Published online Mar 27, 2015. doi: 10.4254/wjh.v7.i3.377
Peer-review started: August 28, 2014
First decision: September 19, 2014
Revised: November 10, 2014
Accepted: November 27, 2014
Article in press: November 27, 2014
Published online: March 27, 2015
Processing time: 215 Days and 7.8 Hours
Recent data indicate that hepatic angiogenesis, regardless of the etiology, takes place in chronic liver diseases (CLDs) that are characterized by inflammation and progressive fibrosis. Because anti-angiogenic therapy has been found to be efficient in the prevention of fibrosis in experimental models of CLDs, it is suggested that blocking angiogenesis could be a promising therapeutic option in patients with advanced fibrosis. Consequently, efforts are being directed to revealing the mechanisms involved in angiogenesis during the progression of liver fibrosis. Literature evidences indicate that hepatic angiogenesis and fibrosis are closely related in both clinical and experimental conditions. Hypoxia is a major inducer of angiogenesis together with inflammation and hepatic stellate cells. These profibrogenic cells stand at the intersection between inflammation, angiogenesis and fibrosis and play also a pivotal role in angiogenesis. This review mainly focuses to give a clear view on the relevant features that communicate angiogenesis with progression of fibrosis in CLDs towards the-end point of cirrhosis that may be translated into future therapies. The pathogenesis of hepatic angiogenesis associated with portal hypertension, viral hepatitis, non-alcoholic fatty liver disease and alcoholic liver disease are also discussed to emphasize the various mechanisms involved in angiogenesis during liver fibrogenesis.
Core tip: Hepatic angiogenesis is closely associated with the progression of fibrosis in chronic liver diseases (CLDs). Recent evidences demonstrated that blocking angiogenesis means also prevention of fibrosis progression. Hypoxia plays a crucial role in eliciting angiogenesis together with hepatic stellate cells being the most prominent sources of vascular endothelial growth factor and Angiopoietin-1. Adipokines, endoplasmic reticulum stress and related unfolded protein response; neuropilins; might be future therapeutical target in the progression of fibrosis in CLDs. Moreover studies on non-alcoholic steatohepatits demonstrated that of angiotensin and renin inhibitors could be effectively used as a new treatment strategy against angiogenesis in the prevention of fibrosis in CLDs.
- Citation: Elpek G&. Angiogenesis and liver fibrosis. World J Hepatol 2015; 7(3): 377-391
- URL: https://www.wjgnet.com/1948-5182/full/v7/i3/377.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i3.377
Angiogenesis, the formation of new vessels from preexisting vasculature, is an active, growth factor dependent and hypoxia induced event that takes place in several organs during growth and repair of injured tissues[1,2]. It should always be distinguished from other characteristic mechanisms of vessel growth that include vasculogenesis, arteriogenesis, and collateral vessel growth[3,4].
Although it is crucial for tissue growth and regeneration, accumulated evidence indicated that angiogenesis develops in many organs during multiple pathologic situations. Angiogenesis is a fundamental part of tumor progression and contributes to the pathogenesis of different inflammatory, fibroproliferative and ischemic diseases[2]. Recent studies demonstrated that chronic liver diseases (CLDs) can not be excluded from this rule, emerging angiogenesis as a promising therapeutic target[5-11].
As a matter of fact angiogenesis does not solely takes place in CLDs but has been clearly documented in many conditions including liver regeneration (after acute liver injury or partial hepatectomy), ischemia, in primary (hepatocellular carcinoma) and metastatic tumors[3,12]. It is still unclear whether angiogenesis represents a simple response to maintain homeostasis or one that exerts a pathological role leading to liver injury. However, recent data in CLDs have been releaved that angiogenesis might contributes to the progression of fibrosis during the wound healing process in chronic liver damage[3,5,7,9,10,12]. Consequently, efforts are being directed to revealing the mechanisms that are involved in angiogenesis in CLDs with different etiology.
In this review first, a consideration of the basic mechanisms and events in angiogenesis will be described. The following section will be focused to give a clear view on the relevant features that communicate angiogenesis with progression of fibrosis in CLDs towards the-end point of cirrhosis. I recommend to the intrested reader to refer to more detailed comprehensive articles on the role of angiogenesis in liver regeneration or liver tumors.
In general two main pathways are determined in the progression of angiogenesis in all tissues: inflammation and hypoxia.
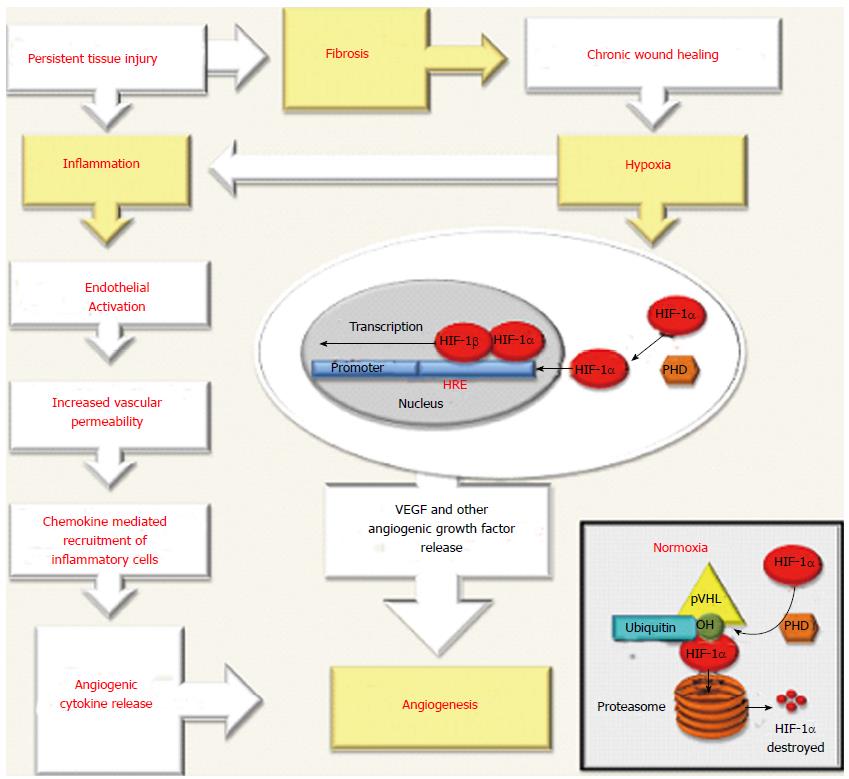
In physiological angiogenesis, an immune response triggered by tissue damage provide the extravasation of immune cells from peripheral blood into the injured tissue leading to the restoration of tissue homeostasis[13]. However, the persistence of tissue damage and accompanying inflammation perpetuates the activation of endothelial cells (ECs) resulting an increase of vascular permeability and promoting chemokine-mediated recruitment of inflammatory cells[13-15]. These cells can produce angiogenic cytokines and growth factors that induce the proliferation and migration of ECs that are necessary for the formation of new vessels[14,16-18]. Besides, during chronic inflammation the accumulation inflammatory cells together with fibrosis may contribute to hypoxia, by increasing the resistance of damaged tissue to blood flow and oxygen (O2) supply[15,19,20]. On the other hand accumulated evidences indicate hypoxia alone could be important in the stimulation of angiogenesis and can also stimulate inflammation leading to a viscous circle between inflammation and angiogenesis[3,21,22] (Figure 1). Hypoxia activates angiogenesis as a result of signaling mediated by hypoxia-inducible factors (HIFs)[12,21-23]. By definition, these critical molecular mediators are transcription factors which promote cells to react to the reduced levels of pO2 in the site of injury by up-regulation of several genes carrying the hypoxia response elements (HRE) sequences in their promoter or enhancer[12,22-24]. HIFs are heterodimers formed by an oxygen sensitive and inducible α subunit and an oxygen-independent β subunit[12,22-24]. Three α subunits, named hypoxia inducible factor-1-α (HIF-1α), HIF-2α and HIF-3α have been described and all bind to a common β subunit named, the aryl hydrocarbo nuclear receptor translocator (ARNT), alternatively, HIF-1β[23,24]. The best-characterized member of this family is HIF-1, is regarded as a main regulator of homeostasis [12,22-24]. Under the normal levels of O2, HIF-1α is incessantly hydroxylated by several enzymes [prolyl-hydroxylases (PHD1,PHD2 or PHD3) and asparaginyl hydroxylase (FIH1)] whose activity is O2 dependent[23,24]. This modified HIF-1α scaffolded on a multimeric protein complex including von Hippel-Lindau protein(VHL) leading to rapid ubiquitination and proteasomal degradation[23,24]. Hypoxia inhibits the activity of O2 dependent enzymes and HIF-1α forms a heterodimer with HIF-1β subunit then phosphorylated and stabilized to form a transcriptional complex able to bind HRE sequences in the promoter region of target genes in the nuclei[23,24]. HIFs activate transcription of a broad range of genes[12,19,22-24]. Although a comprehensive list of HIF targets exist, only oxygen dependent regulation of HIF-1α in normal and hypoxic conditions is in the scope of this article. The target genes in mediating hypoxia-induced angiogenesis are demonstrated in Figure 1.
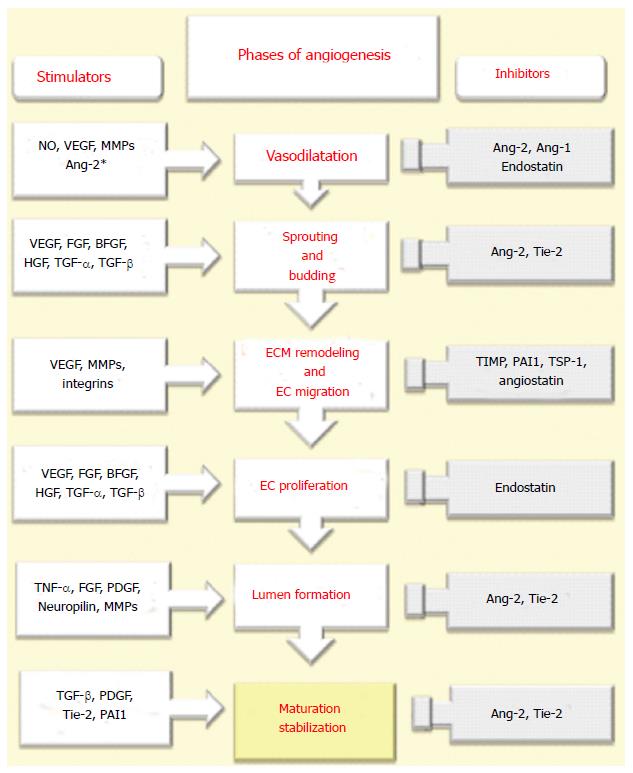
The development of new functional vessels is closely related to precise orchestration of the molecular effectors that stimulate different processes. It comprehends consecutive phases and a large spectrum of proangiogenic mediators. These phases and mediators that are involved in angiogenesis are described in Figure 2.
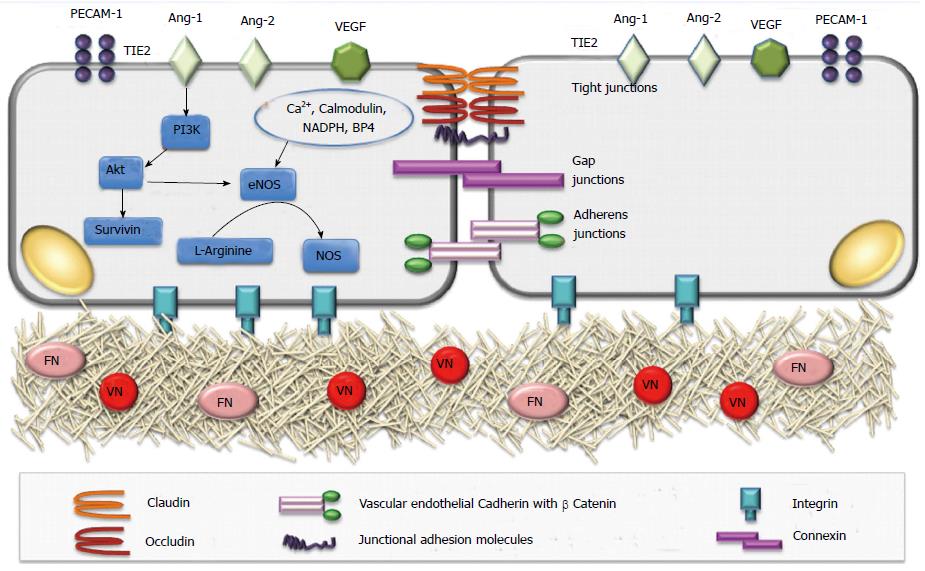
This is the fist step of angiogenesis that involves the changes in structural organization in terms of intercellular and matrix interactions of ECs. In quiescent state ECs adhere to each other and to extracellular matrix (ECM) through inter-endothelial junctions (IEJs) and integrin receptors that provide together a mechanical strength and tightness to establish a barrier, as well as allow intercellular communications[25,26]. IEJs comprise tight junctions, gap junctions and adherens junctions[25-27]. While occludin, claudins, and junctional adhesion molecules are the keystones of tight junctions, VE-cadherin is necessary for formation of adherens junctions and connexins constitute gap junctions[25-27]. The contacts are also relevant through CD31 (PECAM1)[25,26]. The link between ECs with ECM is provided by the connection of integrin receptors with matrix proteins [fibronectin (FN) or vitronectin (VN)][26,27] (Figure 3).
After an initiating stimulus (mainly hypoxia) NO-dependent vasodilation and the influence of Ang-2 and vascular endothelial growth factor (VEGF) on increased vascular permeability with loosening of all those inter-endothelial contacts result in leakiness from vessels[26-28]. The extravasation of plasma proteins together with ECM components constitute a scaffold for migration of ECs[26-28]. The main antagonist to these starting events is represented by Angiopoietin-1 (Ang1), which tightens inter-endothelial contacts[26-29].
In order to allow migration, proliferation of ECs to form new sprouts, the ECM network has to be submitted to a process of proteolytic remodeling. This remodeling are related to the coordinated activity of matrix metalloproteinases (MMPs), plasminogen activators (mainly urokinase plasminogen activator or uPA) and their inhibitors [tissue inhibitors (TIMPs) and plasminogen activator inhibitor (PAI-1)][27,28,30]. Other proteinases, including heparinases and cathepsins are also involved[27,30]. The proteoliytic degradation of ECM gives rise to the exposure of cryptic epitopes and to disruption of integrin-mediated contacts between ECs and ECM leading to migration of ECs. vβ3 and vβ5 integrins regulate the connection of ECs to the ECM and provide their communication with their microenvironment[2,30]. On the other hand they (vβ3 and vβ5) may also act as anti-angiogenic factors by inhibiting VEGF and VEGF receptor Type 2 [VEGFR-2 (Flk-1)][3,31,32]. It should be noted that proteolysis during angiogenesis should be well balanced[32-34]. Insufficient or inadequate proteolysis prevents migration of ECs[32-34]. However, an exaggerated degradation of ECM impairs the migration of ECs through the disorganisation of supporting structures and results in inhibiton of angiogenesis[32-34]. Proteinases can also mediate the release of ECM-bound proangiogenic factors [VEGF, basic fibroblast growth factor (bFGF) and transforming growth factor 1 (TGF 1)] or proteolytically activate other factors, as such facilitate the migration of ECs[30].
Several angiogenic growth factors that are secreted both by ECs or surrounding cells induce the proliferation of ECs. The most relevant angiogenic factor is VEGF, that act mainly on cells expressing two tyrosine kinase receptors, VEGF-R1 (FLT-1) and VEGF-R2[34,35]. Other growth factors including transforming growth factor- (TGF-), fibroblast growth factor (FGF), TGF- and hepatocyte growth factor (HGF) also up-regulate EC proliferation[36]. In addititon, cytokines also provide positive stimuli for proliferation of ECs[17,37]. Certain chemokines, lipid mediators and hormones may stimulate proliferation of ECs[18]. In contrast, angiostatin, endostatin, interferon, platelet-derived growth factor 4, leukemia inhibiting factor and Antithrombin III are potent inhibitors of EC proliferation[37]. Following proliferation of ECs, signaling pathways and mediators that determine tube formation and branching are activated[36,37]. ECs accumulate in the form of tubular structures. VEGF, Ang-1, vβ3 and vβ5 integrins are mainly responsible in the regulation of both the diameter and length of these structures[36]. The most potent antagonist of this phase is thrombospondin[36,37].
A three dimensional of an efficient vessel network requires precise orchestration of signaling pathways that influence branching of new vessels, deposition of ECM and formation of basement membrane[36-39]. Branching is mainly monitored by ephrins and neuropilins[38,39]. TIMPs and MMPs regulates of ECM deposition and formation of basement membrane.
For nascent vessels to mature and to stabilize the recruitment of pericytes is required. This is regulated mainly by the secretion of platelet derived growth factor (PDGF)-BB[40,41]. PDGF-BB and its receptor PDGF-b subunit play a crucial role in the stabilization of nascent vessels[40,41]. PDGF-BB is released by ECs and contributes to recruit of PDGFR- b expressing mesenchymal cells to nascent vessels and leads to their proliferation[40,41]. Besides its role in the regulation of vessel maturation through stimulation of ECM, TGF-1 also induces differentiation of mesenchymal cells into pericytes[42]. Pericytes release Ang-1 that interacts with the corresponding receptor Tie-2 expressed on ECs facilitates the formation of junctions between ECs and pericytes eventually leading to the stabilization of nascent vessels[43]. Whereas lack of migration of mural cells into nascent vessels results in fragile and permeable vessels that results in hypoxia, an excess of Ang-1 ending up with the formation of tightened vessel and prevents angiogenesis[41,43].
It should be noted that Ang-2 acts differently in angiogenesis. In the presence of angiogenic signals (VEGF, Ang-1, PIGF, PDGF-BB) Ang-2 can activate Tie-2[3,36]. However, in the absence of angiogenic signals or in an excess of anti-angiogenic factors in the microenvironment Ang-2 can block Tie-2 allowing to EC death and vessel regression[3,36] .
In liver, angiogenesis proceeds with steps and molecular mechanisms that mostly coincide with those demonstrated in other part of the body. However, a number of differences in liver render angiogenesis more complex[3,5,44,45]. These differences are: (1) liver parenchyma possesses two different microvascular structures: Large vessels such as portal vessels that are lined by a continuous ECs lying on a basement membrane and liver sinusoids that are lined by fenestrated and discontinuous Ecs; (2) the presence of liver derived angiopoietin-like peptide 3 (ANGPTL3). Although no data are available at present on its role in liver angiogenesis, this peptide do not bind to the angiopoietin receptor Tie-2 but can bind αvβ3 integrin, inducing EC adhesion and migration and function to manipulate angiogenesis[45,46]. ANGPTL3 also regulates lipid, glucose, and energy metabolism independent from angiogenic effects[46]; and (3) the “stars” of liver fibrogenesis, hepatic stellate cells (HSCs), especially in their activated and myofibroblast like phenotype may contribute to angiogenesis and vascular remodeling in liver[3,5,15]. Whereas HSCs regarded as liver-specific pericytes, they differ from their microcapillary counterparts because they play an active role in modulating angiogenesis[3,5]. The role of activated HSCs in angiogenesis during liver fibrosis will be described in more details in following sections.
During chronic liver injury, angiogenesis can be interpreted by two basic phenomena. First, many liver diseases are characterized by inflammation and fibrosis leading to progressive tissue hypoxia which in turn stimulates angiogenesis[3,5]. Second, wound healing typical for CLDs is defined by an increase in the expression of some cytokines, growth factors with proangiogenic action[3,5,12,14]. Both pathways contribute to structural and functional chages in liver angioarchitecture[3,5,12,14].
Hypoxia and hepatic angiogenesis: After the first evidence about the parallel development of angiogenesis and fibrosis during liver injury their association with hypoxia has been described by many studies[5,19,24,35,44,47]. Indeed both in humans and in experimental models, chronic liver damage is defined by an increase in EC numbers and microvessels, the latter being particularly prominent in portal tracts and fibrotic septa[3,5,35,47,48]. On the other hand, the co-localization of VEGF-A expressions with hypoxic areas and a parallel increase of VEGF-A expression and hypoxic areas during progression of fibrosis have been supported that hypoxia; angiogenesis and fibrosis are closely related[3,5,19]. Moreover, the response to hypoxia and VEGF expression not only encountered in ECs but also in hepatocytes as well as HSCs in the progression of fibrosis. Currently with accumulated data, it is possible to conclude that hypoxia is one of the most important stimulus to switch on the transcription of pro-angiogenic genes through the action of HIFs[1-4]. This is not surprising because angiogenesis is frequently encountered in any kind of wound healing response and, as previously suggested, in liver chronic activation of this response represents the principal impulsive force for deposition of ECM components, leading ultimately to cirrhosis[3,5,7,12,15,22,49,50].
In chronic liver injury progression of fibrosis by it self can favor the development of hypoxia. During this progression the deposition of fibrillar collagen (type I) instead of sinusoidal collagen (type IV) leads to the formation of regenerative nodules of parenchyma, encircled and divided by fibrotic septa, and closely related with prominent changes in angioarchitecture[12,48,51]. The anatomical changes that follow the progression of fibrosis with an increased participation of the hepatic artery to the generation of sinusoidal blood allows to arterialisation of sinusoidal blood flow with higher oxygen concentration[48-51]. Accordingly, continuous capillarisation of sinusoids occurs and causes the loss of specific endothelial fenestrations[48-51]. This process together with the accumulation of fibrotic tissue provokes vascular resistance and diminishes the transport of oxygen to the parenchyma leading to up-regulation of pro-angiogenic mechanisms via hypoxia[22,48]. Recently it has been noted that the pattern of fibrosis (bridging fibrosis, peri-cellular fibrosis, centrilobular fibrosis) can affect the extent of angiogenesis and favor progression of liver injury, representing at the same time a key limiting factor for fibrosis reversibility[49,50].
In liver, inflammation is a biological response for activation of healing process following cellular injury[51]. However prolonged inflammation in chronic liver injury may affect the extent of angiogenesis and favors fibrosis progression. During injury HSCs may be activated and release inflammatory mediators[3,5,21,49-51]. These mediators can elicit angiogenesis via the induction of HIF-1α and HIF-1-dependent transcriptional activity[3,5,15,21,49-51].
All of these findings reveal the strong relation between angiogenic and inflammatory pathways during chronic liver injury. In hypoxia, HIF-1 not only induces angiogenesis but also stimulates the NF-k pathway, thus induces inflammation[24,51]. Moreover, both events are capable of supporting each other[51] (Figure 1). Therefore neovessels themselves express chemokines as well as adhesion molecules and stimulate the recruitment of inflammatory cells that allows to the prolongation of the inflammatory response[48,51]. Consequently angiogenesis in the earlier phases of liver damage contributes to the progression from acute to chronic inflammation[48,51].
Inflammatory cells in hepatic angiogenesis: Activated Kupffer cells that reside in hepatic sinusoids may contribute to angiogenesis by their ability to release of reactive oxygen species (ROS) and platelet-activating factor (PAF)[51]. An increase of ROS and nitrogen species may induce new vessel formation by the stimulation of TNF-, NO, HIF-1 and VEGF expressions[51-54]. TNF- an inflammatory cytokine that is primarily produced by macrophages can also stimulates the mitogen-activated protein kinase (MAPK)/ERK pathway that can also stimulates angiogenesis[51]. PAF induces nuclear factor (NF)-k activation that stimulates angiogenic factors including VEGF[51,53,54].
Mast cells are involved in the regulation of angiogenesis by releasing mediators (histamine, heparin, tryptase, TNF, TGF-1, cytokines, interleukins) and they also affect the number of ECs , including ECs covering liver sinusoids[51,55,56].
The inflammatory response is not solely induced via the activation of cells that reside in the liver[48,51]. As mentioned above during tissue injury increased vascular permeability promotes chemokine-mediated recruitment of inflammatory cells. Besides their role in angiogenesis chemokines may also regulates the influx of leucocytes[51]. Leucocytes can produce angiogenic factors [VEGF, PlGF, PDGF, FGF, Ang-2, TGF-1, epidermal growth factor (EGF)] and various interleukins[51,55]. Several mediators generated during chronic liver injury such as hepatocyte growth factor (HGF) can also contribute to angiogenesis[51,54,55,57].
HSCs in hepatic angiogenesis: During chronic liver inflammation activated HSCs express different chemokines which are also capable to stimulate angiogenesis[49,51,57-59]. Therefore, the cellular and molecular relations between fibrosis and angiogenesis involve the role of activated HSCs[48,60]. They constitute a crossroad between inflammation, fibrosis and angiogenesis[22]. HSCs are sensitive to hypoxia, can be modulated by both cytokines and chemokines during liver injury[22,35,61]. The expression of chemokines by HSCs is controlled by products of oxydative stress, proteases, growth factors and pro-inflammatory cytokines[51]. HSCs can also regulate sinusoidal calibre and blood flow thereby modulates hepatic microvascular dynamics[51]. Once activated HSCs act as proangiogenic cells and may respond to hypoxia in a HIF-1α related pathway through the increase of VEGF, Ang-1, and their receptors VEGFR-2 and Tie-2[22,35,48,61]. On the other hand, they can be activated by both VEGF and Ang-1[22,48]. VEGF stimulates their proliferation, migration, and chemotaxis[48]. In particular, oriented migration of activated HSCs in response to either hypoxia or proangiogenic mediators has been described as a biphasic mechanism[21,22,35,48,62]. This mechanism first switched on by ROS and proceeds through activation of Ras/ERK and c-Jun-NH2-terminal kinase isoforms (JNKs)[21]. This is followed by a delayed stage of migration that is related to up-regulation of VEGF expression, leading to the chemotactic action of extracellularly released VEGF[21]. These findings are in accordance with the results of a previous study that showed activated HSCs that express both VEGF and Ang-1 were not found in the established bridging septa which is in contrast to the presence of such cells at the edge of incomplete fibrotic septa[22,48]. This finding demonstrates the presence of two different phases of angiogenesis in CLDs[48]. An early phase that is regulated by activated HSCs and a later phase in the large and mature septa with expression of proangiogenic agents only in ECs reflecting the stabilization of newly formed vessels[21,22,48]. Accumulating evidences suggest that HSCs may operate their pro-angiogenic role in a hypoxia-independent manner by responding to a number of stimuli[21,22,48,51]. These include pro-fibrogenic polypeptide mediators like mainly PDGF and leptin (see in adipokines section)
PDGF can promote an angiogenic phenotype of HSC. This phenotype regulates the formation of vascular tube and enhanced coverage of sinusoids in vitro and in vivo, respectively[61,62]. HSCs together with PDGF contribute to the modulation of microvascular structure and function in liver parenchyma[63,64]. PDGF might play also an additional pro-angiogenic role in vascular remodeling in cirrhosis[62]. A previous study in experimental biliary cirrhosis model emphasized that cholangiocytes and HSCs in response to PDGF have been produced and released Hedgehog (Hh) ligands that contained in microparticles[64]. In normal circumstances the effect of low level of Hh ligands released from immature ductular cells antagonized by Hh interacting protein (HIP) expression by quiescent HSCs and sinusoidal ECs[64]. During chronic liver damage HIP expression is supressed and stimulation of ductular-type progenitor cells may lead to PDGF-BB release[22,64]. This event may ends with the production of Hh ligands by HSCs and ductular cells, which may influence the gene expression of sinusoidal ECs resulting in capillarisation of sinusoids and release of NO, then contributing to vascular remodeling in cirrhosis[22,64]. Activated HSCs also induce some inflammatory mediators and the recruitment of inflammatory cells, together with hypoxia stimulate the proangiogenic factor expression from cells in the microenvironment[48]. Moreover HSCs are capable to express a large number of chemokines[65]. These include the CC chemokines (CCL2, CCL3, CCL5)and the CXC chemokines (CXCL8, CXCL9, CXCL10, CXCL12)[65,66]. Many of them have been related to liver fibrosis in CLDs[65-67]. The CXC chemokines also manipulate angiogenesis during iniation and progression of fibrosis[65-67]. Whereas CXC chemokines containing the ELR motif (ELR+) stimulate angiogenesis, chemokines devoid of this motif (ELR-) inhibit it[65-67]. For instance the direct interference of angiostatic CXCR3 ligand CXCL4 with VEGF signaling have been demonstrated in liver angiogenesis during the progression of fibrosis[68]. The evidences indicating the counter-regulation of VEGF-driven angiogenesis by CXCR3 ligand CXCL9 also decribed both in vivo and in vitro experimental studies[69,70]. As pointed out by Yasar et al[65] all of these new findings set the stage for additional investigation of ELR- chemokines as promising therapeutical targets in CLDs.
Adipokines: In CLDs, besides their roles in the regulation of fibrogenesis, metabolic and inflammatory processes, adipokines have been shown to be important regulators of angiogenesis[9,10,48,71-77]. They manipulate and induce the agents responsible for the modulation of angiogenesis[48,72,78,79]. Although, Leptin, visfatin [pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase (PBEF-1/Nampt)], chemerin [retinoic acid receptor responder protein 2 (RARRES2), tazarotene-induced gene two protein (TIG2) or RAR-responsive protein (TIG2)], and resistin have been found to stimulate angiogenesis, adiponectin attenuates the new vessel formation[48,71-77,80]. Until now, the effect of vaspin (visceral adipose tissue-derived serine protease inhibitor, SERPINA12) on ECs has been described in a few study[48,81].
Leptin can directly up-regulate VEGF, Ang-1 and monocyte-chemoattractant protein 1 (MCP-1 or CCL2) in human HSCs[71,80]. An experimental study has been demonstrated that leptin has been operated the pro-angiogenic actions by recruitment/stabilization of HIF-1α and nuclear translocation of HIF-1[71]. Leptin increases gene expression of the VEGF and Ang-1[71]. After induction of fibrosis in rat the specific leptin receptor ObR was found to be co-localized with VEGF and αSMA[71,82]. More recently both leptin and PDGF-BB up-regulated VEGF in HSCs[48,71,82]. Pro-angiogenic role of leptin involves both activation of the mammalian target of rapamycin (mTOR) pathway and generation of ROS via NADPH-oxidase, the latter being relevant for HIF-1α stabilization but not for mTOR activation[71,82,83].
An interesting adipokine, is apelin that has been reported to be markedly increased in cirrhotic livers[84,85]. It is overexpressed by HSCs and the use of an antagonist of the apelin receptor inhibits both angiogenesis and hepatic fibrosis[85]. In HSCs exposure to recombinant apelin allows to an increased synthesis of type I collagen and PDGF-β receptor. It expression is also up-regulated by hypoxia[86].
Endoplasmic reticulum stress and consequent unfolded protein response: The changes in microenvironment, such as hypoxia provoke to cell and consequently endoplasmic reticulum (ER) stress that result in the unfolded protein response (UPR) to maintain cellular homeostasis[87-89]. This response takes place in a number of processes in liver including angiogenesis[88,89]. The role of angiogenesis and ER stress in the pathogenesis of CLDs is reported[89,90]. Although the relation between the UPR and angiogenesis is not fully described evidences indicate that ER stress induces angiogenesis in CLDs[88-90]. It is suggested that further studies concerning the role of the UPR in new vessel formation could provide new anti-angiogenic targets that inhibit specific ER stress mediators[90].
Neuropilins: Neuropilins (NRPs) were originally identified as receptors for class 3 semaphorins, polypeptides with key roles in the nervous system[91-94]. Following studies have been revelead that NRPs are also contribute in many signaling pathways such as PDGF, TGF- and VEGF signaling[91,93,94]. NRP overexpression was also observed in specimens from CLDs and some studies revealed a role for NRP-1 as a regulator of angiogenesis (see above) and may be involved in the progression of liver cirrhosis[39,93]. In some experimental models of liver fibrosis a NRP-1 neutralizing antibody diminished VEGF responses of ECs in cultures[94]. Because antibodies to NRP-1 are under investigation in phase I trials, it is suggested that these antibodies might be available for future antifibrotic therapies that targets PDGF, TGF-β and VEGF in CLDs[91,94,95].
Althought in chronic viral hepatitis (CVH), the molecular mechanisms of angiogenesis have not been fully discovered, accumulated evidences suggest that angiogenesis plays an important role in the progression of disease[44]. The stimulation of migration and proliferation of ECs by sera from patients with CVH [especially in hepatitis C virus (HCV infection)]; the frequent occurence of angiogenesis in CVH when compared to normal tissues and a positive correlation between the intensity of angiogenesis and activity of inflammation are important findings that support an active role of angiogenesis in CVH progression[6-8,96-98]. Other evidences about the contibution of angiogenesis in the progression CVH are the up-regulation of HGF (that induce proliferation of ECs via VEGF pathway), the increase of PDGF expression and its receptors in sinusoidal and HSCs, the overexpression of inducible NO synthase leading to NO overproduction[44,48,78,99,100]. Moreover the amount of angiogenesis and hepatic VEGF expression is associated with the grade of fibrosis indicating the potential role of angiogenesis in the progression of fibrosis in CVH[47,72,97,99]. In patients with CVH as well as in cell cultures an increase in Hh activity was found to be related with poor prognosis suggesting that blocking Hh pathway may be another approach in the inhibition of fibrosis in CVH[101].
During hepatitis, HCV may activates several pathways and systems that are implicated in angiogenesis. The core E1, NS3 and NS5A proteins induce mithochondrial dysfunction resulting to generation of new ROS that leads to induction of HIF-1 and up-regulation of VEGF and placental growth factor (PlGF)[102,103]. The first evidence about the modulation of HIF-1α in HCV infection has been described in 2007 by Nasimuzzaman et al[104] that demonstrated non-structural proteins have the capacity to induce HIF-1α, even in normoxia. The induction of oxydative stress activates several kinase pathways (such as PI3K and MEK) leading to an increase of HIF-1α synthesis that may results in angiogenesis[104,105]. More recently Abe et al[106] observed that under hypoxia, transfection with the HCV Core protein activated NF-kβ signaling (by an unknown mechanism) resulting in transcriptional up-regulation of HIF-1α mRNA and eventually augmented HIF-1α. However, these results were found in cell lines already containing HCV. Therefore the relevance of the Core protein overexpression in a natural HCV infection remains to be elucidated. It is also demonstrated that proangiogenic markers are increased in sera from patients with HCV hepatitis and their concentrations significantly diminished after therapy (pegylated interferon and ribavirin)[48,107,108]. These findings indicate that proangiogenic markers may be valuable in the follow-up of the disease and response to treatment in HCV infection[48]. Besides, these data also highlight the close association of angiogenesis, inflammation and fibrosis during the progression of HCV hepatitis. Recently it is reported that VEGF-A expression promoted by HCV results in the loose of cellular polarization and increased viral entry[109,110]. In HCV infected cell cultures, hepatocytes exposed to VEGF-A inhibitors regain their polarization and viral infection was reduced suggesting that VEGF-A inhibitors may be another treatment option in HCV hepatitis[107-111]. Interestingly, Rowe et al[112] observed a reduced VEGFR-2 activation of sinusoidal ECs and a concomitant increase in bone morphogenetic protein 4 (BMP4) expression in patients with HCV highlighting that EC-hepatocyte crosstalk may reduces the activity of VEGF inhibitors in HCV infection.
Although it will be describted later, it is worhty to note that steatosis that is frequent during the course of HCV hepatitis, by provoking the cellular injury evokes a wound healing which is closely related with angiogenesis. Indeed CD34 expression are more frequent in liver tissues with steatosis from HCV infected patients[6,48]. The number of microvessels are increased parallel to the severity of steatosis[6,48]. In high grade steatosis, angiogenesis is higher in steatotic areas of parenchyma and primarily observed in periportal zone (zone 1)[6,48]. As HCV hepatitis is also recognised as metabolic disease as well, various adipokines are considered to be involved in their progression[48,96,111,113]. Besides their role in the regulation of fibrogenesis, metabolic events and inflammatory conditions adipokines are also important in the manipulation of angiogenesis[48]. Serum leptin levels are higher in HCV infection when compared to controls[96,111]. There is also a positive correlation between level of leptin and the grade of fibrosis[60,99]. These results point out that, leptin contributes to angiogenesis that takes place during the evolution of HCV hepatitis. Besides, a few studies in HCV hepatitis with new adipokines (visfatin and chemerin and vaspin) that exert pro-angiogenic activities also performed[79,99,114,115]. Interestingly, although a negative correlation between visfatin and the severity of angiogenesis described in females patients, such an association did not observed in males[115]. On the other hand, vaspin was significantly down-regulated, but increased in higher stages of fibrosis[114]. In these patients vaspin was positively related to angiogenesis with a strong association in males[114]. Accordingly, it is suggested that the function of some adipokines in neovessel formation may be different depending on gender[115].
HBV virus it self can influence angiogenesis. Of the different proteins coded in HBV genome, the regulatory protein X (Hbx) was found to stimulate angiogenesis directly through the both activation and stabilization of HIF-1[116]. HbX also promotes the induction of Ang-2 and contribute to angiogenesis[116]. More recently in viral induced HCC Yen et al[117] observed that Hbx protein also activates m-TOR and VEGF-A pathways via up-regulation of IKKβ[103]. Although the precise mechanism of IKKβ stimulation by Hbx needs to be further investigated it seems to represents a potential new pathway in angiogenesis that occurs in HBV infection[103,118].
In addition to gross hepatic structural disorders related to diffuse fibrosis and formation of regenerative nodules, the morphological and functional rearrangements of the hepatic vasculature are shown to contribute in portal hypertension (PHT). A hyperdynamic splanchnic circulation is an important constituent of the portal hypertensive syndrome[51,119-121]. An increase of blood flow in splachnic organs that drain into the portal vein leading to the augmentation of portal venous flow provokes the portal hypertensive syndrome[119,120]. Traditionally, it is accepted that this increase has been related to an increased production of endogenous vasodilators together with a decreased vascular reactivity to vasoconstrictors and the development of collaterals has been considered a mechanical consequence of an increased blood pressure[120,122]. However, recent studies object to these opinions by demonstrating that angiogenesis, contributes to the evolution and perpetuation of splanchnic hyperemia and the development of portal-systemic collaterals[120-124]. Many studies revealed that angiogenesis induced by VEGF, PDGF and PIGF actively modulates the occurence of hyperdynamic splanchnic circulation[51,125,126]. It has been also observed that the formation of portal–systemic collaterals are also induced by VEGF and PlGF dependent angiogenesis[51,125,127].
It should be noted that in mice with PHT rapamycin by inhibiting mTOR pathway has been prevented mesenteric neoangiogenesis and reduced the splanchnic blood flow[51,128]. Recently, the effect of PPAR activation in the improvement of hepatic EC dysfunction and the dimunition of fibrosis in PHT developed in cirrhotic rats, suggested that PPARα may represent as a new therapeutic strategy[51,129].
Cannabinoids (CBs) inhibit angiogenesis, but little is known about their influences in CLDs. In an experimental study a CB2 agonist alleviated PHT, severity of angiogenesis, portosystemic shunts, and liver fibrosis[130]. It is suggested that the vascular effects might be mediated by COX and NOS down-regulation and concluded that targeting CB2 receptors might be useful in the control of PHT[129].
Briefly angiogenesis participates in the pathogenesis of PHT by modulating activation of HSCs and fibrogenesis, splanchnic vasodilation and formation of portal-systemic collaterals.
Fatty liver diseases (FLDs) possess a broad spectrum of histopathological findings that ranges from steatosis through non-alcoholic fatty liver disease(NAFLD) to non-alcoholic steatohepatitis (NASH) that can cause cirrhosis[22,48,131]. Although the mechanism of NASH is not completely understood, currently it is pointed out that its pathophysiology should be recognized as multifactorial, including a link between changes in microvasculature and the progression of fibrosis[107,131,132].
Tissue damage related both to lipotoxicity and to fat accumulation results in reduced sinusoidal perfusion as well as changes in sinusoidal architecture. Cytokines activated by lipotoxicity mediates the migration of the inflammatory cells leading to inflammation[107,133]. In inflammatory foci these cells can contribute to angiogenesis. As the disease progress large, fatty hepatocytes, together with inflammation and related perivascular fibrosis constrict the sinusoidal lumens and impair sinusoidal perfusion leading to hypoxia[48,133]. All of these events provoking liver damage can innate angiogenesis.
Recent studies have been demonstrated that angiogenesis in NASH is significantly increased when compared to steatosis and control tissues[72,134]. Moreover, the grade of angiogenesis has been found to be related to the grade of fibrosis both in humans and in experimental studies[73,134]. It is also observed that during the evolution of disease the progression patterns of fibrosis and angiogenesis are parallel[73,134]. All of these findings support that angiogenesis contributes to the progression of fibrosis in NASH[132]. Besides, in a recent study by employing hepatocyte-specific-VHL and HIF-1 or HIF-2 mouse mutants demonstrated that mice with liver conditional disruption of VHL have spontaneous fatty liver and inflammation progressing to fibrosis in a HIF-2 dependent way[135,136]. These observations suggest that in addition to its role in angiogenesis hypoxia together with HIF-2 play a critical role in inflammation that is also necessary for the evolution of FLD, as proposed by the two hit hypothesis[135-137]. More recently in an elegant study, Ciupińska-Kajor et al[10] detected that in morbid obesity the activation of angiogenesis took place at an early stage, when compared to nonobese patients in whom the activation took place at the level of NASH. Besides, in this group the expression of angiogenic factors has been correlated with the progression of fibrosis independently from the development of NASH. These interesting findings highlight that in morbid obesity the progression of liver fibrosis is leaded by angiogenesis independently from steatosis[48].
Recently it is observed that similar to HCV hepatitis, patients with simple steatosis had low level of serum leptin when compared to patients with NASH[10,48]. It has been indicated that leptin could mediate angiogenesis during the progression of steatosis[48]. Increased serum level of new angiogenic adipokines, vaspin and chemerin have been also described in NAFLD[10,138,139].
Recently a role of angiotensin-II (AT-II) in the progression of CLDs, including NASH has been suggested[140-143]. In an experimental model of NASH , inhibition of AT-II by a AT-II-type-I receptor blocker (ARB) was significantly decreased the formation of new vessels[140,144]. Moreover it is observed that liver fibrogenesis might be impaired by both angiotensin converting enzyme inhibitor (ACE) and ARB along with inhibiton of the HSCs[142]. These results indicate that anti-fibrotic effects of these agents were provided by their dual influence both in angiogenesis and HSCs[144,145]. It is also demonstrated that in liver the expressions of AT-II, TGF-β and VEGF were inhibited by DRI (direct renin inhibitor-Aliskiren) indicating an important role of renin during liver fibrogenesis in NASH[144]. Because these agents are frequently used in practice, it is suggested that they could be effectively used as a new strategy against angiogenesis that takes place in the fibrogenic progression of steatohepatitis in the future.
Evidences also indicate that long-term ethanol consumption is closely related to angiogenesis by means of finely coordinated action of various mediators in liver[146,147]. Ethanol up-regulates VEGF and VEGFR-2 and stimulates angiogenesis in the rat liver after 36 weeks of consumption[146,147]. Moreover, higher concentrations of Ang2 and VEGF-A in alcoholic liver disease (ALD) patients as compared to controls were found. It is proposed that angiogenesis-related biomarkers are useful in the noninvasive monitoring of the ALD course. More recently it is observed that alcohol also induces angiogenesis via PECAM-1[147]. It is suggested that these molecular insights could contribute to the evolution of new anti-angiogenic treatment strategies.
In conclusion, angiogenesis contributes to the progression of fibrosis in CLDs. Vascular remodeling leading to capillarization of the sinusoids with generation of intrahepatic shunts characterize hepatic angiogenesis. These changes in angioarchitecture give rise to an increase in vascular resistance and a decrease of hepatocyte perfusion leading to hypoxia. This is the one of the most important stimulus to switch on the transcription of pro-angiogenic genes through the action of HIFs. Cellular and molecular mechanisms implicated in the interactions between angiogenesis and fibrosis and liver cells including hepatocytes, ECs, and activated HSCs have been described. HSCs may constitute a crossroad at the interaction between inflammation, angiogenesis, and fibrosis. Metabolic abnormalities, steatosis and adipokines, may also influence angiogenesis, consequently inflammation and fibrosis.
The relationship between angiogenesis and fibrosis progression in CLDs indicates that determination of proangiogenic factors may be a useful noninvasive approach in follow-up of both disease progression and response to therapy. Although anti angiogenic therapy may be a promising in the prevention of fibrosis in CLDs, it should be well balanced because an excessive blockage may prevent wound healing response. Finally there are still large gaps in our understanding and ability to treat angiogenesis during the progression of CLDs and clinical trials on a large number of patients are needed.
P- Reviewer: Hadianamrei R, Korpanty G S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2389] [Cited by in RCA: 2495] [Article Influence: 191.9] [Reference Citation Analysis (0)] |
| 2. | Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3471] [Cited by in RCA: 4019] [Article Influence: 287.1] [Reference Citation Analysis (0)] |
| 3. | Valfrè di Bonzo L, Novo E, Cannito S, Busletta C, Paternostro C, Povero D, Parola M. Angiogenesis and liver fibrogenesis. Histol Histopathol. 2009;24:1323-1341. [PubMed] |
| 4. | Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 6. | Kukla M, Gabriel A, Waluga M, Budak R, Mazur W. Angiogenesis in chronic viral hepatitis. Gastroenterol Pol. 2009;16:304-309. |
| 7. | Amarapurkar AD, Amarapurkar DN, Vibhav S, Patel ND. Angiogenesis in chronic liver disease. Ann Hepatol. 2007;6:170-173. [PubMed] |
| 8. | Gabriel A, Kukla M, Wilk M, Liszka Ł, Petelenz M, Musialik J. Angiogenesis in chronic hepatitis C is associated with inflammatory activity grade and fibrosis stage. Pathol Res Pract. 2009;205:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Kukla M, Gabriel A, Sabat D, Liszka Ł, Wilk M, Petelenz M, Musialik J, Dzindziora-Frelich I. Association between liver steatosis and angiogenesis in chronic hepatitis C. Pol J Pathol. 2010;61:154-160. [PubMed] |
| 10. | Ciupińska-Kajor M, Hartleb M, Kajor M, Kukla M, Wyleżoł M, Lange D, Liszka L. Hepatic angiogenesis and fibrosis are common features in morbidly obese patients. Hepatol Int. 2013;7:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Shah VH, Bruix J. Antiangiogenic therapy: not just for cancer anymore? Hepatology. 2009;49:1066-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Sanz-Cameno P, Trapero-Marugán M, Chaparro M, Jones EA, Moreno-Otero R. Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. J Oncol. 2010;2010:272170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Bruno A, Pagani A, Pulze L, Albini A, Dallaglio K, Noonan DM, Mortara L. Orchestration of angiogenesis by immune cells. Front Oncol. 2014;4:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 625] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 15. | Novo E, Cannito S, Paternostro C, Bocca C, Miglietta A, Parola M. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Tecchio C, Cassatella MA. Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Chem Immunol Allergy. 2014;99:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Bosisio D, Salvi V, Gagliostro V, Sozzani S. Angiogenic and antiangiogenic chemokines. Chem Immunol Allergy. 2014;99:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Simpson KJ, Henderson NC, Bone-Larson CL, Lukacs NW, Hogaboam CM, Kunkel SL. Chemokines in the pathogenesis of liver disease: so many players with poorly defined roles. Clin Sci (Lond). 2003;104:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G582-G592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Novo E, Povero D, Busletta C, Paternostro C, di Bonzo LV, Cannito S, Compagnone A, Bandino A, Marra F, Colombatto S. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012;226:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. 2013;114:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 25. | Lampugnani MG. Endothelial adherens junctions and the actin cytoskeleton: an ‘infinity net’? J Biol. 2010;9:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 887] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 27. | Chien S, Li S, Shiu YT, Li YS. Molecular basis of mechanical modulation of endothelial cell migration. Front Biosci. 2005;10:1985-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 30. | Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2765] [Cited by in RCA: 2836] [Article Influence: 189.1] [Reference Citation Analysis (0)] |
| 32. | Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med. 2002;8:918-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 389] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Gálvez BG, Matías-Román S, Albar JP, Sánchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001;276:37491-37500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6941] [Article Influence: 315.5] [Reference Citation Analysis (0)] |
| 35. | Novo E, Cannito S, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S, Marra F, Pinzani M. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochim Biophys Acta. 2014;1846:161-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Neufeld G, Kessler O. Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev. 2006;25:373-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 1007] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 39. | Zachary I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy. 2014;99:37-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1837] [Cited by in RCA: 1789] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 41. | Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 777] [Cited by in RCA: 793] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 42. | Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 876] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 43. | Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 949] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 44. | Medina J, Arroyo AG, Sánchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281-17290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. FEBS J. 2011;278:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Bozova S, Elpek GO. Hypoxia-inducible factor-1alpha expression in experimental cirrhosis: correlation with vascular endothelial growth factor expression and angiogenesis. APMIS. 2007;115:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Kukla M. Angiogenesis: a phenomenon which aggravates chronic liver disease progression. Hepatology Int. 2013;7:4-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets. Dig Liver Dis. 2004;36:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 51. | Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Huang Y, Li S. Detection of characteristic sub pathway network for angiogenesis based on the comprehensive pathway network. BMC Bioinformatics. 2010;11 Suppl 1:S32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 778] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 54. | Ko HM, Seo KH, Han SJ, Ahn KY, Choi IH, Koh GY, Lee HK, Ra MS, Im SY. Nuclear factor kappaB dependency of platelet-activating factor-induced angiogenesis. Cancer Res. 2002;62:1809-1814. [PubMed] |
| 55. | Raevens S, Coulon S, Van Steenkiste C, Colman R, Verhelst X, Van Vlierberghe H, Geerts A, Perkmann T, Horvatits T, Fuhrmann V. Role of angiogenic factors/cell adhesion markers in serum of cirrhotic patients with hepatopulmonary syndrome. Liver Int. 2014;Apr 28; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Franceschini B, Ceva-Grimaldi G, Russo C, Dioguardi N, Grizzi F. The complex functions of mast cells in chronic human liver diseases. Dig Dis Sci. 2006;51:2248-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12:171-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 60. | Kukla M, Berdowska A, Gabriel A, Sawczyn T, Mazur W, Sobala-Szczygieł B, Grzonka D, Zajęcki W, Tomaszek K, Bułdak RJ. Association between hepatic angiogenesis and serum adipokine profile in non-obese chronic hepatitis C patients. Pol J Pathol. 2011;62:218-228. [PubMed] |
| 61. | Friedman SL. Transcriptional regulation of stellate cell activation. J Gastroenterol Hepatol. 2006;21 Suppl 3:S79-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Wang YQ, Luk JM, Ikeda K, Man K, Chu AC, Kaneda K, Fan ST. Regulatory role of vHL/HIF-1alpha in hypoxia-induced VEGF production in hepatic stellate cells. Biochem Biophys Res Commun. 2004;317:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Zhang F, Kong D, Chen L, Zhang X, Lian N, Zhu X, Lu Y, Zheng S. Peroxisome proliferator-activated receptor-γ interrupts angiogenic signal transduction by transrepression of platelet-derived growth factor-β receptor in hepatic stellate cells. J Cell Sci. 2014;127:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320-330.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Sahin H, Wasmuth HE. Chemokines in tissue fibrosis. Biochim Biophys Acta. 2013;1832:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 67. | Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 68. | Sulpice E, Contreres JO, Lacour J, Bryckaert M, Tobelem G. Platelet factor 4 disrupts the intracellular signalling cascade induced by vascular endothelial growth factor by both KDR dependent and independent mechanisms. Eur J Biochem. 2004;271:3310-3318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, Mukhopadhyay D, Schuppan D, Bi Y, Simonetto D. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339-1350.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 70. | Sahin H, Borkham-Kamphorst E, Kuppe C, Zaldivar MM, Grouls C, Al-samman M, Nellen A, Schmitz P, Heinrichs D, Berres ML. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 71. | Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, Vizzutti F, Anania FA, Milani S, Rombouts K. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 72. | Kitade M, Yoshiji H, Kojima H, Ikenaka Y, Noguchi R, Kaji K, Yoshii J, Yanase K, Namisaki T, Asada K. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology. 2006;44:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008;42:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 333] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 75. | Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 76. | Robertson SA, Rae CJ, Graham A. Induction of angiogenesis by murine resistin: putative role of PI3-kinase and NO-dependent pathways. Regul Pept. 2009;152:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 78. | Kukla M, Mazur W, Bułdak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol Med. 2011;17:1397-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Raucci R, Rusolo F, Sharma A, Colonna G, Castello G, Costantini S. Functional and structural features of adipokine family. Cytokine. 2013;61:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Fu BD, Yamawaki H, Okada M, Hara Y. Vaspin can not inhibit TNF-alpha-induced inflammation of human umbilical vein endothelial cells. J Vet Med Sci. 2009;71:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Aleffi S, Navari N, Delogu W, Galastri S, Novo E, Rombouts K, Pinzani M, Parola M, Marra F. Mammalian target of rapamycin mediates the angiogenic effects of leptin in human hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2011;301:G210-G219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2298] [Cited by in RCA: 2187] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 84. | Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004;325:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 85. | Principe A, Melgar-Lesmes P, Fernández-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, Bernardi M, Arroyo V, Jiménez W. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology. 2008;48:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Melgar-Lesmes P, Casals G, Pauta M, Ros J, Reichenbach V, Bataller R, Morales-Ruiz M, Jimenez W. Apelin mediates the induction of profibrogenic genes in human hepatic stellate cells. Endocrinology. 2010;151:5306-5314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Isom HC, McDevitt EI, Moon MS. Elevated hepatic iron: a confounding factor in chronic hepatitis C. Biochim Biophys Acta. 2009;1790:650-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 89. | Paridaens A, Laukens D, Vandewynckel YP, Coulon S, Van Vlierberghe H, Geerts A, Colle I. Endoplasmic reticulum stress and angiogenesis: is there an interaction between them? Liver Int. 2014;34:e10-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 949] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 91. | Tripathi D. Primary prophylaxis against gastric variceal bleeding: is there a sticky solution at last? Hepatology. 2011;54:1094-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 92. | Plein A, Fantin A, Ruhrberg C. Neuropilin regulation of angiogenesis, arteriogenesis, and vascular permeability. Microcirculation. 2014;21:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 93. | Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest. 2010;120:2379-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 94. | Troeger JS, Schwabe RF. Neuropilin and liver fibrosis: hitting three birds with one stone? Hepatology. 2011;54:1091-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 95. | Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 96. | Mazzanti R, Messerini L, Monsacchi L, Buzzelli G, Zignego AL, Foschi M, Monti M, Laffi G, Morbidelli L, Fantappié O. Chronic viral hepatitis induced by hepatitis C but not hepatitis B virus infection correlates with increased liver angiogenesis. Hepatology. 1997;25:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Messerini L, Novelli L, Comin CE. Microvessel density and clinicopathological characteristics in hepatitis C virus and hepatitis B virus related hepatocellular carcinoma. J Clin Pathol. 2004;57:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Mazzanti R, Messerini L, Comin CE, Fedeli L, Ganne-Carrie N, Beaugrand M. Liver angiogenesis as a risk factor for hepatocellular carcinoma development in hepatitis C virus cirrhotic patients. World J Gastroenterol. 2007;13:5009-5014. [PubMed] |
| 99. | Zwirska-Korczala K, Kukla M, Ziołkowski A. Leptin, neopterin and hepatocyte growth factor as markers of fibrosis and inflammatory activity in chronic hepatitis C. Exp Clin Hep. 2005;1:60-65. |
| 100. | Medina J, Caveda L, Sanz-Cameno P, Arroyo AG, Martín-Vílchez S, Majano PL, García-Buey L, Sánchez-Madrid F, Moreno-Otero R. Hepatocyte growth factor activates endothelial proangiogenic mechanisms relevant in chronic hepatitis C-associated neoangiogenesis. J Hepatol. 2003;38:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Pereira Tde A, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Vrancken K, Paeshuyse J, Liekens S. Angiogenic activity of hepatitis B and C viruses. Antivir Chem Chemother. 2012;22:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 103. | Cuninghame S, Jackson R, Zehbe I. Hypoxia-inducible factor 1 and its role in viral carcinogenesis. Virology. 2014;456-457:370-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Nasimuzzaman M, Waris G, Mikolon D, Stupack DG, Siddiqui A. Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. J Virol. 2007;81:10249-10257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 105. | Tardif KD, Waris G, Siddiqui A. Hepatitis C virus, ER stress, and oxidative stress. Trends Microbiol. 2005;13:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 106. | Abe M, Koga H, Yoshida T, Masuda H, Iwamoto H, Sakata M, Hanada S, Nakamura T, Taniguchi E, Kawaguchi T. Hepatitis C virus core protein upregulates the expression of vascular endothelial growth factor via the nuclear factor-κB/hypoxia-inducible factor-1α axis under hypoxic conditions. Hepatol Res. 2012;42:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Salcedo X, Medina J, Sanz-Cameno P, García-Buey L, Martín-Vilchez S, Borque MJ, López-Cabrera M, Moreno-Otero R. The potential of angiogenesis soluble markers in chronic hepatitis C. Hepatology. 2005;42:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Salcedo X, Medina J, Sanz-Cameno P, García-Buey L, Martín-Vilchez S, Moreno-Otero R. Review article: angiogenesis soluble factors as liver disease markers. Aliment Pharmacol Ther. 2005;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 109. | Mee CJ, Farquhar MJ, Harris HJ, Hu K, Ramma W, Ahmed A, Maurel P, Bicknell R, Balfe P, McKeating JA. Hepatitis C virus infection reduces hepatocellular polarity in a vascular endothelial growth factor-dependent manner. Gastroenterology. 2010;138:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Hassan M, Selimovic D, Ghozlan H, Abdel-kader O. Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways. Hepatology. 2009;49:1469-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 111. | Liaskou E, Karikoski M, Reynolds GM, Lalor PF, Weston CJ, Pullen N, Salmi M, Jalkanen S, Adams DH. Regulation of mucosal addressin cell adhesion molecule 1 expression in human and mice by vascular adhesion protein 1 amine oxidase activity. Hepatology. 2011;53:661-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Rowe IA, Galsinh SK, Wilson GK, Parker R, Durant S, Lazar C, Branza-Nichita N, Bicknell R, Adams DH, Balfe P. Paracrine signals from liver sinusoidal endothelium regulate hepatitis C virus replication. Hepatology. 2014;59:375-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 113. | Piche T, Vandenbos F, Abakar-Mahamat A, Vanbiervliet G, Barjoan EM, Calle G, Giudicelli J, Ferrua B, Laffont C, Benzaken S. The severity of liver fibrosis is associated with high leptin levels in chronic hepatitis C. J Viral Hepat. 2004;11:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Szczygiel B, Berdowska A, Mazur W, Wozniak-Grygiel E, Kryczka W. Chemerin, vaspin and insulin resistance in chronic hepatitis C. J Viral Hepat. 2010;17:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 115. | Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Berdowska A, Rybus-Kalinowska B, Kalinowski M, Janczewska-Kazek E, Woźniak-Grygiel E. Visfatin serum levels in chronic hepatitis C patients. J Viral Hepat. 2010;17:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Chau DK, Chen GG, Zhang H, Leung BC, Chun S, Lai PB. Differential functions of C- and N-terminal hepatitis B x protein in liver cells treated with doxorubicin in normoxic or hypoxic condition. PLoS One. 2012;7:e50118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 117. | Yen CJ, Lin YJ, Yen CS, Tsai HW, Tsai TF, Chang KY, Huang WC, Lin PW, Chiang CW, Chang TT. Hepatitis B virus X protein upregulates mTOR signaling through IKKβ to increase cell proliferation and VEGF production in hepatocellular carcinoma. PLoS One. 2012;7:e41931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Demidenko ZN, Blagosklonny MV. The purpose of the HIF-1/PHD feedback loop: to limit mTOR-induced HIF-1α. Cell Cycle. 2011;10:1557-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 119. | Cichoz-Lach H, Celiński K, Słomka M, Kasztelan-Szczerbińska B. Pathophysiology of portal hypertension. J Physiol Pharmacol. 2008;59 Suppl 2:231-238. [PubMed] |
| 120. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 121. | Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 122. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 123. | D’Amico M, Mejías M, García-Pras E, Abraldes JG, García-Pagán JC, Fernández M, Bosch J. Effects of the combined administration of propranolol plus sorafenib on portal hypertension in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1191-G1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 124. | Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 125. | Hernández-Guerra M, García-Pagán JC, Turnes J, Bellot P, Deulofeu R, Abraldes JG, Bosch J. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Matei V, Rodríguez-Vilarrupla A, Deulofeu R, Colomer D, Fernández M, Bosch J, Garcia-Pagán JC. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 127. | Chan CC, Tsai SC, Cheng LY, Lee FY, Lin HC. Hemodynamic assessment of the development of portal-systemic collaterals in portal hypertensive rats. Dig Dis Sci. 2011;56:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 128. | Geerts AM, Vanheule E, Van Vlierberghe H, Leybaert L, Van Steenkiste C, De Vos M, Colle I. Rapamycin prevents mesenteric neo-angiogenesis and reduces splanchnic blood flow in portal hypertensive mice. Hepatol Res. 2008;38:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Rodríguez-Vilarrupla A, Laviña B, García-Calderó H, Russo L, Rosado E, Roglans N, Bosch J, García-Pagán JC. PPARα activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 130. | Huang HC, Wang SS, Hsin IF, Chang CC, Lee FY, Lin HC, Chuang CL, Lee JY, Hsieh HG, Lee SD. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology. 2012;56:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 131. | Bellentani S, Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8 Suppl 1:S4-S8. [PubMed] |
| 132. | Sanal MG. The blind men ‘see’ the elephant-the many faces of fatty liver disease. World J Gastroenterol. 2008;14:831-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 133. | McCuskey RS, Ito Y, Robertson GR, McCuskey MK, Perry M, Farrell GC. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 134. | Kitade M, Yoshiji H, Noguchi R, Ikenaka Y, Kaji K, Shirai Y, Yamazaki M, Uemura M, Yamao J, Fujimoto M. Crosstalk between angiogenesis, cytokeratin-18, and insulin resistance in the progression of non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:5193-5199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, Shah YM. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 136. | Liu Y, Ma Z, Zhao C, Wang Y, Wu G, Xiao J, McClain CJ, Li X, Feng W. HIF-1α and HIF-2α are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1α-mediated fatty acid β-oxidation. Toxicol Lett. 2014;226:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 137. | Fletcher AM, Bregman CL, Woicke J, Salcedo TW, Zidell RH, Janke HE, Fang H, Janusz WJ, Schulze GE, Mense MG. Incisor degeneration in rats induced by vascular endothelial growth factor/fibroblast growth factor receptor tyrosine kinase inhibition. Toxicol Pathol. 2010;38:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 138. | Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 139. | Kukla M, Zwirska-Korczala K, Hartleb M, Waluga M, Chwist A, Kajor M, Ciupinska-Kajor M, Berdowska A, Wozniak-Grygiel E, Buldak R. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2010;45:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 140. | Shirai Y, Yoshiji H, Noguchi R, Kaji K, Aihara Y, Douhara A, Moriya K, Namisaki T, Kawaratani H, Fukui H. Cross talk between toll-like receptor-4 signaling and angiotensin-II in liver fibrosis development in the rat model of non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2013;28:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 141. | Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Shirai Y, Aihara Y, Namisaki T, Yoshii J, Yanase K. Combination treatment of angiotensin II type I receptor blocker and new oral iron chelator attenuates progression of nonalcoholic steatohepatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1094-G1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | Noguchi R, Yoshiji H, Ikenaka Y, Kaji K, Aihara Y, Shirai Y, Namisaki T, Kitade M, Douhara A, Moriya K. Dual blockade of angiotensin-II and aldosterone suppresses the progression of a non-diabetic rat model of steatohepatitis. Hepatol Res. 2013;43:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Tamaki Y, Nakade Y, Yamauchi T, Makino Y, Yokohama S, Okada M, Aso K, Kanamori H, Ohashi T, Sato K. Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis. J Gastroenterol. 2013;48:491-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Aihara Y, Yoshiji H, Noguchi R, Kaji K, Namisaki T, Shirai Y, Douhara A, Moriya K, Kawaratani H, Fukui H. Direct renin inhibitor, aliskiren, attenuates the progression of non-alcoholic steatohepatitis in the rat model. Hepatol Res. 2013;43:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 145. | Yoshiji H, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, Nakatani T, Imazu H, Yanase K, Kuriyama S, Fukui H. Inhibition of renin-angiotensin system attenuates liver enzyme-altered preneoplastic lesions and fibrosis development in rats. J Hepatol. 2002;37:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 146. | Das SK, Mukherjee S, Vasudevan DM. Effects of long term ethanol consumption mediated oxidative stress on neovessel generation in liver. Toxicol Mech Methods. 2012;22:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Raskopf E, Gonzalez Carmona MA, Van Cayzeele CJ, Strassburg C, Sauerbruch T, Schmitz V. Toxic damage increases angiogenesis and metastasis in fibrotic livers via PECAM-1. Biomed Res Int. 2014;2014:712893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |