Published online Dec 28, 2015. doi: 10.4254/wjh.v7.i30.2933
Peer-review started: May 25, 2015
First decision: August 16, 2015
Revised: October 7, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: December 28, 2015
Processing time: 216 Days and 22.2 Hours
Gadoxetic acid- or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) achieves excellent lesion detection and characterization for both hypervascular hepatocellular carcinoma (HCC) in arterial phase imaging and hypovascular early HCC (small well-differentiated HCC of the vaguely nodular type) in hepatobiliary phase imaging, and has become an indispensable imaging modality in the treatment of HCC. Early HCCs have been detected more frequently since the introduction of EOB-MRI into daily clinical practice. Early HCC is known to progress to conventional hypervascular HCC, and many risk factors have been identified for the hypervascularization of early HCC including the diameter of the tumor, presence of fat, and imaging findings of EOB-MRI. The rate of the development of hypervascular HCC was previously reported to be high in patients with chronic liver disease and early HCC. The presence of early HCC is regarded as a predictor for the recurrence of HCC following hepatic resection. On the other hand, although early HCC itself is currently not regarded as a target lesion for hepatic resection, early HCC at high risk of hypervascularity needs to be treated by local ablation therapy. If concomitant early HCC with progressed HCC is at high risk of hypervascularization and the functional liver reserve of a patient is sufficient, its simultaneous treatment at the time of hepatic resection for progressed HCC is recommended. Further studies on larger numbers of patients are needed before this strategy is adopted.
Core tip: Gadoxetic acid- or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging has excellent lesion detection and characterization for both hypervascular hepatocellular carcinomas (HCC) in arterial phase imaging and hypovascular early HCC in hepatobiliary phase imaging, and has become an indispensable imaging modality in the treatment of HCC. Early HCC is known to progress to conventional hypervascular HCC. Although early HCC itself is currently not considered to be a target lesion for hepatic resection, if concomitant early HCC with progressed HCC is at high risk of hypervascularization, its simultaneous treatment at the time of hepatic resection is recommended.
- Citation: Matsuda M. Clinical value of gadoxetic acid-enhanced magnetic resonance imaging in surgery for hepatocellular carcinoma - with a special emphasis on early hepatocellular carcinoma. World J Hepatol 2015; 7(30): 2933-2939
- URL: https://www.wjgnet.com/1948-5182/full/v7/i30/2933.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i30.2933
Hepatocellular carcinoma (HCC) is one of the most malignant tumors worldwide, and hepatic resection still represents the most effective treatment; however, the recurrence rate of HCC is very high even after curative resection. The postoperative 5-year recurrence rate was previously reported to be higher than 70%, with 80% to 95% of recurrence being confined to the liver[1-3].
A proper preoperative evaluation of intrahepatic tumor progression by imaging modalities and appropriate hepatic resection, in addition to the early diagnosis of recurrent HCC followed by treatment[4,5], are needed in order to achieve a favorable prognosis after hepatic resection.
A new magnetic resonance imaging (MRI) contrast medium, gadoxetic acid, or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA), which has the properties of both an extracellular gadolinium chelate and liver-specific (hepatocyte-targeting) contrast material, has recently become available. The injection of a bolus of Gd-EOB-DTPA allows tumor vascularity to be assessed using arterial phase imaging and enables hepatobiliary phase imaging approximately 20 min after its administration, with approximately 50% of the contrast material being taken up by hepatocytes[6-11]. Gd-EOB-DTPA-enhanced MRI (EOB-MRI), which includes a gradient dual echo sequence and diffusion-weighted imaging, has been recommended for the pretherapeutic evaluation of patients with HCC[12]. We previously showed that EOB-MRI was the most useful imaging technique for evaluating small HCC, including early HCC[11].
Early HCCs have been detected more frequently in daily clinical practice since the introduction of EOB-MRI in Japan.
The treatment of early HCCs including hepatic resection will become a very important issue in the near future.
Current understanding on the clinical value of EOB- MRI in surgery for HCC and the significance of newly diagnosed early HCC from several clinical viewpoints have been outlined in this review. The prospect of treatment strategies for newly diagnosed early HCC concomitantly with progressed HCC has also been discussed.
Ichikawa et al[13] analyzed the findings of multicenter phase III studies in order to evaluate the safety and efficacy of EOB-MRI for the detection and characterization of focal liver lesions. They showed that EOB-MRI was safe and improved the detection and characterization of focal hepatic lesions over that with unenhanced MRI. EOB-MRI also appeared to be more beneficial than spiral computed tomography (CT), especially for the detection of smaller lesions or HCC underlying cirrhotic liver. Therefore, they concluded that EOB-MRI enabled excellent lesion detection and characterization for both hypervascular HCCs in arterial phase imaging and hypovascular HCCs in hepatobiliary phase imaging[13]. EOB-MRI is recommended every 3-4 mo in selected cases of HCC ultrahigh-risk groups, and at least once during the first visit in all HCC ultrahigh-risk groups[14].
Therefore, we routinely perform EOB-MRI together with other imaging modalities prior to hepatic resection for HCC. EOB-MRI is also used in postoperative follow-ups for patients with HCC after hepatic resection.
Two types of human hepatocarcinogenesis are now considered: De novo hepatocarcinogenesis and multistep carcinogenesis from a low-grade dysplastic nodule (DN) to a high-grade DN followed by early HCC and hypervascular HCC (progressed HCC)[15-17].
However, difficulties have been associated with the precise histological diagnosis of early HCC and accurate differentiation between early HCC and DN because of similarities in their pathological features[18].
The International Consensus Group for Hepatocellular Neoplasia (ICGHN), which was composed of 34 world-renowned pathologists and two clinicians, finally announced a consensus on the pathological criteria of early HCC (small well-differentiated HCC of the vaguely nodular type) after significant debate in 2009. In this report, early HCC was characterized by various combinations of the following major histological features: (1) a cell density more than 2-fold higher than that of the surrounding tissue, with a higher nuclear/cytoplasm ratio and irregularly thin trabecular pattern; (2) varying numbers of portal tracts within the nodule (intratumoral portal tracts); (3) pseudoglandular pattern; (4) diffuse fatty change; and (5) varying numbers of unpaired arteries.
Since all of these features may be found in high-grade DN, stromal invasion remains the most helpful objective pathological finding for differentiating early HCC from DN[19].
Among the various imaging modalities currently used, the role of EOB-MRI has become increasingly important in the diagnosis of early HCC.
We conducted a review on the imaging findings obtained from multi-imaging modalities of early HCC cases diagnosed according to the pathological criteria of the ICGHN by only including surgically resected nodules in order for pathologists to thoroughly investigate the whole nodule. These multi-imaging modalities included EOB-MRI, contrast-enhanced CT, CT during arterioportography, and CT during hepatic arteriography. EOB-MRI is the only imaging modality that has sufficient resolution for the detection and classification of early HCC. The most significant imaging feature in the diagnosis of early HCC was hypointensity on hepatobiliary phase images of EOB-MRI; all cases of early HCC that were detected on the hepatobiliary phase images of EOB-MRI showed hypointensity, while all of the images of DN showed isointensity or hyperintensity relative to the liver parenchyma. The findings of the diagnostic performance analysis showed that EOB-MRI had excellent sensitivity (97%) for detecting early HCC and exceptional specificity (100%) for distinguishing early HCC from DN[11,20].
Hypovascular nodules that appear hypointense on hepatobiliary phase EOB-MRI (hypovascular hypointense nodules) may progress to conventional hypervascular HCC[21]. Therefore, identifying the risk factors for the hypervascularization of these nodules, most of which are early HCCs, is important for decision making on the timing of treatment.
We previously reported that nodules that were more than 10 mm in diameter and contained fat were at a higher risk of developing hypervascularization[22]. In addition, a maximum diameter of more than 10 mm[23] or 15 mm or greater[24], increased growth rate, hyperintensity on T1-weighted images[25], hyperintensity on T2-weighted and diffusion-weighted images[26], and a tumor volume doubling time of less than 542 d[27] were identified as risk factors for hypervascularization in early HCC.
In order to determine whether the presence of a hypovascular hypointense nodule was a risk factor for hypervascular HCC in patients with chronic liver disease, we retrospectively selected 41 patients with pathologically confirmed hypervascular HCC and 41 age- and gender-matched controls and evaluated risk factors for hypervascular HCC. A multivariate analysis revealed that serum albumin levels (OR = 0.19, 95%CI: 0.06-0.57; P = 0.0024), a history of hypervascular HCC (OR = 8.62 95%CI: 2.71-32.8; P = 0.0001), and the presence of a hypovascular hypointense nodule (OR = 4.18, 95%CI: 1.18-17.2; P = 0.0256) were significant risk factors for hypervascular HCC. We concluded that the risk of developing HCC was high in patients with chronic liver disease showing a hypovascular hypointense nodule[28].
Komatsu et al[29] selected 127 patients with chronic hepatitis B or C and no history of HCC, including 68 with liver cirrhosis, divided them into those with (non-clean liver group, n = 18) and without (clean liver group, n = 109) hypovascular hypointense nodules, and investigated whether the risk of hepatocarcinogenesis was higher in patients with these nodules. Seventeen patients (10 in the non-clean liver group and seven in the clean liver group) developed typical HCC. The cumulative 3-year rates of HCC development were 55.5% in the non-clean liver group and 6.4% in the clean liver group (P < 0.001), and those at different sites from the initial nodules were also higher in the non-clean liver group (22.2%) than in the clean liver group (6.4%) (P = 0.003). A multivariate analysis identified an older age (P = 0.024), low platelet count (P = 0.017), and non-clean liver (P < 0.001) as independent risk factors for the subsequent development of HCC[29].
The imaging findings of HCC detected by EOB-MRI are useful predictors of recurrence after hepatic resection. Ariizumi et al[30] determined the tumor margins of HCC from 61 patients preoperatively based on the hepatobiliary phase images of EOB-MR and found that a non-smooth tumor margin in the hepatobiliary phase of EOB-MRI predicted microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatic resection in patients with HCC.
The presence of non-hypervascular hypointense hepatic nodules detected during the hepatobiliary phase of EOB-MRI was identified as another predictor of the recurrence of HCC after hepatic resection. Toyoda et al[31] prospectively examined 77 patients who underwent hepatic resection for primary, non-recurrent, hypervascular HCC after hepatic resection and compared postoperative recurrence rates according to the presence of non-hypervascular hypointense hepatic nodules detected during the hepatobiliary phase of EOB-MRI. They showed that recurrence rates after hepatic resection were higher in patients with non-hypervascular hypointense nodules (RR = 1.9396, 95%CI: 1.3615-2.7222), the presence of non-hypervascular hypointense nodules was an independent factor associated with postoperative recurrence (RR = 2.1767, 95%CI: 1.5089-3.1105) in addition to HCC differentiation and portal vein invasion, and intrahepatic recurrence was mainly multicentric in origin[31]. This group subsequently reported that the presence of concurrent non-hypervascular hypointense hepatic nodules in the hepatobiliary phase of pretreatment EOB-MRI in patients with early-stage typical HCC was an indicator of the higher likelihood of recurrence after treatments including hepatic resection and radiofrequency ablation (RFA), and may be a marker for an unfavorable outcome[32].
The signal intensity of HCC in the hepatobiliary phase of EOB-MRI was found to be another prognostic maker of HCC after hepatic resection.
Kitao et al[33] classified 180 surgically resected hypervascular HCCs in 180 patients as either hypointense (n = 158) or hyperintense (n = 22) relative to the signal intensity of the background liver in the hepatobiliary phase of EOB-MRI and compared clinical and pathological features as well as recurrence and survival rates after hepatic resection between the two groups. The grade of differentiation was higher (P = 0.028) and portal vein invasion was less frequent in hyperintense HCCs (13.6%) than in hypointense HCCs (36.7%) (P = 0.039). Serum levels of alpha-fetoprotein (AFP), the Lens culinaris agglutinin reactive fraction of AFP, and prothrombin induced by vitamin K absence or antagonist II (PIVKA-II) were lower in hyperintense than in hypointense HCCs (P = 0.003, 0.004 and 0.026, respectively). Immunohistochemical AFP and PIVKA-II expression levels were lower in hyperintense than in hypointense HCCs (both P < 0.001) and organic anion transporting polypeptide 8 (OATP8, synonymous with OATP1B3) expression was significantly lower in hypointense HCCs than in hyperintense HCCs (P < 0.001). The recurrence rate was lower in hyperintense than in hypointense HCCs (P = 0.039). They concluded that hyperintense HCC in the hepatobiliary phase of EOB-MRI was less aggressive than hypointense HCCs[33].
It has not yet been established whether early HCC need to be treated by hepatic resection.
Takayama et al[34] prospectively examined 70 patients diagnosed with a single HCC of 2 cm or less in diameter who underwent curative hepatic resection and a long-term follow-up. They found that the time to recurrence was longer in the early HCC group than in the overt HCC group (3.9 years vs 1.7 years; P < 0.001) and there was no local recurrence. Therefore, they concluded that early HCC is a distinct clinical entity with a high rate of surgical cure. This group subsequently applied the concept of lead time to chronic liver disease, which is the length of time between screen-detected and symptom-detected disease. In order to evaluate the prolongation of survival with the treatment of early HCC, they compared the survival of patients with early and overt HCCs smaller than 2.0 cm treated with liver resection and concluded that the survival benefit of resection for early HCC was marginal because of a long lead time, and, thus, demonstrated that early HCC was not a target lesion for surgery[35].
Early HCC itself is not a target lesion for hepatic resection. However, early HCC at risk of hypervascularity needs to be treated by local ablation therapy including RFA and not surgery because early HCC seldom causes intrahepatic metastasis.
Although early HCC itself is not a target lesion for hepatic resection, the treatment of concomitant early HCC with progressed HCC remains controversial.
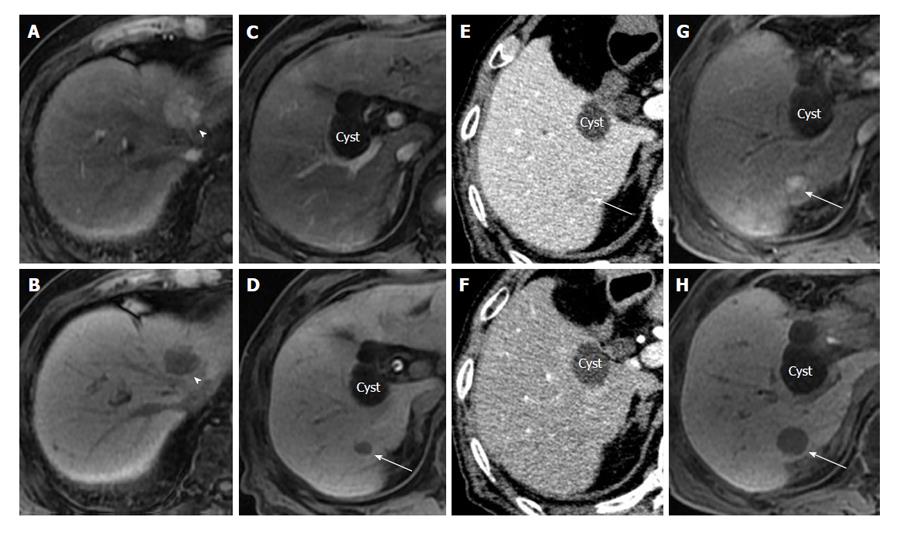
We sometimes encounter patients who developed the recurrence of hypervascular HCC that originated from residual early HCC in a short period of time after hepatic resection (Figure 1).
Therefore, we examined 147 patients undergoing hepatic resection for HCC and determined whether pre-operative imaging with EOB-MRI and the simultaneous treatment of concomitant early HCC by resection or ablation at the time of resection for progressed HCC improved the prognosis of patients following hepatic resection. Of the 147 consecutive patients undergoing their first resection for HCC, 77 received EOB-MRI before resection. Additional treatments for early HCC were more frequent in EOB-MRI patients. Recurrence-free survival was similar at 1 year (81.4% vs 82.1%), but improved with EOB-MRI at 3 and 5 years (62.6% and 48.7% vs 41.5% and 25.5%, respectively, P < 0.01). We speculated that recurrence within one year of hepatic resection was mainly due to the enlargement of preoperatively-undetectable intrahepatic micrometastasis of resected progressed HCC, while recurrence after one year was multicentric HCC that progressed from early HCC to hypervascular HCC or was de novo hypervascular HCC. We estimated that the simultaneous treatment of early HCC at the time of resection for progressed HCC reduced multicentric HCC by removing early HCC, which may have progressed to hypervascular HCC. Overall survival slightly improved with EOB-MRI at all time points (1-, 3-, and 5-year survival rates: 98.7%, 90.7% and 80.8% vs 97.0%, 86.3% and 72.4%, respectively, P = 0.38). One of the reasons why preoperative EOB-MRI and the simultaneous treatment of early HCC at the time of resection prolonged recurrence-free survival but not overall survival after hepatic resection for HCC was an early diagnosis and the prompt treatment of recurrent HCC detected by our postoperative close follow-up[36].
We subsequently examined the 5-year survivors of both groups (41 with EOB-MRI and 48 without) in order to investigate the frequency of treatments for recurrent HCC until 5 years after surgery in both groups. The mean frequency of treatments for recurrent HCC until 5 years after surgery was significantly lower in patients with EOB-MRI than in those without EOB-MRI (0.83 vs 1.65 respectively, P < 0.05). Although the overall survival rate was not significantly different, reductions in the frequency of treatments for recurrent HCC may have been physically and economically beneficial for patients.
Further studies on larger numbers of patients are needed before this strategy is adopted.
EOB-MRI has excellent lesion detection and characterization for hypervascular HCC in arterial phase imaging and hypovascular early HCC in hepatobiliary phase imaging, and has become an indispensable imaging modality in the treatment of HCC. Early HCCs have been detected more frequently since the introduction of EOB-MRI into daily clinical practice. Although optimal timing for the treatment of early HCC currently remains unclear, early HCC, which has particular risk factors, progresses to conventional hypervascular HCC that requires treatment in a short period of time. Early HCC at high risk of hypervascularity need to be treated by local ablation therapy including RFA. If concomitant early HCC with progressed HCC is at a high risk of hypervascularization and the functional liver reserve of a patient is sufficient, its simultaneous treatment at the time of hepatic resection for progressed HCC is recommended. Further studies on larger numbers of patients are needed before this strategy is adopted. Furthermore, since the risk of hypervascular HCC development or recurrence is high in patients with hypovascular hypointense nodules (almost all nodules are early HCC), close follow-ups are needed in these patients.
P- Reviewer: Morana G, Schmeding M, Tarazov PG S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
| 1. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 467] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 3. | Takayama T. Surgical treatment for hepatocellular carcinoma. Jpn J Clin Oncol. 2011;41:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Matsuda M, Fujii H, Kono H, Matsumoto Y. Surgical treatment of recurrent hepatocellular carcinoma based on the mode of recurrence: repeat hepatic resection or ablation are good choices for patients with recurrent multicentric cancer. J Hepatobiliary Pancreat Surg. 2001;8:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Matsuda M, Asakawa M, Amemiya H, Fujii H. Lens culinaris agglutinin-reactive fraction of AFP is a useful prognostic biomarker for survival after repeat hepatic resection for HCC. J Gastroenterol Hepatol. 2011;26:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, Schwarz W, Müller PK, Bechstein WO, Mack MG. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996;200:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 365] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Reimer P, Rummeny EJ, Shamsi K, Balzer T, Daldrup HE, Tombach B, Hesse T, Berns T, Peters PE. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 244] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010;255:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 10. | Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging--correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, Nakano M, Sakamoto M, Nakazawa T, Asakawa M. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 12. | Ooka Y, Kanai F, Okabe S, Ueda T, Shimofusa R, Ogasawara S, Chiba T, Sato Y, Yoshikawa M, Yokosuka O. Gadoxetic acid-enhanced MRI compared with CT during angiography in the diagnosis of hepatocellular carcinoma. Magn Reson Imaging. 2013;31:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, Kamura T, Gabata T, Murakami T, Ito K. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Kudo M, Matsui O, Sakamoto M, Kitao A, Kim T, Ariizumi S, Ichikawa T, Kobayashi S, Imai Y, Izumi N. Role of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in the management of hepatocellular carcinoma: consensus at the Symposium of the 48th Annual Meeting of the Liver Cancer Study Group of Japan. Oncology. 2013;84 Suppl 1:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kojiro M. Pathology of Hepatocellular Carcinoma. United States: Blackwell Publishing Ltd., MA 2006; . |
| 16. | Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44 Suppl 19:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology. 2009;252:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991;22:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 369] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | International Consensus Group for Hepatocellular NeoplasiaThe International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 589] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 20. | Ichikawa T, Sano K, Morisaka H. Diagnosis of Pathologically Early HCC with EOB-MRI: Experiences and Current Consensus. Liver Cancer. 2014;3:97-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Kobayashi S, Matsui O, Gabata T, Koda W, Minami T, Ryu Y, Kozaka K, Kitao A. Relationship between signal intensity on hepatobiliary phase of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced MR imaging and prognosis of borderline lesions of hepatocellular carcinoma. Eur J Radiol. 2012;81:3002-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Motosugi U, Ichikawa T, Sano K, Sou H, Onohara K, Muhi A, Amemiya F, Enomoto N, Matsuda M, Fujii H. Outcome of hypovascular hepatic nodules revealing no gadoxetic acid uptake in patients with chronic liver disease. J Magn Reson Imaging. 2011;34:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Takechi M, Tsuda T, Yoshioka S, Murata S, Tanaka H, Hirooka M, Mochizuki T. Risk of hypervascularization in small hypovascular hepatic nodules showing hypointense in the hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with chronic liver disease. Jpn J Radiol. 2012;30:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kumada T, Toyoda H, Tada T, Sone Y, Fujimori M, Ogawa S, Ishikawa T. Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2011;197:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Higaki A, Ito K, Tamada T, Teruki S, Yamamoto A, Higashi H, Kanki A, Sato T, Noda Y. High-risk nodules detected in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging in cirrhosis or chronic hepatitis: incidence and predictive factors for hypervascular transformation, preliminary results. J Magn Reson Imaging. 2013;37:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kim YK, Lee WJ, Park MJ, Kim SH, Rhim H, Choi D. Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology. 2012;265:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Hyodo T, Murakami T, Imai Y, Okada M, Hori M, Kagawa Y, Kogita S, Kumano S, Kudo M, Mochizuki T. Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology. 2013;266:480-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Ichikawa S, Ichikawa T, Motosugi U, Sano K, Morisaka H, Enomoto N, Matsuda M, Fujii H, Araki T. Presence of a hypovascular hepatic nodule showing hypointensity on hepatocyte-phase image is a risk factor for hypervascular hepatocellular carcinoma. J Magn Reson Imaging. 2014;39:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Komatsu N, Motosugi U, Maekawa S, Shindo K, Sakamoto M, Sato M, Tatsumi A, Miura M, Amemiya F, Nakayama Y. Hepatocellular carcinoma risk assessment using gadoxetic acid-enhanced hepatocyte phase magnetic resonance imaging. Hepatol Res. 2014;44:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Ariizumi S, Kitagawa K, Kotera Y, Takahashi Y, Katagiri S, Kuwatsuru R, Yamamoto M. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;18:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Sone Y, Kaneoka Y, Maeda A. Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol. 2013;58:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Toyoda H, Kumada T, Tada T, Sone Y, Maeda A, Kaneoka Y. Non-hypervascular hypointense nodules on Gd-EOB-DTPA-enhanced MRI as a predictor of outcomes for early-stage HCC. Hepatol Int. 2015;9:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, Gabata T, Yamashita T, Kaneko S, Nakanuma Y. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology. 2012;265:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, Kosuge T, Okada S, Takayasu K, Yamasaki S. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Midorikawa Y, Takayama T, Shimada K, Nakayama H, Higaki T, Moriguchi M, Nara S, Tsuji S, Tanaka M. Marginal survival benefit in the treatment of early hepatocellular carcinoma. J Hepatol. 2013;58:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Matsuda M, Ichikawa T, Amemiya H, Maki A, Watanabe M, Kawaida H, Kono H, Sano K, Motosugi U, Fujii H. Preoperative gadoxetic Acid-enhanced MRI and simultaneous treatment of early hepatocellular carcinoma prolonged recurrence-free survival of progressed hepatocellular carcinoma patients after hepatic resection. HPB Surg. 2014;2014:641685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |