Published online Nov 18, 2015. doi: 10.4254/wjh.v7.i26.2688
Peer-review started: March 1, 2015
First decision: April 13, 2015
Revised: May 3, 2015
Accepted: September 2, 2015
Article in press: September 7, 2015
Published online: November 18, 2015
Processing time: 263 Days and 14.5 Hours
AIM: To investigate the efficacy of virological response (VR) to telaprevir (TVR)-based triple therapy in predicting treatment outcome of hepatitis C.
METHODS: This prospective, multicenter study consisted of 253 Japanese patients infected with hepatitis C virus (HCV) genotype 1b. All received 12 wk of TVR in combination with 24 wk of pegylated-interferon-α (IFN-α) and ribavirin. Serum HCV RNA was tested at weeks 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24. VR was defined as undetectable serum HCV RNA. Sustained virological response (SVR) was VR at 24 wk after the end of treatment and was regarded as a successful outcome.
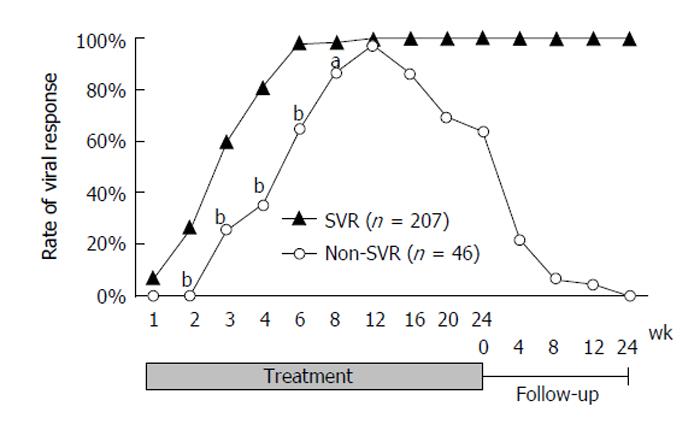
RESULTS: Of 253 patients, 207 (81.8%) achieved SVR. The positive predictive value of VR for SVR was 100% at week 2, after which it gradually decreased, and was over 85% to week 12. The negative predictive value (NPV) gradually increased, reaching 100% at week 12. The upslope of the NPV showed a large increase from week 4 (40.6%) to week 6 (82.4%). There was a moderate concordance between the SVR and VR at week 6 (kappa coefficient = 0.44), although other VRs had poor concordance to SVR. Multiple logistic regression analysis extracted VR at week 6 (P < 0.0001, OR = 63.8) as an independent factor contributing to SVR. In addition, the interleukin-28B single nucleotide polymorphism and response to previous pegylated-IFN-α and ribavirin therapy were identified as independent factors for SVR.
CONCLUSION: VR at week 6, but not at week 4, is an efficient predictor of both SVR and non-SVR to TVR-based triple therapy.
Core tip: Although an undetectable viral level at week 4 or 12 is a good predictor of the outcome of hepatitis C for conventional interferon therapy without direct-acting antiviral agents (DAAs); the transition of the viral level during DAA therapy has not been well documented. In this prospective multicenter study, we frequently tested 253 patients to investigate viral activity during triple therapy containing telaprevir, the first approved DAA, and found that an undetectable viral level at week 6 was the most effective predictor of disease outcome. Our findings suggest that the most predictive time point in DAA therapy is different from conventional therapy markers.
- Citation: Hiramine S, Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A, Koyanagi T, Kotoh K, Shimoda S, Hayashi J. Importance of virological response in the early stage of telaprevir-based triple therapy for hepatitis C. World J Hepatol 2015; 7(26): 2688-2695
- URL: https://www.wjgnet.com/1948-5182/full/v7/i26/2688.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i26.2688
Since the approval of interferon-α (IFN-α) for the treatment of hepatitis C virus (HCV) infected patients in 1991, treatment regimens have greatly evolved and improved. The rate of sustained virological response (SVR) to dual therapy with ribavirin (RBV) and pegylated IFN (PegIFN) of patients with HCV genotype 1 has remained approximately 50%[1-3], but with telaprevir (TVR), the first direct-acting antiviral agent (DAA) approved in the United States, Canada, the European Union, and Japan, the rate of SVR to triple therapy of PegIFN-α, RBV, and TVR against HCV genotype 1 has reached over 70%[4-6]. New DAAs have since been developed and approved, and it has become common for patients to be treated with IFN therapy that contains a DAA or a DAA based IFN-free oral therapy. Unfortunately, the cost of DAAs can be prohibitive, and some have serious side effects. If patients who will not achieve SVR can be identified before or in the early stage of treatment, they can avoid starting or continuing an expensive treatment that has no possibility of success. Therefore, studies of factors that can be used to predict the outcome of DAA based therapies are needed.
For dual therapy with PegIFN-α/RBV, it has been consistently reported that virological response (VR: undetectable serum HCV RNA) at week 4 or 12 of therapy is strongly associated with outcome[7-10]. Rapid VR (RVR), VR at week 4, and early VR (EVR), VR at week 12, were terms coined before the approval of DAAs, and this criterion is still used for determining the best form of antiviral treatment management, as recommended by international consensus conferences such as the American Association for the Study of Liver Diseases (AASLD)[11] and the European Association for the Study of the Liver (EASL)[12]. However, the viral kinetics during DAA therapy are unclear, and it is possible that the time point most predictive of success might be different than the older regimens.
To clarify the timing of VR most predictive of SVR during DAA based treatment, we measured serum HCV RNA at seven time points during the early stage of TVR-based triple therapy for Japanese patients.
Since 2004, the Kyushu University Liver Disease Study Group has conducted prospective, multicenter studies to investigate the efficacy and safety of antiviral treatment for chronic hepatitis C patients[3,6]. For this study, we recruited 253 chronic hepatitis C patients infected with HCV genotype 1b who started TVR-based triple therapy between December 2011 and December 2012 and completed 24 wk post-therapy follow-up by June 2013. Exclusion criteria were as reported previously[6]. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of our hospital. Informed consent was obtained from all patients before enrollment. The study was registered as a clinical trial on the University Hospital Medical Information Network (ID 000009711).
VR was defined as undetectable serum HCV RNA. Successful treatment was SVR at 24 wk after the end of treatment. Relapse was defined as VR during the treatment but non-SVR. Patients with HCV RNA detectable throughout treatment were classified as non-responders. Patients who had not been previously treated with PegIFN-α/RBV therapy were classified as treatment naïve.
Clinical parameters included hemoglobin, platelet count, serum albumin, aspartate aminotransferase (AST), alanine aminotransferase, γ-glutamyl-transpeptidase, low-density lipoprotein (LDL) cholesterol, ferritin, and estimated glomerular filtration rate. HCV RNA was tested at baseline, weeks 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 during the treatment and at weeks 4, 8, 12, and 24 after the end of treatment. We defined the early stage of treatment as the period between day 1 and week 12. The timing of VR in the early stage of treatment was evaluated for candidate predictors of SVR. Liver biopsy was done for 154 (60.9%) patients before the induction of therapy. For each specimen, the stage of fibrosis (F0-4) and grade of activity (A0-3) were established according to the Metavir score[13].
The baseline and follow-up tests for HCV viremia were done by real-time polymerase chain reaction (PCR) assay (COBAS TaqMan HCV test, Roche Diagnostics, Basel, Switzerland), with a detectability of ≥ 15 IU/mL and a linear dynamic range of 1.2-7.8 log IU/mL. HCV genotype and the core amino acid substitution at position 70 of the HCV genome were determined before treatment for all patients. HCV genotype was determined by sequence determination in the 5’ non-structural region of the HCV genome, followed by phylogenetic analysis[14].
Human genomic DNA was extracted from peripheral blood. Genotyping by the single-nucleotide polymorphism (SNP) of the interleukin 28B (IL28B) (rs8099917) gene was done using the TaqMan Allelic Discrimination Demonstration Kit (7500 Real-Time PCR System; Applied Biosystems, Foster City, CA). Patients were genotyped as TT, TG, or GG at the polymorphic site. Similarly, genotyping by the SNP of the inosine triphosphate pyrophosphatase (ITPA) (rs1127354) gene was done using the TaqMan Allelic Discrimination Demonstration Kit. Patients were genotyped as CC, CA, or AA at the polymorphic site. IL28B and ITPA SNPs were not available for only two patients (1.2%). Although rs12979860, another IL28B SNP that is also strongly correlated to the therapeutic outcome, has been reported[15], we determined only rs8099917 because it was previously reported that rs8099917 and rs12979860 represent 98.6% of the Japanese population[16].
All patients received 12 wk triple therapy that included TVR (2250 mg/day) (Telavic; Mitsubishi Tanabe Pharma, Osaka, Japan), PegIFN-α-2b (60-150 μg/wk) (PEG-Intron; MSD, Tokyo, Japan), and RBV (600-1000 mg/d) (Rebetol; MSD), followed by a 12 wk dual therapy that included PegIFN-α-2b and RBV. TVR (750 mg) was administered orally three times a day at 8 h intervals after each meal. PegIFN-α-2b was injected subcutaneously once weekly at a dose of 1.5 μg/kg. RBV was given orally at a daily dose of 600-1000 mg based on body weight (600 mg for patients weighing < 60 kg, 800 mg for those weighing 60-80 kg, and 1000 mg for those weighing > 80 kg). The above durations and dosages are those approved by the Japanese Ministry of Health, Labor, and Welfare. If marked anorexia, an elevation of serum creatinine, or severe anemia developed, the TVR dose could be reduced to 1500 mg/d (750 mg at a 12 h interval, after meals). The method of RBV/TVR dose reduction in the case of anemia was as reported[17]. The completed assigned total cumulative dosages of each drug were calculated by reviewing the patients’ medical records and by counting the pills not consumed by each patient. The actual dosage of TVR given was calculated as the percentage of target TVR (2250 mg/d). The dosages of PegIFN-α-2b and RBV were calculated individually as averages on the basis of body weight at baseline.
To evaluate the precision rate of on-treatment VR for predicting outcome, we calculated the positive predictive value (PPV) and the negative predictive value (NPV). PPV is defined as the probability that a patient with a given on-treatment VR will achieve SVR. In contrast, NPV is defined as the probability that a patient without a given on-treatment VR will not achieve SVR.
Statistical analyses were performed using the SAS system, version 9.1.3 (SAS Institute, Cary, NC, United States). Continuous data are expressed as median with interquartile range. Univariate analyses were performed using the χ2 test, Fisher’s exact test, paired t-test, or Mann-Whitney U test, as appropriate, with SVR as the outcome. Kappa coefficient was used for the analysis of the concordance between SVR and VR at the seven time points. To identify independent factors predictive of SVR, variables that reached the P < 0.1 level in univariate tests were used as candidates in the multiple logistic regression analysis. Continuous parameters that were significant in univariate analysis were converted into categorical variables by dichotomizing at the round number closest to their median for analysis in the multiple logistic regression model. Because liver histology data was missing for 99 (39.1%) patients, it was excluded from the multiple logistic regression model. A P value less than 0.05 was regarded as statistically significant in all analyses.
Of the 253 patients, 207 (81.8%) achieved SVR, 37 (14.6%) relapsed, and nine (3.6%) were non-responders. The VR rates increased dramatically over the first 6 wk (5.9%, 22.0%, 53.4%, 74.0%, and 93.1% at weeks 1, 2, 3, 4, and 6, respectively). Two hundred and forty-four patients (96.4%) had achieved VR by week 12. The rate gradually decreased to 81.8% after the end of treatment. A graph of the VR rates classified by SVR status is shown in Figure 1. Comparison of the VR rates of the SVR and non-SVR groups in the early stage of treatment showed that although there was no statistical difference at weeks 1 or 12 (7.3% vs 0.0% and 100% vs 97.3%, respectively), the rates were significantly higher for the SVR than for the non-SVR group for weeks 2 to 8 (26.9% vs 0.0%, 59.8% vs 25.6%, 81.6% vs 35.0%, 98.5% vs 65.0%, and 98.5% vs 86.5% at weeks 2, 3, 4, 6, and 8, respectively. P < 0.0001 at weeks 2, 3, 4, and 6. P = 0.0027 at week 8).
The patient characteristics are summarized by the clinical outcome in Table 1. Sex, age, genotype of IL28B SNP (rs8099917), hemoglobin level, platelet count, serum albumin, AST, and LDL-cholesterol at baseline were significantly correlated with SVR in the univariate analysis (all P < 0.05). The rate of non-responders to previous PegIFN-α/RBV therapy was significantly higher in the non-SVR group than in the SVR group (44.8% vs 12.5%, P < 0.0001). The SVR rate significantly decreased as the stage of fibrosis progressed but was not related to the grade of activity.
| All (n = 253) | SVR (n = 207) | Non-SVR (n = 46) | P value | |
| Sex, male (%) | 123 (48.6) | 108 (52.2) | 15 (32.6) | 0.0153 |
| Age (yr) | 61 (12.5) | 60 (12) | 63.5 (11.25) | 0.0340 |
| Body mass index (kg/m2) | 23.4 (3.9) | 23.4 (3.9) | 23.9 (3.8) | 0.2198 |
| Baseline HCV RNA (log10 IU/mL) | 6.5 (0.9) | 6.5 (0.9) | 6.4 (0.7) | 0.4468 |
| IL28B SNP (rs8099917), TT/TG or GG (%)1 | 186/65 (74.1/25.9) | 166/40 (80.6/19.4) | 20/25 (44.4/55.6) | < 0.0001 |
| ITPA SNP (rs1127354), CC/CA or AA (%)1 | 193/58 (76.9/23.1) | 157/49 (76.2/23.8) | 36/9 (80.0/20.0) | 0.5802 |
| Hemoglobin level (g/L) | 138 (22) | 140 (21) | 134 (20) | 0.0031 |
| Platelet count (× 109/L) | 157 (69) | 159 (65) | 129 (69) | 0.0006 |
| Serum albumin (g/L) | 40 (6.0) | 40 (6.0) | 39 (5.0) | 0.0143 |
| Aspartate aminotransferase (U/L) | 48 (42) | 46 (43) | 59 (34.5) | 0.0350 |
| Alanine aminotransferase (U/L) | 54 (58) | 53 (64) | 58 (44) | 0.4955 |
| γ-glutamyl-transpeptidase (U/L) | 40 (51) | 39 (47) | 46 (59) | 0.1270 |
| LDL-cholesterol (mg/dL) | 95 (38) | 98 (36) | 75 (35) | < 0.0001 |
| Ferritin (μg/L) | 164.6 (232.3) | 160.5 (223.2) | 181.7 (253.9) | 0.3583 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 79.4 (19.1) | 79.7 (18.9) | 77.7 (19.4) | 0.6210 |
| Response to previous PegIFN-α/RBV therapy | < 0.0001 | |||
| Treatment naïve, n (%) | 92 (36.4) | 81 (39.4) | 11 (23.9) | |
| Prior relapse, n (%) | 113 (44.7) | 100 (48.1) | 13 (28.3) | |
| Prior non-response, n (%) | 48 (19.0) | 26 (12.5) | 22 (44.8) | |
| Liver histology | ||||
| Stage, F0-2/F3-4 (%) | 96/58 (62.3/37.7) | 87/38 (69.6/30.4) | 9/20 (31.0/69.0) | < 0.0001 |
| Grade, A0-1/A2-3 (%) | 54/100 (35.1/64.9) | 45/80 (36.0/64.0) | 9/20 (31.0/69.0) | 0.614 |
| Not determined, n | 99 | 82 | 17 |
The PPV and NPV, calculated on the basis of VR in the early stage of treatment, are shown in Table 2. The PPV of VR for SVR was 100% at week 2, after which it gradually decreased, and it was over 85% to week 12. The NPV gradually increased, reaching 100% at week 12. The upslope of the NPV showed a large increase from week 4 (40.6%) to week 6 (82.4%). Kappa coefficients were calculated to evaluate the concordance between SVR and VRs (Table 2). There was a moderate concordance between the SVR and VR at week 6 (kappa coefficient = 0.44, 95%CI: 0.24-0.76), although the other VRs had poor concordance to SVR.
| Patients who achieved SVR/patients with VR, n | PPV (%) | Patients who did not achieve SVR/patients without VR, n | NPV (%) | Kappa coefficient (95%CI) | |
| Week 1 | 14/14 | 100 | 45/222 | 20.3 | 0.03 (0.01-0.05) |
| Week 2 | 52/52 | 100 | 44/185 | 23.8 | 0.12 (0.08-0.16) |
| Week 3 | 113/124 | 91.1 | 33/109 | 30.3 | 0.22 (0.12-0.33) |
| Week 4 | 168/182 | 92.3 | 26/64 | 40.6 | 0.38 (0.24-0.51) |
| Week 6 | 202/228 | 88.6 | 14/17 | 82.4 | 0.44 (0.27-0.61) |
| Week 8 | 200/232 | 86.2 | 5/8 | 62.5 | 0.18 (0.02-0.34) |
| Week 12 | 198/234 | 84.6 | 1/1 | 100 | - |
Multiple logistic regression analysis was done to determine factors predictive of SVR. VR at week 6, which had the highest kappa coefficient, was included as a candidate in order to compare its predictive power. IL28B SNP (rs8099917) genotype [P < 0.0001, odds ratio (OR) = 8.24, 95%CI: 2.81-26.8], response to previous PegIFN-α/RBV therapy (P = 0.0281, OR = 3.29, 95%CI: 1.14-9.46), and VR at week 6 (P < 0.0001, OR = 63.8, 95%CI: 10.8-563) were extracted as factors contributing to SVR. VR at week 6 had a high statistical correlation with SVR (Table 3).
| Univariate analysis | Multivariable analysis | |||
| OR | P value | OR (95%CI) | P value | |
| Sex (male to female) | 2.25 | 0.0153 | ||
| Age (< 60 yr to ≥ 60 yr) | 1.79 | 0.0822 | ||
| IL28B SNPs (rs8099917) (TT to TG/GG) | 5.19 | < 0.0001 | 8.24 (2.81-26.8) | < 0.0001 |
| Hemoglobin level (≥ 140 g/L to < 140 g/L) | 2.13 | 0.0245 | ||
| Platelet count (≥ 150 × 109/L to < 150 × 109/L) | 3.21 | 0.0005 | ||
| Serum albumin (> 35 g/L to ≤ 35 g/L) | 2.51 | 0.0308 | ||
| Aspartate aminotransferase (< 50 U/L to ≥ 50 U/L) | 2.30 | 0.0123 | ||
| LDL-cholesterol (≥ 95 mg/dL to < 95 mg/dL) | 4.39 | < 0.0001 | ||
| Response to previous PegIFN-α/RBV therapy (naïve/relapse to non-response) | 6.38 | < 0.0001 | 3.29 (1.14-9.46) | 0.0281 |
| VR at week 6 | 31.1 | < 0.0001 | 63.8 (10.8-563) | < 0.0001 |
VR in the early stage of treatment has in the past been used to manage the treatment of patients with HCV. Since the advent of DAAs, no studies have been published that describe the detailed transition of serum HCV RNA during DAA therapy. Although the guidelines of AASLD and EASL recommend checking VR at weeks 4 (RVR) and 12 (EVR) for the assessment of initial response to therapy and adherence, other time points, such as weeks 1, 2, 3, 6, and 10, were not mentioned in these guidelines[11,12]. It is likely that RVR and EVR were chosen because they have been traditionally used as markers for PegIFN-α/RBV therapy and because the efficacy of other time points in DAA-containing therapy have not yet been fully investigated. By testing at frequent intervals in this prospective multicenter study of 253 patients infected with HCV genotype 1b, we were able to show that the transition during treatment with a DAA is different than what was seen in the past with PegIFN-α/RBV therapy. VR at week 6 had a high PPV (88.6%), NPV (82.4%), and kappa coefficient (0.44), which indicates its usefulness as a single time point for predicting both SVR and non-SVR during TVR-based triple therapy. In addition, multiple logistic regression analysis that included pretreatment factors, such as the patient’s genotype, laboratory parameters at baseline, and response to previous therapy, extracted VR at week 6 as an independent factor contributing to SVR.
For dual therapy with PegIFN-α and RBV, RVR and EVR correlate with outcome and have traditionally been utilized as predictors. It has consistently been reported that RVR has a high PPV, around 90%[7-9], making it a useful marker for the prediction of SVR. In contrast, EVR has a high NPV, over 90%, making it a useful predictor of non-SVR[10]. In our study, the rates of EVR were not significantly different between the SVR and the non-SVR group. Although RVR had a high PPV, the NPV showed a sharp rise, from 45.7% at week 4% to 87.0% at week 6. This suggests that in DAA therapy, which has a direct mechanism and much stronger power to eliminate HCV than dual therapy, the most useful and meaningful time points for predicting the outcome may be different than in PegIFN-α/RBV therapy.
Although the DAAs strongly eliminate HCV, they are costly and some have serious side effects, such as the rash and anemia that often accompany TVR. To avoid unproductive expenditures and side effects, attempts have been made to establish response-guided treatment regimens that include early termination rules for unproductive DAA therapy[5,18]. It has been suggested that patients who have a rapid decline in their viral level can be treated with a shorter treatment duration, while preserving the high rate of SVR, and that treatment can be discontinued earlier for patients who are unlikely to respond the treatment. Our results showed that checking VR at week 6 would contribute to shortening the duration of TVR-based triple therapy. Furthermore, because both SVR and non-SVR can be predicted at a single time point (week 6), unnecessary testing can be eliminated, which will contribute to patient comfort and economic efficiency.
One of the limitations of our study is that TVR is no longer the standard of care in many countries. It is not recommended for the treatment of patients with decompensated cirrhosis or in a post-liver transplantation setting, and it should not be administered as co-medication. In addition, TVR can cause serious rash and anemia. In Japan, IFN-based therapy with RBV and simeprevir, a new nonstructural protein (NS)3/4A inhibitor, has become the standard of care against HCV genotype 1[19]. More recently, a number of novel DAAs, such as NS5A and NS5B inhibitors, have been developed and approved, and the current standard of care in the United States is an IFN-free DAA regimen[11]. Although our results might seem late to the game, TVR-containing treatment will continue to be an option in regions of the world where the newly approved DAAs are not available or in those patients with no other alternative. Another limitation of our study is that the patients were all Japanese and infected with HCV genotype 1b. The rate of SVR significantly differs by the race of the patient and the genotype of HCV[20]. Hence, our results may not be broadly applicable to the up-to-date IFN-free DAA regimens or to every patient with chronic hepatitis C. However, the results are useful because 253 patients were enrolled and frequent HCV RNA testing during DAA-containing therapy was analyzed that included numerous variables, including the genotype and laboratory parameters of each patient in this study. We believe that our study is sufficiently reliable to show that the most efficient time point for checking VR in DAA therapy might be different than the RVR and EVR that was developed for earlier therapies. Our results will need to be validated for the current DAA regimens, and further studies of patients with other HCV genotypes and of other racial cohorts will be necessary.
It is also a limitation of our study that we did not test for mutations of various HCV strains. Many studies have revealed that the variations in the amino acid sequences of HCV affect the antiviral activity of DAAs. Bartels et al[21], using a direct-sequencing technique, reported that the mutant strain resistant to NS3/4A protease inhibitors was detected in 2% of treatment naïve patients and that it was the pre-existing dominant strain in some of the patients. Nasu et al[22], using an ultra-deep sequencing technique, found some resistant mutations in a surprisingly high percentage of treatment naïve patients. In the coming era of IFN-free regimens, it will be essential to determine the mutations of the patients’ HCV strains before treatment.
In conclusion, VR at week 6 is the time point most predictive of both SVR and non-SVR in the early stage of TVR-based triple therapy. This result shows the possibility that the most efficient time point for checking VR in DAA therapy might be different than the conventional RVR and EVR. Our results will need to be validated in light of the newly developed DAA regimens.
Since direct-acting antiviral agents (DAAs) were approved for the treatment of chronic hepatitis C, the treatment success rate has greatly improved. However, DAAs are costly, and some have serious side effects. To avoid unproductive expenditures and side effects, attempts have been made to predict the treatment response by checking the serum viral load of patients during the early stage of treatment. It has been suggested that patients who have a rapid decline in viral level can be treated with a shorter treatment duration while preserving the high rate of success and that patients who are unlikely to respond treatment should discontinue it early.
An undetectable viral level at week 4 or 12 has consistently been correlated with outcome of conventional interferon therapy without DAAs, with rapid virological response and early virological response commonly used as predictors of treatment success. The transition of the viral level during DAA therapy has not been well documented. In this prospective multicenter study, the authors did frequent testing of 253 patients to investigate viral activity during triple therapy containing telaprevir, the first approved DAA.
This is the first study to report the detailed transition of the viral level during the early stage of DAA therapy for chronic hepatitis C. Importantly, it was found that an undetectable serum viral level at week 6, and not at week 4 or 12, is the most efficient predictor of outcome.
Checking the serum viral level at week 6 would be useful for establishing a response-guided treatment regimen for patients treated with DAAs, which would help reduce the total duration of treatment.
Virological response (VR) is defined as undetectable serum hepatitis C virus (HCV) RNA. Sustained virological response (SVR) is VR at 24 wk after the end of treatment and is regarded as successful treatment. The authors evaluated the ability of the VR between weeks 1 and 12 during the treatment to predict SVR or non-SVR.
Hiramine et al in this article describe in detail the factors that can be used for the prediction of therapeutic outcome. The limitation of the study is, as mentioned by the authors, that all the results are only for HCV genotype 1 Japanese patients. Interleukin 28B (IL28B) polymorphism is now a known factor influencing treatment response, and the authors have identified this as a predictive factor too. Although the polymorphism at IL28B rs8099917 is studied, another very important polymorphism rs12979860, which is well documented to influence the therapeutic outcome, is not studied. It would be helpful if the authors studied that polymorphism as well in these patients. Overall, the study is well-designed and well written.
P- Reviewer: Grammatikos G, Khaliq S S- Editor: Yu J L- Editor: Zhou B E- Editor: Liu SQ
| 1. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 2. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 3. | Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, Masumoto A, Maruyama T, Nakamuta M, Enjoji M, Azuma K. Association between the treatment length and cumulative dose of pegylated interferon alpha-2b plus ribavirin and their effectiveness as a combination treatment for Japanese chronic hepatitis C patients: project of the Kyushu University Liver Disease Study Group. J Gastroenterol Hepatol. 2008;23:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1861] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 5. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 6. | Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A. Telaprevir can be successfully and safely used to treat older patients with genotype 1b chronic hepatitis C. J Hepatol. 2013;59:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, Ferenci P, Ackrill AM, Willems B. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, Lee LP, Hsieh MY, Lin ZY, Chen SC. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47:1884-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Poordad F, Reddy KR, Martin P. Rapid virologic response: a new milestone in the management of chronic hepatitis C. Clin Infect Dis. 2008;46:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J Hepatol. 2005;43:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 11. | Recommendations for Testing, Managing, and Treating Hepatitis C. In: The American Association for the Study of Liver Diseases. 2015;. |
| 12. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 13. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 14. | Simmonds P, Alberti A, Alter HJ, Bonino F, Bradley DW, Brechot C, Brouwer JT, Chan SW, Chayama K, Chen DS. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology. 1994;19:1321-1324. [PubMed] |
| 15. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 16. | Ito K, Higami K, Masaki N, Sugiyama M, Mukaide M, Saito H, Aoki Y, Sato Y, Imamura M, Murata K. The rs8099917 polymorphism, when determined by a suitable genotyping method, is a better predictor for response to pegylated alpha interferon/ribavirin therapy in Japanese patients than other single nucleotide polymorphisms associated with interleukin-28B. J Clin Microbiol. 2011;49:1853-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Ogawa E, Furusyo N, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A. Clinical milestones for the prediction of severe anemia by chronic hepatitis C patients receiving telaprevir-based triple therapy. J Hepatol. 2013;59:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Jacobson IM, Marcellin P, Zeuzem S, Sulkowski MS, Esteban R, Poordad F, Bruno S, Burroughs MH, Pedicone LD, Boparai N. Refinement of stopping rules during treatment of hepatitis C genotype 1 infection with boceprevir and peginterferon/ribavirin. Hepatology. 2012;56:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis C Virus Infection: A 2014 Update for Genotype 1. Hepatol Res. 2014;44 Suppl S1:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Zhu Y, Chen S. Antiviral treatment of hepatitis C virus infection and factors affecting efficacy. World J Gastroenterol. 2013;19:8963-8973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Bartels DJ, Zhou Y, Zhang EZ, Marcial M, Byrn RA, Pfeiffer T, Tigges AM, Adiwijaya BS, Lin C, Kwong AD. Natural prevalence of hepatitis C virus variants with decreased sensitivity to NS3.4A protease inhibitors in treatment-naive subjects. J Infect Dis. 2008;198:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Nasu A, Marusawa H, Ueda Y, Nishijima N, Takahashi K, Osaki Y, Yamashita Y, Inokuma T, Tamada T, Fujiwara T. Genetic heterogeneity of hepatitis C virus in association with antiviral therapy determined by ultra-deep sequencing. PLoS One. 2011;6:e24907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |