Published online Dec 18, 2015. doi: 10.4254/wjh.v7.i29.2871
Peer-review started: May 8, 2015
First decision: September 8, 2015
Revised: October 24, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: December 18, 2015
Processing time: 224 Days and 21.4 Hours
Hepatic encephalopathy (HE) is a major complication of cirrhosis resulting in significant socioeconomic burden, morbidity, and mortality. HE can be further subdivided into covert HE (CHE) and overt HE (OHE). CHE is a subclinical, less severe manifestation of HE and requires psychometric testing for diagnosis. Due to the time consuming screening process and lack of standardized diagnostic criteria, CHE is frequently underdiagnosed despite its recognized role as a precursor to OHE. Screening for CHE with the availability of the Stroop test has provided a pragmatic method to promptly diagnose CHE. Management of acute OHE involves institution of lactulose, the preferred first-line therapy. In addition, prompt recognition and treatment of precipitating factors is critical as it may result in complete resolution of acute episodes of OHE. Treatment goals include improvement of daily functioning, evaluation for liver transplantation, and prevention of OHE recurrence. For secondary prophylaxis, intolerance to indefinite lactulose therapy may lead to non-adherence and has been identified as a precipitating factor for recurrent OHE. Rifaximin is an effective add-on therapy to lactulose for treatment and prevention of recurrent OHE. Recent studies have demonstrated comparable efficacy of probiotic therapy to lactulose use in both primary prophylaxis and secondary prophylaxis.
Core tip: Hepatic encephalopathy (HE) is a major complication of cirrhosis resulting in significant socioeconomic burden, morbidity, and mortality. Management of acute overt HE (OHE) involves institution of lactulose, the preferred first-line therapy. In addition, prompt recognition and treatment of precipitating factors may result in complete resolution of acute episodes of OHE. Treatment goals include improvement of daily functioning and prevention of OHE recurrence. For secondary prophylaxis, intolerance to indefinite lactulose therapy may lead to non-adherence and has been identified as a precipitating factor for recurrent OHE. Rifaximin is an effective add-on therapy to lactulose for treatment and prevention of recurrent OHE.
- Citation: Liu A, Perumpail RB, Kumari R, Younossi ZM, Wong RJ, Ahmed A. Advances in cirrhosis: Optimizing the management of hepatic encephalopathy. World J Hepatol 2015; 7(29): 2871-2879
- URL: https://www.wjgnet.com/1948-5182/full/v7/i29/2871.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i29.2871
The prevalence of cirrhosis both worldwide and in the United States is unknown[1], although experts estimate that 5.5 million people in the United States have cirrhosis[2]. One of the primary complications of cirrhosis is hepatic encephalopathy (HE)[3]. HE represents a continuum of clinical features involving cerebral dysfunction in cirrhotic patients with hepatic decompensation[4]. The spectrum can be further divided into preclinical stage, covert HE (CHE), or a clinically symptomatic state known as overt HE (OHE).
According to a study utilizing data from the Nationwide Inpatient Sample, annual inpatient incidence of HE ranged from 20918 in 2005 to 22931 in 2009[5]. This accounted for 0.33% of all hospitalizations in the United States. A 2010 study of 170 cirrhotic patients from United States reported 56% of patients with underlying CHE, while 30% developed an episode of OHE[6]. Among 1348 consecutive patients with cirrhosis in a multi-center European study, 34% patients were diagnosed with OHE[7]. Other studies utilizing data from British and Danish cohorts report OHE in 10% to 11% of patients in the setting of alcoholic cirrhosis[8,9]. Prevalence of CHE in patients with cirrhosis range from 27% to 70%[10,11].
The onset of OHE is associated with high mortality rates as reported by Bustamante et al[12] with 42% survival at 1 year follow-up and 23% at 3 years. Based on comparison with other complications of cirrhosis, the mortality rates are comparable to bleeding from varices[13]. OHE has been associated with 54% mortality at one year in patients needing admission to intensive care unit[14], while data from the inpatient samples report a 14% to 15% mortality over the 5-year period[5]. Jepsen et al[9] describe 169 patients with a median survival time of 2.4 mo from disease onset with mortality rates of 45% within 1 mo, 64% within 1 year, and 85% within 5 years. In addition to morbidity and mortality associated with HE, the socioeconomic and emotional burden on patients, families and the healthcare system are significant. Patients with HE who were discharged to nursing homes or rehabilitation centers increased from 2.6% to 23.3% between 1993 and 2009[15]. A study of 104 cirrhotic patients with previous history of HE was associated with unemployment, decline in financial status, and higher caregiver burden[16].
Cerebral dysfunction in liver failure is a varied phenomenon that can is termed HE. Numerous, complementary mechanisms have been stipulated to underlie HE. Ammonia and other toxins, typically filtered by the liver, play a role in conjunction with altered blood-brain transport of neurotransmitter precursors, metabolism of amino acid neurotransmitters, and cerebral glucose oxidation[17-19]. These alterations result in propagation of inhibitory signals, mediated by gamma-aminobutyric acid and serotonin, and inhibition of excitatory signals, mediated by glutamate and catecholamines[20,21]. The overall effect is neural inhibition. Other contributing mechanisms include neuroinflammation and altered gut flora[22,23].
The diagnosis of OHE is complex, beginning with a clinical recognition of the distinctive neurologic features of HE. The most common features are confusion or coma, asterixis, loss of fine motor skills, and hyper-reflexia. Also required for the diagnosis of HE are the presence of underlying cirrhosis and exclusion of all other etiologies for neurologic or metabolic abnormalities. The portal-systemic encephalopathy score (PSE score; West Haven Criteria) is used to evaluate overall severity.
The two forms of HE include CHE, which has also been called subclinical encephalopathy or minimal encephalopathy (MHE) in the past, and OHE. In contrast to clinically symptomatic OHE, the diagnosis of CHE is made difficult by a lack of agreement in which tests should be utilized[4]. CHE was originally used as the label for patients who performed poorly on psychometric tests[11]. Noted to be more sensitive than observation; psychometric tests have since served as the standard for diagnosis of CHE[24]. Recent studies have also established the usefulness of the neurophysiological critical flicker frequency (CFF) test in diagnosing CHE[25,26]. A study of 132 cirrhotic patients looking at inter-index agreement and predictive validity showed that CFF did not predict CHE reliably, while abnormal electroencephalogram and psychometric HE score (PHES) were predictive of subsequent CHE episodes[27]. The availability of neuroimaging to measure brain activity adds to the variability in tests used to detect CHE. In 2013, Zheng et al[28] used neuroimaging to report that the gray matter cerebral blood flow of CHE patients was significantly higher than that of non-HE patients. Arterial-spin labeling magnetic resonance imaging was used to characterize patterns of cerebral blood flow. Comparing CHE, non-HE, and control patients, they identified particular brain regions such as the right putamen where cutoff values for cerebral blood flow was 93.8% sensitive for characterization of CHE. The study was limited by its small sample size but further research can elucidate the potential for non-invasive CHE screening.
The presence of CHE is associated with higher re-hospitalization and lower survival rates as shown by Patidar et al[6] who followed 170 cirrhotic patients to assess the rates of CHE, hospitalizations, need for liver transplantation and survival. The authors reported that patients diagnosed with CHE via≥ 2 psychometric tests were at a higher risk of developing OHE (HR = 2.1, P = 0.05), hospitalization (HR = 2.5, P = 0.002), needing liver transplantation or death (HR = 3.4, P = 0.01). Despite these observations, the clinical significance of CHE is under appreciated and diagnosis is not universally pursued. The PHES includes five pencil and paper tests, but is not widely used. In a survey of American Association for the Study of Liver Diseases physicians, 38% never tested for CHE, while only 14% tested for CHE in > 80% of patients with cirrhosis[29]. The major reason cited for lack of screening for CHE was time constraints (85%), followed by tests requiring trained personnel (75%) and lack of standardization in testing protocols (69%). Additional work is needed to establish reliable and user-friendly diagnostic criteria for CHE.
The clinical classification of HE can be established according to type of underlying disease, severity of manifestations, time course, and precipitating factors[30]. Based on underlying disease HE is further subdivided into: Type A: HE resulting from acute liver failure; Type B: HE resulting from portosystemic bypass or shunting; and Type C: Resulting from cirrhosis. Clinical manifestations of Type B and Type C are similar, whereas type A includes increased intracranial pressure and risk of cerebral herniation. Grading describes severity of manifestations, with CHE covering minimal HE and grade 1, and OHE covering grades 2-4. Time course is subdivided into three types: episodic, recurrent, and persistent. Lastly, the episode of HE is described as spontaneous or precipitated by specific factors (Table 1).
| Noncompliance with lactulose |
| Dehydration from lactulose overuse |
| Gastrointestinal bleeding |
| Infection/sepsis |
| Medications (narcotics, sedatives, etc.) |
| Recreational drugs (cocaine, marijuana, etc.) |
| Transjugular intrahepatic portosystemic shunt |
| Alcohol intoxication |
| Electrolyte dysfunction |
| Constipation |
| Renal failure |
| Constipation |
The management of HE is mainly guided by clinical impression. In cases where HE is suspected, normal or slightly elevated blood ammonia levels cannot rule out a diagnosis of HE[31]. However, when evidence of underlying chronic liver disease is lacking, elevated blood ammonia levels may provide helpful prognostic information for patients in acute liver failure, or may serve as the basis for further evaluation of uncommon metabolic disorders such as urea cycle disorders[31]. Furthermore, other causes of altered mental status should be ruled out. Laboratory tests and brain imaging can diagnose other causes such as intracranial hematomas, thyroid dysfunction, electrolyte imbalances, and sepsis (Table 2).
| Acidosis |
| Drug intoxication |
| Encephalitis |
| Hyperglycemia |
| Hypoglycemia |
| Hypocapnia |
| Hypoxia |
| Intracranial hematoma |
| Severe sepsis |
| Thyroid dysfunction |
| Uremia |
| Wernicke encephalopathy and korsakoff syndrome (vitamin B1 deficiency) |
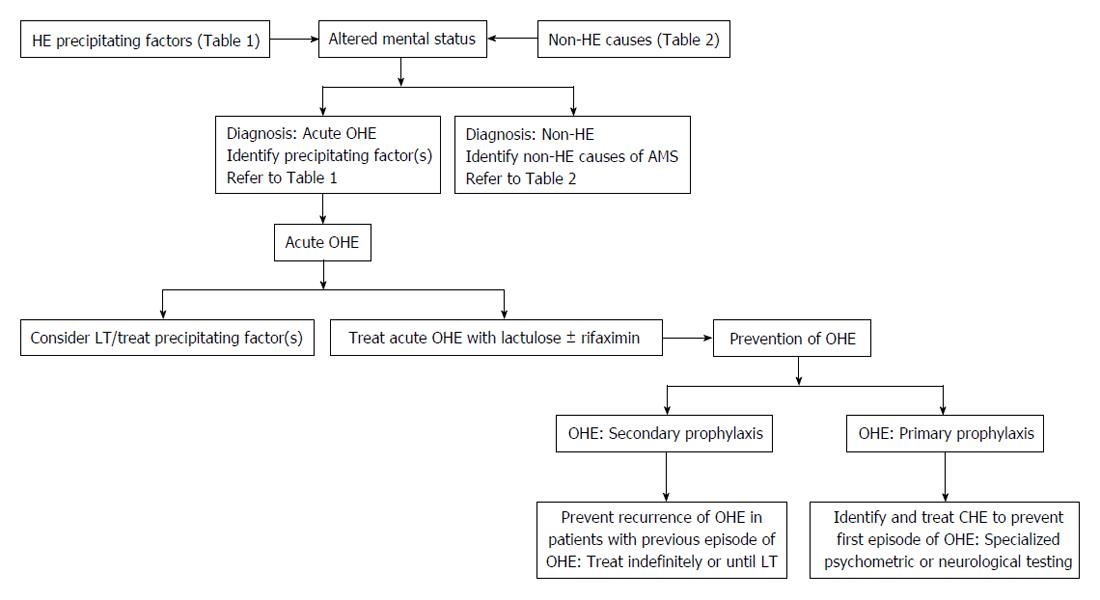
The approach to a patient with acute OHE involves empiric treatment for HE and a focus on identifying the precipitating factor (Figure 1)[32,33]. The precipitating factors may include gastrointestinal bleeding, infection, mind-altering medications (sedatives, narcotics, recreational agents, etc.), electrolyte imbalances, constipation, and renal failure (Table 1)[2]. Identification and treatment of precipitating factors of HE is critical. An early study found 90% of all cases of acute OHE were successfully resolved with prompt management of the precipitating factor(s) alone[34,35]. In one sample, spontaneous bacterial peritonitis was the most common precipitant of HE (20.5%), followed by constipation (18.3%), and urinary tract infection (15.3%)[36]. Therefore, an evaluation to search for a potential infection with a diagnostic paracentesis, blood cultures, urine culture and chest X-ray is warranted. In another study, infection (44%), gastrointestinal bleeding (38%), and constipation (38%) were the most common precipitating factors[37].
Currently, the preferred first-line treatment for an episode of acute OHE is lactulose, a poorly absorbed disaccharide that works in several ways. Lactulose decreases the blood ammonia concentration by promoting elimination of ammonia. Fermentation of cleaved lactulose by bacteria into lactic acid and hydrogen ions acidifies the colon, resulting in protonation of ammonia molecules into non-absorbable ammonium and facilitates movement of ammonia from the blood stream into the colon lumen[2]. Alteration to the gut flora also causes reduction of urease-producing bacteria. Lactitol, a second-generation unabsorbed dissacharide, is an alternative with an analogous mechanism of action to lactulose in the management of HE. Lactitol, which is formulated as a powder that can be dissolved in water for administration, is known to have superior taste properties and a defined laxative threshold. In addition, lactitol has demonstrated comparable efficacy with lactulose[38,39].
Rifaximin is an alternative first-line treatment option for OHE. It is a broad-spectrum antibiotic and it works by altering the bowel flora with net reduction in blood ammonia concentration. A 2013 study by Sharma et al[40] was the first prospective double-blind randomized control trial to compare rifaximin plus lactulose to lactulose alone. A total of 120 patients were randomized to the rifaximin group or placebo group, with lactulose dosed to achieve 2 to 3 semisoft stools per day. Treatment was administered through nasogastric tube and continued for a maximum of 10 d or until recovery. The rifaximin group demonstrated 76% reversal of OHE vs 44% reversal of OHE in the placebo group (P = 0.004). Mortality rates were lower in the rifaximin group when compared to the placebo group (24% vs 49%, P≤ 0.05). The lower mortality was due to a reduction in sepsis-related deaths. The authors hypothesize that the anti-bacterial effect of rifaximin inhibited the release of gut-related endotoxins and their transport into the bloodstream. In addition, this study demonstrated a shorter duration of hospital stay with rifaximin treatment (5.8 ± 3.4 d vs 8.2 ± 4.6 d, P = 0.001). The study was limited by lack of serial ammonia level monitoring, though this is not routinely performed in clinical practice.
Second-line agents for the treatment of acute OHE include neomycin and metronidazole. These antibiotics have fallen out of favor due to their systemic adverse effects such as ototoxicity and nephrotoxicity for neomycin, and neurotoxicity for metronidazole.
Branched-chain amino acids (BCAA) have been studied as therapeutic agents targeting HE. Randomized controlled trials have evaluated the use of BCAA-rich parenteral nutrition in patients with HE[41,42]. Based on a meta-analysis, patients receiving BCAA-rich infusions demonstrated superior mental recovery compared to controls[43]. The impact on mortality was mixed among the constituent trials, with three suggesting improved survival and two suggesting worse survival. Data regarding oral BCAA supplements is controversial. Whereas some trials have demonstrated significant benefit in mental performance with oral BCAA dietary supplementation, others have not revealed consistent benefit[44,45]. A recent meta-analysis including 16 randomized controlled trials of both intravenous and oral BCAA supplementation determined that BCAA had a clinically beneficial effect on OHE (RR = 0.73, 95%CI: 0.61-0.88); however, there was no impact on mortality, quality of life, or nutritional parameters[46]. Increased risk of nausea and vomiting was noted. Les et al[45] found improvement in performance on neuropsychometric tests and an increase in mid-arm muscle circumference in patients randomized to BCAA. The design of this study was limited by its inability to discriminate between the effects of the quantity and quality of nitrogen intake. Marchesini et al[47] found improved benefits of BCCA in average hospital admission rate, nutritional parameters and liver function tests. The major limitation of this study was the withdrawal of a significantly greater number of patients form the BCAA arm compared to control study, primarily due to a combination of adverse effects and noncompliance.
Polyethylene glycol (PEG), a cathartic agent best known for management of constipation, results in increased fecal ammonia excretion. In a randomized controlled trial, PEG was compared head-to-head with lactulose among inpatients with HE. Among patients who were randomly assigned to four liters of PEG over four hours, there was greater improvement in HE after 24 h compared to those who were given three or more doses of lactulose, each 20 to 30 g, over 24 h. Furthermore, median time to resolution of HE was shorter in the PEG cohort[48]. The study was limited by its single-center design and lack of blinding.
Patients with hepatic decompensation in the setting of cirrhosis and HE have been noted to exhibit decreased serum zinc levels[49,50]. Based on the potential role of zinc in neurotransmission[51,52], zinc supplementation has been considered in the treatment or prevention of acute OHE. Yoshida et al[49] compared zinc serum levels in 10 patients with cirrhosis-related hepatic decompensation to patients with compensated cirrhosis and healthy volunteers. Zinc supplementation was found to decrease serum ammonia levels with an unknown impact on OHE clinical course. The study noted that the percentage increase in serum zinc levels was lower in cirrhotic patients. Reduced absorption and higher urinary excretion due to diuretic administration was deemed partially responsible for this observation. The relatively lower percentage increase in serum zinc levels could partially explain an earlier study of zinc-supplementation in 15 cirrhotic patients with chronic HE with no improvement in symptoms associated with chronic HE[53]. A 2013 systematic review of zinc in the treatment of HE found an improvement on the number connection test, but no reduction in recurrence of HE (RR = 0.64; 95%CI: 0.26-1.59)[54].
Secondary prophylaxis is the prevention of recurrent episodes of OHE following resolution of acute OHE. Outpatient management after an episode of acute OHE includes improvement of daily function and evaluation for liver transplant candidacy. Secondary prophylaxis should continue indefinitely or until liver transplantation. In the 2009 study from New Delhi, India, Sharma et al[55] conducted an open-label randomized control trial to study recurrence of OHE in cirrhotic patients after recovery from a prior episode. Primary end point was development of OHE and follow-up ranged from 1 to 20 mo. The proportion of patients with recurrent episodes was found to be lower in the lactulose group compared to the placebo (19.6% vs 46.8%, P = 0.001). The most common adverse effects of lactulose were found to be diarrhea (23%), abdominal bloating (10%), and distaste (13%). In this study from New Delhi, all patients tolerated treatment and remained adherent to therapy. In the United States, adherence to lactulose therapy is a concern because many patients find the adverse effects difficult to tolerate[2]. These include severe diarrhea leading to dehydration, hypokalemia, hyponatremia, and other electrolyte disturbance. Other adverse effects related to lactulose therapy include bloating, flatulence, nausea, vomiting, and unpleasant sweet taste. Bajaj et al[56] studied adherence in 137 patients treated with lactulose therapy after initial episode of OHE Among the 103 patients who experienced a recurrent episode of OHE while on lactulose therapy, 38% were non-adherent, 54% were adherent, and 8% experienced lactulose-associated dehydration leading to recurrence. All non-adherent patients in the study developed recurrence, while 64% of those who were adherent to lactulose therapy developed OHE recurrence. Despite adherence to lactulose in this study, precipitating events such as sepsis and gastrointestinal bleeding resulted in recurrence of OHE. Multivariate regression demonstrated lactulose non-adherence (OR = 3.26) and MELD score (OR = 1.14) as predictive factors for recurrence. A retrospective study by Pantham et al[57] noted that lactulose non-compliance (39%), constipation (22%), opioids and benzodiazepines (17%), dehydration (16%), and infections (15%) as the leading precipitating factors for OHE.
Rifaximin can be used as an effective add-on therapy with lactulose to prevent OHE recurrence. After a second recurrent episode of OHE, rifaximin is recommended as an add-on therapy to lactulose for secondary prophylaxis[30]. Bass et al[58] performed a randomized, double-blind, placebo-controlled trial to study the effect of rifaximin plus lactulose vs lactulose and placebo in patients who were in remission from OHE. Patients were followed for the treatment period of 6 mo with the primary endpoint being time until a breakthrough episode of OHE. In the rifaximin group, 22.1% of patients reported a breakthrough episode vs 45.9% in the placebo group with a relative risk reduction of 58% for recurrence with rifaximin vs placebo. These data estimated the number-needed-to-treat is 4 patients for 6 mo to prevent one episode of OHE. Hospitalizations were also lower in the rifaximin group (13.6%) compared to the placebo group (22.6%). The background use of lactulose in both groups was 91%. In another study, long term effects of rifaximin treatment were studied in 23 cirrhotic patients for up to 5 years, death, or liver transplantation[59]. Compared with controls, patients on rifaximin therapy experienced lower rates of variceal bleeding (35% vs 60%, P = 0.011), HE (32% vs 47%, P = 0.034), spontaneous bacterial peritonitis (5.5% vs 46%, P = 0.027), and hepatorenal syndrome (4.5% vs 51%, P = 0.037). The 5-year cumulative probability of survival in the rifaximin group was 61% vs 13.5% in the controls.
The concerns over bacterial-resistance with long-term rifaximin use is mitigated by studies demonstrating minimal long-term effects on intestinal flora[60]. In vitro, rifaximin is minimally absorbed and predominantly concentrated in the lumen of the gastrointestinal tract[61]. In a study that found rifaximin resistance in individuals treated with rifaximin 800 mg orally per day for 5 d, discontinuation of treatment for 1 to 2 wk resulted in reduction in resistance rates to less than 20% of intestinal flora[62]. Resistant strains were unable to maintain their presence in the intestinal flora[61]. In contrast to plasmid-mediated resistance often seen with aminoglycoside antibiotics[63], rifaximin resistance occurs through a chromosomal mutation affecting bacterial RNA synthesis.
Treatment of patients with CHE to prevent development of a first episode of OHE is referred to as primary prophylaxis and it has important prognostic implications[55]. Sharma et al[64] randomized 120 cirrhotic patients with no previous episodes of OHE to study primary prophylaxis with and without lactulose therapy. Patients were followed for 12 mo and the number of patients with CHE at baseline was similar in both groups. In the lactulose group, 11% of patients developed OHE vs 28% of patients in the no-lactulose group (P = 0.02). Reduction in overall mortality was found to be non-statistically significant (9% vs 20%, P = 0.16). Lactulose was found to improve CHE in 66% of patients. These findings demonstrate lactulose as an effective option for primary prophylaxis of OHE. Current guidelines do not recommend lactulose or rifaximin use as primary prophylactic therapy for the prevention of OHE following post-transjugular intrahepatic portosystemic shunt (TIPS). Lunia et al[65] performed an open-label, randomized-control trial in 2014 to study the role of probiotics in primary prophylaxis of OHE in patients with cirrhosis. At a single site in New Delhi, India, 160 patients were randomized to either probiotics or no treatment. Probiotics alter intestinal microflora by increasing non-urease producing bacteria, thus reducing the amount of ammonia production. In patients with MHE or CHE, an absolute risk reduction of 23.8% was observed, demonstrating a number-needed-to-treat of 4.2 to prevent an episode of OHE in patients with cirrhosis. In addition, a significant reduction in the number of patients with CHE was also observed in the probiotics treatment group compared to no treatment. However, the study was limited by potential treatment bias given lack of blinding the investigators to the administered treatments.
An earlier study published in 2012 by Agrawal et al[66] also compared probiotic therapy to lactulose and no therapy for secondary prophylaxis. Using an open-label, randomized controlled study, patients who had recovered from an episode of OHE were followed until development of recurrent OHE or for 12 mo. Development of OHE in both the lactulose group and probiotics group were significantly lower than that of no treatment (26.2% and 34.4%, respectively, vs 56.9% P = 0.001, P = 0.02). Nevertheless, this study was limited by lack of blinding of the randomized intervention. The improved side effect profile of probiotics compared with lactulose offers a compelling alternative therapy for patients with difficulty tolerating lactulose therapy. In this study, all of the patients in the lactulose group were able to tolerate treatment despite the usual adverse effects; 26.4% diarrhea, 16.2% abdominal bloating, and 17.6% distaste to lactulose. In comparison, the most common adverse effect in the probiotics group was constipation (21.8%) and abdominal distention (14%). At 21.8%, the percentage of patients reporting constipation in the probiotics group paralleled that of the non-therapy group (21.5%). No patients in the probiotics group experienced fever, rash, or increased frequency of stools. While probiotic strains may differ and further studies are needed for validation, data demonstrating effective secondary prophylaxis with decreased risk of non-adherence are encouraging.
New screening test for CHE include Stroop smartphone application[67]. The Stroop application involves a test of mental speed and flexibility that asks the user to correctly identify the color of the presented stimuli. The stimuli can be presented in three forms, a neutral non-verbal cue such as “###” in red ink, a congruent stimuli such as “red” written in red ink, or an incongruent cue such as “red” in green ink. Bajaj et al[67] studied Stroop-app reported scores in 125 cirrhotic patients and 134 controls. Performance was significantly impaired in patients with CHE compared to patients without CHE, as measured using psychometric tests. Furthermore, scores correlated with MELD scores and were worst in those with prior OHE episodes. The study was limited by a relatively high educational status in patients. However, the findings are promising for quick, valid, and reliable screening for CHE.
Management of acute OHE involves institution of lactulose, preferred first-line therapy. In addition, prompt recognition and treatment of precipitating factors is critical as it may result in complete resolution of acute episode of OHE. Treatment goals include improvement of daily functioning, evaluation for liver transplantation, and prevention of OHE recurrence. For secondary prophylaxis, intolerance to indefinite lactulose therapy may lead to non-adherence and has been identified as a precipitating factor for recurrent OHE. Rifaximin is an effective add-on therapy to lactulose for treatment and prevention of recurrent OHE. Recent studies have demonstrated comparable efficacy of probiotic therapy to lactulose use in both primary prophylaxis and secondary prophylaxis.
P- Reviewer: Arai M, Karatapanis S, Lens S, Shimizu Y, Smith MA S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 2. | Khungar V, Poordad F. Management of overt hepatic encephalopathy. Clin Liver Dis. 2012;16:73-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787-799, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1410] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 5. | Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034-1041.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Patidar KR, Thacker LR, Wade JB, Sterling RK, Sanyal AJ, Siddiqui MS, Matherly SC, Stravitz RT, Puri P, Luketic VA. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. 2014;109:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Cordoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, Angeli P, Domenicali M, Ginés P, Bernardi M. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol. 2014;60:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 8. | Saunders JB, Walters JR, Davies AP, Paton A. A 20-year prospective study of cirrhosis. Br Med J (Clin Res Ed). 1981;282:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 254] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW. Screening of subclinical hepatic encephalopathy. J Hepatol. 2000;32:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Gitlin N, Lewis DC, Hinkley L. The diagnosis and prevalence of subclinical hepatic encephalopathy in apparently healthy, ambulant, non-shunted patients with cirrhosis. J Hepatol. 1986;3:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 368] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2132] [Article Influence: 112.2] [Reference Citation Analysis (3)] |
| 14. | Fichet J, Mercier E, Genée O, Garot D, Legras A, Dequin PF, Perrotin D. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care. 2009;24:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Neff GW, Kemmer N, Duncan C, Alsina A. Update on the management of cirrhosis - focus on cost-effective preventative strategies. Clinicoecon Outcomes Res. 2013;5:143-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 18. | Grippon P, Le Poncin Lafitte M, Boschat M, Wang S, Faure G, Dutertre D, Opolon P. Evidence for the role of ammonia in the intracerebral transfer and metabolism of tryptophan. Hepatology. 1986;6:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Weissenborn K, Ahl B, Fischer-Wasels D, van den Hoff J, Hecker H, Burchert W, Köstler H. Correlations between magnetic resonance spectroscopy alterations and cerebral ammonia and glucose metabolism in cirrhotic patients with and without hepatic encephalopathy. Gut. 2007;56:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Cauli O, Rodrigo R, Llansola M, Montoliu C, Monfort P, Piedrafita B, El Mlili N, Boix J, Agustí A, Felipo V. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis. 2009;24:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Yurdaydin C, Hörtnagl H, Steindl P, Zimmermann C, Pifl C, Singer EA, Roth E, Ferenci P. Increased serotoninergic and noradrenergic activity in hepatic encephalopathy in rats with thioacetamide-induced acute liver failure. Hepatology. 1990;12:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 836] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 24. | Riggio O, Ridola L, Pasquale C, Pentassuglio I, Nardelli S, Moscucci F, Merli M, Montagnese S, Amodio P, Merkel C. A simplified psychometric evaluation for the diagnosis of minimal hepatic encephalopathy. Clin Gastroenterol Hepatol. 2011;9:613-616.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 261] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, de Madaria E, Montoliu C, Nuñez D, Flavia M. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Montagnese S, Balistreri E, Schiff S, De Rui M, Angeli P, Zanus G, Cillo U, Bombonato G, Bolognesi M, Sacerdoti D. Covert hepatic encephalopathy: agreement and predictive validity of different indices. World J Gastroenterol. 2014;20:15756-15762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Zheng G, Zhang LJ, Zhong J, Wang Z, Qi R, Shi D, Lu GM. Cerebral blood flow measured by arterial-spin labeling MRI: a useful biomarker for characterization of minimal hepatic encephalopathy in patients with cirrhosis. Eur J Radiol. 2013;82:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology. 2007;45:833-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1407] [Article Influence: 127.9] [Reference Citation Analysis (1)] |
| 31. | Elgouhari HM, O’Shea R. What is the utility of measuring the serum ammonia level in patients with altered mental status? Cleve Clin J Med. 2009;76:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 34. | Strauss E, Tramote R, Silva EP, Caly WR, Honain NZ, Maffei RA, de Sá MF. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39:542-545. [PubMed] |
| 35. | Khungar V, Poordad F. Hepatic encephalopathy. Clin Liver Dis. 2012;16:301-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Mumtaz K, Ahmed US, Abid S, Baig N, Hamid S, Jafri W. Precipitating factors and the outcome of hepatic encephalopathy in liver cirrhosis. J Coll Physicians Surg Pak. 2010;20:514-518. [PubMed] |
| 37. | Maqsood S, Saleem A, Iqbal A, Butt JA. Precipitating factors of hepatic encephalopathy: experience at Pakistan Institute of Medical Sciences Islamabad. J Ayub Med Coll Abbottabad. 2006;18:58-62. [PubMed] |
| 38. | Morgan MY, Hawley KE. Lactitol vs. lactulose in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double-blind, randomized trial. Hepatology. 1987;7:1278-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Blanc P, Daures JP, Rouillon JM, Peray P, Pierrugues R, Larrey D, Gremy F, Michel H. Lactitol or lactulose in the treatment of chronic hepatic encephalopathy: results of a meta-analysis. Hepatology. 1992;15:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 41. | Cerra FB, Cheung NK, Fischer JE, Kaplowitz N, Schiff ER, Dienstag JL, Bower RH, Mabry CD, Leevy CM, Kiernan T. Disease-specific amino acid infusion (F080) in hepatic encephalopathy: a prospective, randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr. 1985;9:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 117] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Michel H, Bories P, Aubin JP, Pomier-Layrargues G, Bauret P, Bellet-Herman H. Treatment of acute hepatic encephalopathy in cirrhotics with a branched-chain amino acids enriched versus a conventional amino acids mixture. A controlled study of 70 patients. Liver. 1985;5:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Naylor CD, O’Rourke K, Detsky AS, Baker JP. Parenteral nutrition with branched-chain amino acids in hepatic encephalopathy. A meta-analysis. Gastroenterology. 1989;97:1033-1042. [PubMed] |
| 44. | Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, Martines D, Abbiati R. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990;11:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 127] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Gluud LL, Dam G, Les I, Córdoba J, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2015;9:CD001939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 48. | Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 49. | Yoshida Y, Higashi T, Nouso K, Nakatsukasa H, Nakamura SI, Watanabe A, Tsuji T. Effects of zinc deficiency/zinc supplementation on ammonia metabolism in patients with decompensated liver cirrhosis. Acta Med Okayama. 2001;55:349-355. [PubMed] |
| 50. | Loomba V, Pawar G, Dhar KL, Setia MS. Serum zinc levels in hepatic encephalopathy. Indian J Gastroenterol. 1995;14:51-53. [PubMed] |
| 51. | Ebadi M, Iversen PL, Hao R, Cerutis DR, Rojas P, Happe HK, Murrin LC, Pfeiffer RF. Expression and regulation of brain metallothionein. Neurochem Int. 1995;27:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Agus ZS, Dukes ID, Morad M. Divalent cations modulate the transient outward current in rat ventricular myocytes. Am J Physiol. 1991;261:C310-C318. [PubMed] |
| 53. | Riggio O, Ariosto F, Merli M, Caschera M, Zullo A, Balducci G, Ziparo V, Pedretti G, Fiaccadori F, Bottari E. Short-term oral zinc supplementation does not improve chronic hepatic encephalopathy. Results of a double-blind crossover trial. Dig Dis Sci. 1991;36:1204-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Chavez-Tapia NC, Cesar-Arce A, Barrientos-Gutiérrez T, Villegas-López FA, Méndez-Sanchez N, Uribe M. A systematic review and meta-analysis of the use of oral zinc in the treatment of hepatic encephalopathy. Nutr J. 2013;12:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885-891, 891.e1. [PubMed] |
| 56. | Bajaj JS, Sanyal AJ, Bell D, Gilles H, Heuman DM. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Pantham G, Waghray N, Prakash R, Biyyani RSRS, Mullen KD. Overt Hepatic Encephalopathy in Cirrhosis: Influence of Multiple Clinical Precipitants Resulting in Hospitalization. Gastroenterology. 2012;142:S-950 [Abstract]. [DOI] [Full Text] |
| 58. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 867] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 59. | Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 60. | Jiang ZD, DuPont HL. Rifaximin: in vitro and in vivo antibacterial activity--a review. Chemotherapy. 2005;51 Suppl 1:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005;3:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | De Leo C, Eftimiadi C, Schito GC. Rapid disappearance from the intestinal tract of bacteria resistant to rifaximin. Drugs Exp Clin Res. 1986;12:979-981. [PubMed] |
| 63. | Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1888] [Cited by in RCA: 1739] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 64. | Sharma P, Sharma BC, Agrawal A, Sarin SK. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: an open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol. 2012;27:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003-1008.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Bajaj JS, Thacker LR, Heuman DM, Fuchs M, Sterling RK, Sanyal AJ, Puri P, Siddiqui MS, Stravitz RT, Bouneva I. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |