 years, and 4%-27% of these patients may have HCC (Figure 1)[59]. Therefore, NAFLD/NASH will gradually become the major etiology of chronic liver disease worldwide[60].
Most subjects with NAFLD are clinically silent and asymptomatic, but can manifest with non-specific symptoms such as right upper quadrant discomfort or fatigue. Liver enzymes are usually minimally perturbed with mostly increased levels of alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase. The diagnosis is often made incidentally in these individuals because of either abnormal liver enzyme levels or radiological features of a fatty liver. In others, NAFLD may be diagnosed either as a result of an unusual appearance of the liver during abdominal surgery or because of persistent hepatomegaly. It is important to recognize that only a minority of subjects has NAFLD been diagnosed and that it currently remains undiagnosed in the great majority of afflicted individuals[61]. NAFLD is a diagnosis of exclusion, so its workup needs to exclude other causes such as significant alcohol consumption (defined as > 30 g/d of ethanol for men and 20 g/d of ethanol for women), hepatitis B and/or C infection, drug abuse, autoimmune liver disease, haemochromatosis or Wilson’s disease[62].
The principal risk factors for developing NAFLD are obesity and insulin resistance. More generally, any elements constituting the metabolic syndrome such as type 2 diabetes, dyslipidaemia and hypertension are linked to the development of NAFLD, and approximately 85% of patients with NAFLD have at least one such constituent. The metabolic syndrome itself is present in 30% of patients with NAFLD[62]. The association of NAFLD with obesity, diabetes, hypertriglyceridemia, and hypertension is well known. However, other associations include cardiovascular morbidity and mortality[63-65], sleep abnormalities[66], psychiatric illness[62], chronic fatigue and pain syndrome[67] and abnormalities of the coagulation cascade[68].
Suspicion for NAFLD is triggered by abnormalities of liver chemistry tests that are usually performed for non-liver-related reasons. About 50% of patients with simple steatosis have higher liver biochemical test levels, which occur in 80% of patients with advanced NAFLD. Also, serum aspartate aminotransferase or ALT level, or both is usually increased up to 1.5- to 4-fold and levels rarely exceed 10 times the upper limit of normal. However, the gamma glutamyl transpeptidase and alkaline phosphatase levels may be elevated, but the serum prothrombin time, bilirubin level and serum albumin level are normal, except in patients with NAFLD-associated cirrhosis[69]. Moreover, about one fourth of NAFLD patients may have antinuclear antibodies (ANA) in low titers (less than 1:320)[70]. Serum ferritin level may be higher in 20% to 50% of NAFLD patients and can be considered a marker for advanced disease[24,71]. Hyperglycemia and dyslipidemia may be detected in 30% to 50% of NAFLD subjects[40]. Laboratory and clinical findings do not correlate with NAFLD histologic severity[68].
The radiologic features of fatty liver disease stem from the increased fat content of the liver parenchyma. The spatial pattern may be diffuse and homogeneous or heterogeneous, with focal fat deposition in an otherwise normal liver or areas of focal fat sparing in a diffusely fatty liver. The homogeneous form is the most common; the heterogeneous and focal forms may simulate perfusion abnormalities, diffusely infiltrative disease, nodular lesions, or masses[72,73]. Therefore, it is not only important to recognize fatty liver on imaging but also to discriminate it from other pathologic processes. The most important modalities used in the assessment of hepatic steatosis are ultrasonography, computed tomography (CT), and magnetic resonance (MR) imaging and MR spectroscopy. However, plain radiography has no significant role in the assessment of NAFLD[74].
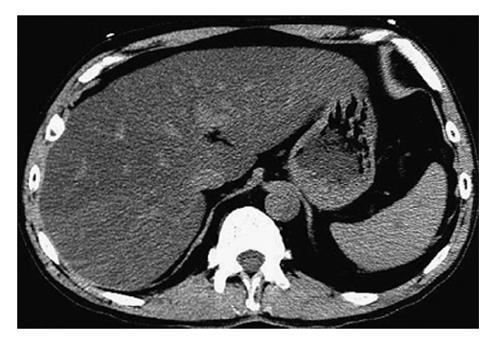
CT has been widely used in evaluation of NAFLD in adults. Use of ionizing radiation precludes its use as a research tool in children, although fatty liver may be observed in children on scans done for clinical purposes[75]. Deposition of fat in the liver is characterized by a reduction in the attenuation of the hepatic parenchyma. On unenhanced CT, normal liver parenchyma has slightly greater attenuation than the spleen or blood. However, with increasing hepatic steatosis, liver attenuation decreases and the liver may become less dense than the intrahepatic vessels, simulating the appearance on a contrast-enhanced scan (Figure 2)[76]. Liver attenuation may be affected by a variety of factors other than liver fat, such as iron, copper, glycogen, fibrosis, edema, or amiodarone use. Assessment of liver fat by CT attenuation may be unreliable, and CT methods are insensitive to mild steatosis. The reported sensitivity and specificity of unenhanced CT for detection of moderate/severe steatosis (> 30% on histology) range from 73% to 100% and 95% to 100%, respectively[75].
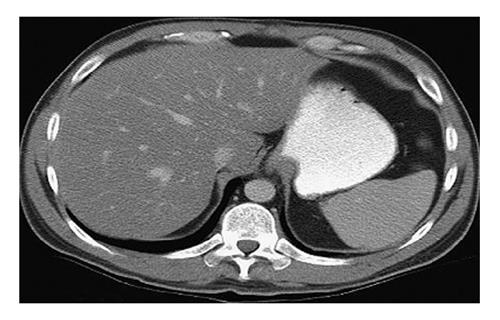
At enhanced CT, the presence of iodine contrast interferes with attenuation, adding a new confounding factor. Perfusion alterations, timing of acquisitions, and contrast type, dosage, and injection rate all may influence hepatic and splenic attenuation. Nevertheless, criteria have been proposed to detect hepatic steatosis at enhanced CT, including a liver-spleen attenuation difference of at least 20 HU between 80 to 100 s, or at least 18.5 HU between 100 to 120 s, after intravenous contrast injection (Figure 3). Sensitivity and specificity of these attenuation differences range from 54% to 93% and 87% to 93%, respectively. Ultimately, however, the quantitative criteria for diagnosing fatty liver at enhanced CT are protocol specific and have significant overlap of liver-spleen attenuation values between normal and fatty liver, thereby limiting its clinical role[75].
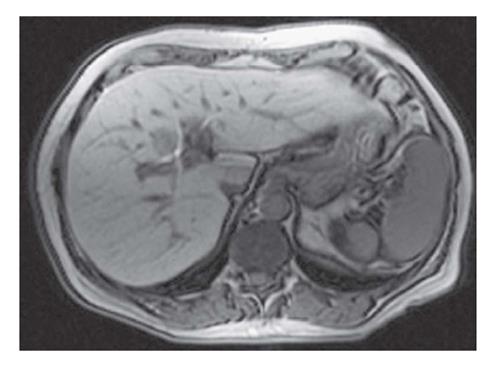
MR imaging is more sensitive than CT for hepatic steatosis assessment. Recently, MR imaging provides a highly validated and reproducible hepatic triglyceride content measurement[77]. MR imaging is generally considered the most definitive radiologic modality for the qualitative and quantitative assessment of fatty liver disease but is relatively costly (Figure 4)[74]. However, proton MR spectroscopy is evolving to detect not only the full spectrum of steatosis but also other features such as the degree of fibrosis[13,78].
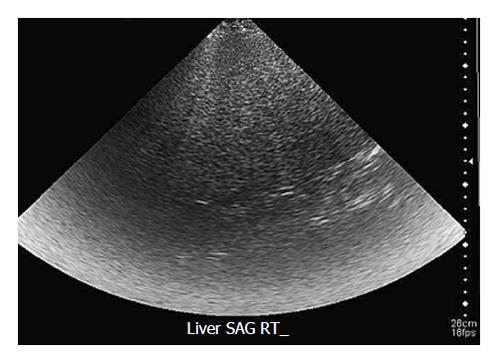
Transabdominal ultrasonography is the most common imaging technique to diagnose hepatic steatosis due to its widespread availability, noninvasiveness and low cost[76]. At ultrasonography, diffuse fatty liver is characterized by hyperechogenicity of the liver parenchyma relative to the adjacent right kidney or spleen (the so-called bright liver). Focal fat deposition appears as a hyperechoic area in an otherwise normal liver, whereas focal fat sparing is represented by a hypoechoic area within diffusely hyperechoic liver parenchyma[73]. Other frequently described ultrasound features of fatty liver include decreased visualization of vascular margins, attenuation of the ultrasound beam, loss of definition of the diaphragm, and hepatomegaly (Figure 5)[79].
Ultrasonography has several limitations in the detection of both diffuse and focal hepatic steatosis. It is highly operator dependent, nonreproducible, and limited by abdominal gas and patient body habitus. The last inadequacy is highlighted in this patient population because the majority of cases of fatty liver disease occur in overweight or obese individuals. Similar to CT, however, ultrasonography is not a quantitative method and may be unable to distinguish simple steatosis from advanced fibrosis or early cirrhosis. Ultrasonography has low sensitivity and specificity for detecting small amounts of fat in the liver[75].
Transient elastography, a recently developed technique based on ultrasound monitoring of the passage of a low frequency pressure wave through tissues, has been found to be a promising non-invasive technique for the detection of advanced fibrosis caused by chronic viral hepatitis and NASH[80], although abdominal obesity may compromise its utility in the NASH patient population[81].
Scintigraphy with xenon-133 (133Xe) as a nuclear medicine imaging technique was used to detect hepatic steatosis in the 1980s and 1990s but is now no longer incorporated in diagnostic algorithms. 133Xe is a highly fat-soluble gas that, after being inhaled or injected, remains in the fatty tissue after blood pool clearing. The 133Xe hepatic retention ratio is increased in patients with fatty liver[82].
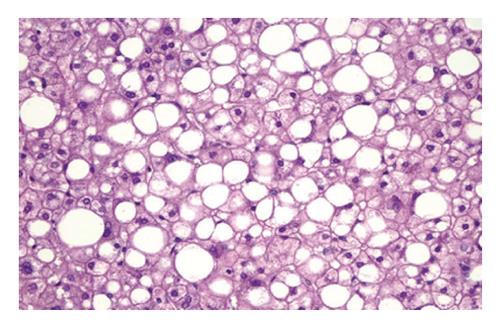
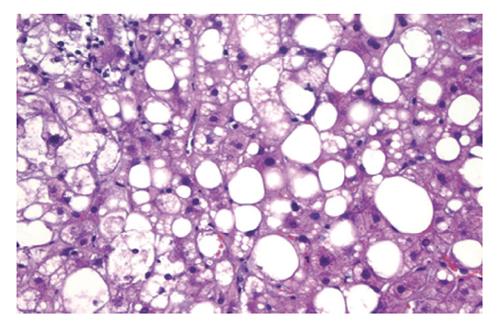
The main histologic features of NAFLD are similar to those of alcohol-induced liver disease and include steatohepatitis (fatty liver plus parenchymal inflammation with or without accompanying focal necrosis), steatosis (fatty liver) and varying degrees of fibrosis, including cirrhosis. Steatosis is predominantly macrovesicular and usually is distributed diffusely throughout the liver lobule, although prominent microvesicular steatosis and zone 3 (perivenular) steatosis have been reported occasionally (Figure 6). Mild neutrophilic, lymphocytic, or mixed inflammatory infiltrates also may be observed, and glycogenated nuclei are common[69]. NASH, which is an advanced form of NAFLD, is indistinguishable histologically from alcoholic hepatitis (Figure 7)[83].
Literature reviews indicate that similar factors and markers of inflammation are present in paediatric NAFLD as in adults[84-86]. However, many differences are noted in comparison to adult histology and include: (1) greater severity of steatosis; (2) less or no ballooning or Mallory-Denk bodies; (3) less lobular inflammation; (4) few or no polymorphonuclear leukocytes; and (5) increased portal tract inflammation and fibrosis[87]. Moreover, cirrhosis in children is rare but is reported[88].
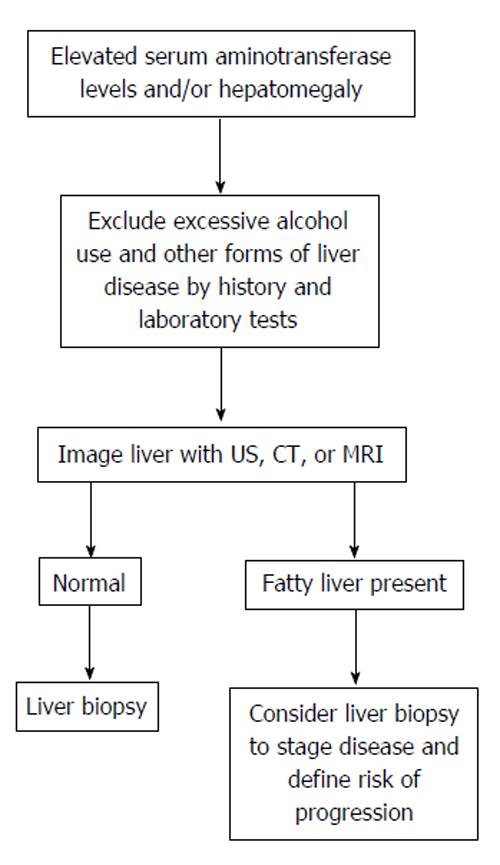
Establishing a definitive diagnosis of NAFLD requires both clinical and histologic data (Figure 8). Most patients with NAFLD are evaluated because of chronically elevated liver biochemical test levels, with or without hepatomegaly. The combination of the patient’s history, clinical examination, radiologic findings and blood test results is essential for accurate diagnosis of NAFLD[89]. Anti-smooth muscle antibodies and ANA are common in patients with NASH and most frequently represent a nonspecific antibody response that is not associated with the pattern or severity of injury on liver biopsy[90]. However, alcoholic liver disease must be excluded in order to establish NAFLD diagnosis.
The diagnosis of fatty liver is confirmed by imaging studies and the clinician is challenged with establishing the etiology of hepatic steatosis. Among patients with elevated serum aminotransferase values, the etiology is usually established through a careful evaluation of their history (medication use, risk factors for viral hepatitis, history of alcohol and drug use, and review of comorbidities), a series of screening blood tests for causes of chronic liver disease (viral serologic studies, iron studies, autoimmune markers, ceruloplasmin, and α1-antitrypsin), supportive imaging studies (initial evaluation usually by ultrasonography), and, sometimes, liver biopsy[91,92].
Alcoholic liver disease includes a spectrum of conditions provoked by alcohol ingestion, including alcoholic hepatitis, fatty liver disease and cirrhosis. It has been estimated that almost all patients with heavy alcohol consumption develop fatty liver, although only 10% to 35% develop alcoholic hepatitis and 8% to 20% progress to alcoholic cirrhosis[93]. In individuals who admit to moderate alcohol intake, the differentiation between NAFLD and alcoholic fatty liver disease is difficult because laboratory, imaging, and histologic findings are similar. Unfortunately, strong data are lacking to determine accurate thresholds for alcohol consumption required to cause fatty liver. Historically, daily alcohol intake of 30 g in men and 20 g in women has been used to distinguish NAFLD from alcoholic fatty liver disease, although the validity of these thresholds is unknown[94]. If liver biopsy specimens are obtained, individuals with alcoholic liver disease tend to have more Mallory’s hyaline and acidophil bodies and less glycogenated nuclei than those with NAFLD, although these are not reliable findings[74].
Because the radiologic findings of hepatic steatosis are common to its diverse causes, the differential diagnosis is largely discriminated on clinical and laboratory grounds[95]. Special attention needs to be given to the possible imaging overlap between simple steatosis and advanced fibrosis or early cirrhosis; these disparate conditions are often, but not always, easily distinguished clinically. A major challenge in the differential diagnosis of hepatic steatosis occurs when the radiologic findings of focal fat deposition or focal fat sparing simulate hepatic nodular lesions such as abscess, benign neoplasm, or primary or metastatic malignancy. The diagnosis of focal fat deposition or sparing is supported by their occurrence in typical locations, a wedge shape, the lack of mass effect, and the absence of vascular displacement or distortion inside the lesion. When there is still doubt, MR imaging may be performed[74].
Certain drugs may produce de novo steatohepatitis (e.g., amiodarone, perhexiline maleate, diethylaminoethoxyhexestrol) and others may exacerbate NASH (tamoxifen, corticosteroids, diethylstilbestrol, estrogens)[96]. Oxaliplatin and irinotecan administered as preoperative chemotherapy before surgical resection of hepatic metastases have been associated with steatohepatitis, with irinotecan-associated steatohepatitis associated with poorer outcomes after hepatic resection[97,98]. Other conditions capable of eliciting fatty liver include intestinal bypass surgery for weight loss (classically seen with jejunoileal bypass surgery), human immunodeficiency virus (HIV) infection with lipodystrophy, and parenteral nutrition[3]. If any of the these secondary causes of fatty liver are excluded (alcohol, viral hepatitis, drug-induced, jejunoileal bypass surgery, HIV infection, and parenteral nutrition support), a diagnosis of NAFLD can be made[74].
The standardized schema of NAFLD staging and grading was published by Brunt and associates in 1999, who assigned the overall grade of mild, marked, or severe (grades 1, 2, and 3, respectively), based on the degree of ballooning degeneration, steatosis and lobular and portal inflammation as listed in Table 2[99]. The Pathology Committee of the National Institute of Diabetes and Digestive and Kidney Diseases sponsored NASH Clinical Research Network maintained features of the Brunt schema for NAFLD grading as found in Table 3[9].
| Severe (grade 3) | Moderate (grade 2) | Mild (grade 1) | |
| Typically > 66% (panacinar); commonly mixed steatosis | Any degree and usually mixed macrovesicular and microvesicular | Predominantly macrovesicular; involves < 33%-66% of the lobules | Steatosis |
| Predominantly zone 3; marked | Obvious and present in zone 3 | Occasionally observed; zone 3 hepatocytes | Ballooning |
| Scattered acute and chronic inflammation; polymorphs may appear concentrated in zone 3 areas of ballooning and perisinusoidal fibrosis | Polymorphs may be noted associated with ballooned hepatocytes, pericellular fibrosis; mild chronic inflammation may be seen | Scattered and mild acute (polymorphs) inflammation and occasional chronic inflammation (mononuclear cells) | Lobular inflammation |
| Mild or moderate | Mild to moderate | None or mild | Portal inflammation |
To date, there are no established treatment guidelines and no single approved therapy for NAFLD treatment. Historically, the principal treatment for NAFLD consisted of removal of offending drugs and toxins, weight loss, and control of associated metabolic disorders as hyperlipidemia and diabetes. The focus of NAFLD management is to ameliorate the NASH risk factors (i.e., insulin resistance and obesity), with the objective of preventing disease progression or regression of already established fatty liver or NASH. Lifestyle changes and dietary modification are the main methods for weight management.
The ultimate weight management goal is to achieve the ideal body weight. However, significant insulin resistance improvement could be attained by modest weight loss[100].
The National Heart, Lung, and Blood Institute guidelines for weight management in obsess subjects are the best evidence-based treatment guidelines, which generally recommended that the diet should be planned to achieve a daily caloric deficit of 500 to 1000 calories along with an increase in everyday activities[101]. Furthermore, for subjects with a BMI higher than 30 kg/m2 or with a BMI higher than 27 along with other comorbid conditions (e.g., sleep apnea), pharmacologic weight management with orlistat or sibutramine may be considered as these agents could produce a beneficial effect on NAFLD[99]. However, both vertical banded gastroplasty and proximal gastric bypass have been shown to be safe in NASH subjects[102]. Moreover, the severity of hepatic steatosis, fibrosis and cell injury regresses once the weight stabilizes following these operations[103].
Several drug therapies have been tried in both research and clinical settings, yet no agent has been approved by the Food and Drug Administration for the treatment of NAFLD[61].
Vitamin E, an inexpensive potent antioxidant, has been examined as a treatment agent for NAFLD in many adult and pediatric studies, with varying results. In all studies, vitamin E was well tolerated, and most studies showed modest improvements in ultrasonographic appearance of the liver, serum aminotransferase levels and histologic findings[97,104,105]. In one published series of 11 pediatric patients with NASH who received vitamin E (d-α-tocopherol), 400 to 1200 IU, ALT improved [105-107].
Few small trials assessed the usefulness of lipid-lowering and cytoprotective drugs for NAFLD treatment, with varying results. In one controlled trial, gemfibrozil improved liver chemistry in 74% of NAFLD patients in the treatment group, compared with 30% of untreated control subjects with no available histologic data. So, in general, lipid-lowering agents are not used for NASH treatment[108].
The association between hyperinsulinemic insulin resistance and NAFLD provides a logical target for treatment. Two classes of drugs have been shown to correct insulin resistance: biguanides (e.g., metformin) and thiazolidinediones. Metformin, a biguanide that reduces hyperinsulinemia and improves hepatic insulin sensitivity, reduces hepatomegaly and hepatic steatosis in ob/ob mice[109], but results in human studies have been less impressive[110,111] as in human studies, although ALT improved and liver size decreased, metformin was not consistently found to improve liver histology[110,112].
Ursodeoxycholate (UDCA) is a hydrophilic bile acid that is associated with hepatoprotective properties. In one study, UDCA produced improvement in liver enzymes and a decrease in hepatic steatosis. The long-term benefits of UDCA and the optimal dose of UDCA remain to be established[113].
Taurine is believed to function as a lipotropic factor and to improve the mobilization of hepatic fat. In another single uncontrolled series, 10 children treated with taurine supplements orally had radiologic resolution of their fatty liver[114].
Betaine is a hepatoprotective factor, and liver histology and aminotransferase activity were improved in ten NAFLD subjects who received betaine for one year[115,116]. In a recent randomized placebo-control study, 55 NASH patients received betaine (20 g daily). Patients randomized to betaine had a decrease in steatosis grade without a significant change in intragroup or intergroup differences in NAS or fibrosis stage. Moreover, there was no significant change in adiponectin, insulin, glucose, proinflammatory cytokines, or oxidant stress in NASH patients receiving betaine therapy[116].
Pentoxifylline antagonizes TNF-α and is orally available for long-term use. In two small pilot studies, ALT improved after several months of treatment at a dose of 400 mg three times a day. In addition, although the drug was well tolerated in one study, 9 of 20 subjects in the other study dropped out because of side effects, especially nausea[117,118].
Angiotensin II has been implicated in hepatic stellate cell activation and matrix production[119]. In a small pilot study of an angiotensin receptor blocker, losartan, an improvement in ALT was noted[120].
Bariatric surgery is the primary surgical intervention for NAFLD in patients with an BMI more than 40 kg/m2 or of 35 kg/m2 with comorbidities[121]. Current bariatric surgical techniques include vertical banded gastroplasty, adjustable gastric banding, Roux-en-Y gastric bypass, biliopancreatic bypass, and biliopancreatic diversion with duodenal switch. Based on a recent meta-analysis, bariatric surgery is associated with significant histologic improvements in steatosis, steatohepatitis, and fibrosis, with more than 50% of patients experiencing complete resolution of their fatty liver disease after surgery. Although these results are compelling, these observational studies showed no relationship between histologic improvement and the amount of weight loss[122].
As with other causes of cirrhosis, liver transplantation is a viable option for patients with end-stage liver disease due to fatty liver disease[123]. The outcome of liver transplantation in these patients is good, although NAFLD can recur after liver transplantation[124,125].
P- Reviewer: Ahmed M, Liang J, Mascitelli L, Marzuillo P, Sutti S
S- Editor: Song XX L- Editor: Wang TQ E- Editor: Liu SQ
| 1. | Sonsuz A, Basaranoglu M, Ozbay G. Relationship between aminotransferase levels and histopathological findings in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:1370-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 3. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 4. | Charlton MR, Kondo M, Roberts SK, Steers JL, Krom RA, Wiesner RH. Liver transplantation for cryptogenic cirrhosis. Liver Transpl Surg. 1997;3:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17-S29. [PubMed] |
| 8. | Serfaty L, Lemoine M. Definition and natural history of metabolic steatosis: clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab. 2008;34:634-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8233] [Article Influence: 411.7] [Reference Citation Analysis (5)] |
| 10. | Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006;43:413-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 11. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 652] [Article Influence: 28.3] [Reference Citation Analysis (2)] |
| 12. | Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5-10. [PubMed] |
| 13. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2695] [Article Influence: 128.3] [Reference Citation Analysis (3)] |
| 14. | Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Soejima Y, Shimada M, Suehiro T, Kishikawa K, Yoshizumi T, Hashimoto K, Minagawa R, Hiroshige S, Terashi T, Ninomiya M. Use of steatotic graft in living-donor liver transplantation. Transplantation. 2003;76:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1056] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 17. | Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008;10:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [PubMed] |
| 19. | Crespo J, Fernández-Gil P, Hernández-Guerra M, Cayón A, Mayorga M, Domínguez-Diez A, Fernández-Escalante JC, Pons-Romero F. Are there predictive factors of severe liver fibrosis in morbidly obese patients with non-alcoholic steatohepatitis? Obes Surg. 2001;11:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Dixon JB, Bhathal PS, O‘Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 21. | Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg. 2003;138:1240-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Gholam PM, Kotler DP, Flancbaum LJ. Liver pathology in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2002;12:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 539] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 24. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1090] [Article Influence: 41.9] [Reference Citation Analysis (1)] |
| 25. | Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III). Am J Med Sci. 2005;329:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1917] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 27. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] |
| 28. | Schwimmer JB. Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin Liver Dis. 2007;27:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Ruhl CE, Everhart JE. Relation of elevated serum alanine aminotransferase activity with iron and antioxidant levels in the United States. Gastroenterology. 2003;124:1821-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 946] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 31. | Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 32. | Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL; Asia-Pacific Working Party on NAFLD. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 33. | Moretto M, Kupski C, Mottin CC, Repetto G, Garcia Toneto M, Rizzolli J, Berleze D, de Souza Brito CL, Casagrande D, Colossi F. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obes Surg. 2003;13:622-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Hsiao PJ, Kuo KK, Shin SJ, Yang YH, Lin WY, Yang JF, Chiu CC, Chuang WL, Tsai TR, Yu ML. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. J Gastroenterol Hepatol. 2007;22:2118-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Cheung O, Kapoor A, Puri P, Sistrun S, Luketic VA, Sargeant CC, Contos MJ, Shiffman ML, Stravitz RT, Sterling RK. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology. 2007;46:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 700] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 39. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 893] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 40. | Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 815] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 41. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 42. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3129] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 43. | Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 719] [Article Influence: 55.3] [Reference Citation Analysis (1)] |
| 45. | Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 444] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 46. | Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 332] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 47. | Le TH, Caldwell SH, Redick JA, Sheppard BL, Davis CA, Arseneau KO, Iezzoni JC, Hespenheide EE, Al-Osaimi A, Peterson TC. The zonal distribution of megamitochondria with crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2004;39:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int. 2013;7:771-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 50. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 617] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 51. | Nijhuis J, Rensen SS, Slaats Y, van Dielen FM, Buurman WA, Greve JW. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity (Silver Spring). 2009;17:2014-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 52. | Rensen SS, Slaats Y, Nijhuis J, Jans A, Bieghs V, Driessen A, Malle E, Greve JW, Buurman WA. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 53. | Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 54. | Csak T, Dolganiuc A, Kodys K, Nath B, Petrasek J, Bala S, Lippai D, Szabo G. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology. 2011;53:1917-1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Chitturi S, Farrell GC, George J. Non-alcoholic steatohepatitis in the Asia-Pacific region: future shock? J Gastroenterol Hepatol. 2004;19:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1013] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 58. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1659] [Article Influence: 118.5] [Reference Citation Analysis (2)] |
| 59. | Hsu CS, Kao JH. Non-alcoholic fatty liver disease: an emerging liver disease in Taiwan. J Formos Med Assoc. 2012;111:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 61. | Boyer TD, Manns MP, Sanyal AJ. Zakim& Boyer’s Hepatology: A Textbook of Liver Disease. Nonalcoholic Fatty Liver Disease. Saunders: an imprint of Elsevier Inc 2012; 941-968. |
| 62. | Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic fatty liver disease and insulin resistance: from bench to bedside. Diabetes Metab. 2013;39:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 331] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 64. | Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Kempf J, Zinman B, Haffner SM; insulin resistance atherosclerosis study. Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 65. | Stranges S, Trevisan M, Dorn JM, Dmochowski J, Donahue RP. Body fat distribution, liver enzymes, and risk of hypertension: evidence from the Western New York Study. Hypertension. 2005;46:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Tanné F, Gagnadoux F, Chazouillères O, Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R, Serfaty L. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 67. | Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol. 2005;42 Suppl:S2-12. [PubMed] |
| 68. | Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 822] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 69. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Nonalcoholic Fatty Liver Disease. Saunders: an imprint of Elsevier Inc 2010; 1401-1411. |
| 70. | Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2004;99:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Bugianesi E, Manzini P, D’Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 302] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 72. | Karcaaltincaba M, Akhan O. Imaging of hepatic steatosis and fatty sparing. Eur J Radiol. 2007;61:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 74. | Mazhar SM, Patton HM, Scuderi RT, Yokoo T, Fari CS, Sirlin CB. Fatty Liver Disease. Abdominal Imaging. Saunders: an imprint of Elsevier Inc 2011; 595-606. |
| 75. | Rofsky NM, Fleishaker H. CT and MRI of diffuse liver disease. Semin Ultrasound CT MR. 1995;16:16-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis. 2007;11:37-54, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 77. | Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD, Bell JD, Taylor-Robinson SD. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Elias J, Altun E, Zacks S, Armao DM, Woosley JT, Semelka RC. MRI findings in nonalcoholic steatohepatitis: correlation with histopathology and clinical staging. Magn Reson Imaging. 2009;27:976-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Yokoo T, Bydder M, Hamilton G. Hepatic fat quantification by low flip-angle multi-echo gradient-echo MR imaging: a clinical study with validation with MR spectroscopy. Presented before the annual meeting of the International Society for Magnetic Resonance in Medicine. Canada: Toronto 2008; 706. |
| 80. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 81. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [PubMed] |
| 82. | Lall CG, Aisen AM, Bansal N, Sandrasegaran K. Nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2008;190:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Cortez-Pinto H, Baptista A, Camilo ME, De Moura MC. Nonalcoholic steatohepatitis--a long-term follow-up study: comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci. 2003;48:1909-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 85. | Barshop NJ, Francis CS, Schwimmer JB, Lavine JE. Nonalcoholic fatty liver disease as a comorbidity of childhood obesity. Ped Health. 2009;3:271-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Roberts EA. Non-alcoholic steatohepatitis in children. Clin Liver Dis. 2007;11:155-172, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, Xanthakos SA, Whitington PF, Charatcharoenwitthaya P, Yap J. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 88. | Ozturk Y, Soylu OB. Fatty liver in childhood. World J Hepatol. 2014;6:33-40. [PubMed] |
| 89. | Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 90. | Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non-alcoholic steatohepatitis. J Clin Gastroenterol. 2004;38:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Itai Y, Saida Y. Pitfalls in liver imaging. Eur Radiol. 2002;12:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Rinella ME, McCarthy R, Thakrar K, Finn JP, Rao SM, Koffron AJ, Abecassis M, Blei AT. Dual-echo, chemical shift gradient-echo magnetic resonance imaging to quantify hepatic steatosis: Implications for living liver donation. Liver Transpl. 2003;9:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Pilleul F, Chave G, Dumortier J, Scoazec JY, Valette PJ. Fatty infiltration of the liver. Detection and grading using dual T1 gradient echo sequences on clinical MR system. Gastroenterol Clin Biol. 2005;29:1143-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Lelbach WK. Epidemiology of alcoholic liver disease. Prog Liver Dis. 1976;5:494-515. [PubMed] |
| 95. | Kawamori Y, Matsui O, Takahashi S, Kadoya M, Takashima T, Miyayama S. Focal hepatic fatty infiltration in the posterior edge of the medial segment associated with aberrant gastric venous drainage: CT, US, and MR findings. J Comput Assist Tomogr. 1996;20:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Patton HM, Patel K, Behling C, Bylund D, Blatt LM, Vallée M, Heaton S, Conrad A, Pockros PJ, McHutchison JG. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 97. | Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 98. | Fernandez FG, Ritter J, Goodwin JW, Linehan DC, Hawkins WG, Strasberg SM. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 99. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2886] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 100. | Niskanen L, Uusitupa M, Sarlund H, Siitonen O, Paljärvi L, Laakso M. The effects of weight loss on insulin sensitivity, skeletal muscle composition and capillary density in obese non-diabetic subjects. Int J Obes Relat Metab Disord. 1996;20:154-160. [PubMed] |
| 101. | Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855-1867. [PubMed] |
| 102. | Luyckx FH, Desaive C, Thiry A, Dewé W, Scheen AJ, Gielen JE, Lefèbvre PJ. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 317] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 103. | Luyckx FH, Scheen AJ, Desaive C, Thiry A, Lefébvre PJ. Parallel reversibility of biological markers of the metabolic syndrome and liver steatosis after gastroplasty-induced weight loss in severe obesity. J Clin Endocrinol Metab. 1999;84:4293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 105. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 484] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 106. | Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 107. | Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 108. | Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31:384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 200] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 109. | Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 522] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 110. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 479] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 111. | Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 112. | Ozturk ZA, Kadayifci A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J Hepatol. 2014;6:199-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 113. | Angulo P. Use of ursodeoxycholic acid in patients with liver disease. Curr Gastroenterol Rep. 2002;4:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 114. | Obinata K, Maruyama T, Hayashi M, Watanabe T, Nittono H. Effect of taurine on the fatty liver of children with simple obesity. Adv Exp Med Biol. 1996;403:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 116. | Abdelmalek MF, Sanderson SO, Angulo P, Soldevila-Pico C, Liu C, Peter J, Keach J, Cave M, Chen T, McClain CJ. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 117. | Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 118. | Satapathy SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946-1952. |
| 119. | Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 120. | Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 121. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 122. | NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956-961. [PubMed] |
| 123. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 352] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 124. | Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 125. | Burke A, Lucey MR. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |