Published online Oct 28, 2015. doi: 10.4254/wjh.v7.i24.2551
Peer-review started: June 17, 2015
First decision: July 3, 2015
Revised: July 28, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: October 28, 2015
Processing time: 135 Days and 11.2 Hours
AIM: To study pentoxifylline effects in liver and adipose tissue inflammation in obese mice induced by high-fat diet (HFD).
METHODS: Male swiss mice (6-wk old) were fed a high-fat diet (HFD; 60% kcal from fat) or AIN-93 (control diet; 15% kcal from fat) for 12 wk and received pentoxifylline intraperitoneally (100 mg/kg per day) for the last 14 d. Glucose homeostasis was evaluated by measurements of basal glucose blood levels and insulin tolerance test two days before the end of the protocol. Final body weight was assessed. Epididymal adipose tissue was collected and weighted for adiposity evaluation. Liver and adipose tissue biopsies were homogenized in solubilization buffer and cytokines were measured in supernatant by enzyme immunoassay or multiplex kit, respectively. Hepatic histopathologic analyses were performed in sections of paraformaldehyde-fixed, paraffin-embedded liver specimens stained with hematoxylin-eosin by an independent pathologist. Steatosis (macrovesicular and microvesicular), ballooning degeneration and inflammation were histopathologically determined. Triglycerides measurements were performed after lipid extraction in liver tissue.
RESULTS: Pentoxifylline treatment reduced microsteatosis and tumor necrosis factor (TNF)-α in liver (156.3 ± 17.2 and 62.6 ± 7.6 pg/mL of TNF-α for non-treated and treated obese mice, respectively; P < 0.05). Serum aspartate aminotransferase levels were also reduced (23.2 ± 6.9 and 12.1 ± 1.6 U/L for non-treated and treated obese mice, respectively; P < 0.05) but had no effect on glucose homeostasis. In obese adipose tissue, pentoxifylline reduced TNF-α (106.1 ± 17.6 and 51.1 ± 9.6 pg/mL for non-treated and treated obese mice, respectively; P < 0.05) and interleukin-6 (340.8 ± 51.3 and 166.6 ± 22.5 pg/mL for non-treated and treated obese mice, respectively; P < 0.05) levels; however, leptin (8.1 ± 0.7 and 23.1 ± 2.9 ng/mL for non-treated and treated lean mice, respectively; P < 0.05) and plasminogen activator inhibitor-1 (600.2 ± 32.3 and 1508.6 ± 210.4 pg/mL for non-treated and treated lean mice, respectively; P < 0.05) levels increased in lean adipose tissue. TNF-α level in the liver of lean mice also increased (29.6 ± 6.6 and 75.4 ± 12.6 pg/mL for non-treated and treated lean mice, respectively; P < 0.05) while triglycerides presented a tendency to reduction.
CONCLUSION: Pentoxifylline was beneficial in obese mice improving liver and adipose tissue inflammation. Unexpectedly, pentoxifylline increased pro-inflammatory markers in the liver and adipose tissue of lean mice.
Core tip: Pentoxifylline is prescribed to patients with severe alcoholic hepatitis, which suggest that this drug could also be beneficial to non-alcoholic steatohepatitis (NASH) patients. However, experimental results with pentoxifylline have shown conflicting data depending on the NASH model employed. Considering that obesity is strongly associated with the development of NASH, our study evaluated the effects of pentoxifylline in a high-fat diet induced obesity model. Our results showed that pentoxifylline was beneficial in obesity-associated NASH improving liver and adipose tissue inflammation. Unexpectedly, pentoxifylline treatment resulted in undesirable effects in adipose tissue and liver inflammatory markers in lean mice.
- Citation: Acedo SC, Caria CREP, Gotardo &MF, Pereira JA, Pedrazzoli J, Ribeiro ML, Gambero A. Role of pentoxifylline in non-alcoholic fatty liver disease in high-fat diet-induced obesity in mice. World J Hepatol 2015; 7(24): 2551-2558
- URL: https://www.wjgnet.com/1948-5182/full/v7/i24/2551.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i24.2551
Non-alcoholic fatty liver disease (NAFLD) defines a spectrum of hepatic disorders including steatosis or uncomplicated fatty liver and non-alcoholic steatohepatitis (NASH). NAFLD is frequently associated with metabolic syndrome establishment and it occurs more often in males than in females and primarily affects the middle aged and the elderly[1].
Several factors are associated with the development of fatty liver, and NAFLD diagnosis requires the exclusion of secondary etiologies, including alcohol consumption, drug usage, hepatitis B and C[2]. Although simple steatosis is considered benign, NASH can progress to end-stage liver disease, such as fibrosis, cirrhosis and hepatic cancer[3]. The mechanism of steatosis progression to more severe liver injuries is not fully understood, but it is associated with several risk factors, including elevated serum transaminases, inflammation upon liver biopsy, old age, diabetes mellitus, high body mass index (≥ 28 kg/m2), presence of ballooning plus Mallory hyaline or fibrosis upon biopsy and increased visceral adipose tissue[2,4].
The growing epidemic of obesity and an aging population have led to an important demand for a medical therapy for NAFLD, but several decades of pharmacological research have resulted in very few options[2]. As NAFLD is considered a hepatic manifestation of a metabolic syndrome, the first treatment approach is a lifestyle change, including dietary alterations and increased physical activity to reduce adiposity and body weight[5]. Therapeutic drugs are an adjunctive approach to lifestyle changes. Statins are used to control dyslipidemias, metformin and glitazones are used to control diabetes mellitus, and angiotensin receptor blockers are used to control inflammatory cell recruitment and hepatic fibrosis development in addition to their anti-hypertensive effects. In total, these drugs aim to control the symptoms of the metabolic syndrome[1,2,6].
Pentoxifylline is a non-selective phosphodiesterase inhibitor that has been reported to have antioxidant activity and decrease tumor necrosis factor (TNF)-α gene transcription. Pentoxifylline treatment improved the 6-mo survival rate of patients with severe alcoholic hepatitis compared with placebo[7]. Recent studies have shown that pentoxifylline may be a promising drug therapy for NASH treatment[8-10]. Experimental results with pentoxifylline have shown conflicting data depending on the model employed. In NAFLD induced by a choline- and methionine-deficient diet, pentoxifylline treatment was beneficial because it decreased hepatic inflammation and alanine aminotransferase (ALT) levels[11]. However, in a genetic obesity model, pentoxifylline worsened fatty liver in ob/ob mice because it increased intestinal glucose absorption, and thus, hyperglycemia[12].
Considering that obesity is strongly associated with the development of NAFLD and one of the main causes of epidemic obesity is a hyperlipidic and hypercaloric diet, our study evaluated the effects of pentoxifylline in a high-fat diet (HFD)-induced obesity model. Metabolic parameters, hepatic inflammation and adipose tissue alteration were studied after 2 wk of pentoxifylline treatment in mice after 12 wk of a HFD.
Specific pathogen-free, 4-wk-old male Swiss mice were obtained from CEMIB (State University of Campinas, Campinas, São Paulo, Brazil). All experiments were performed in accordance with the principles outlined by the National Council for the Control of Animal Experimentation (CONCEA, Brazil) and received approval from the Ethics Committee of São Francisco University, Bragança Paulista, SP, Brazil (Protocol CEA/USF 00.02.11). The animal protocol was designed to minimize pain or discomfort to the animals.
The animals were individually housed and acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. After random selection, 6-wk-old mice were introduced to control AIN-93 or HFD ad libitum for 12 wk (Table 1). The mice received 100 mg/kg per day ip pentoxifylline (Sigma Aldrich, Co. - Saint Louis, Missouri, United States) diluted in 0.9% NaCl during weeks 10-12 before being sacrificed. Each experimental group used 5 animals.
| Control (AIN-93) | HFD | |||
| g/kg | kcal/kg | g/kg | kcal/kg | |
| Cornstarch (QSP) | 397.5 | 1590 | 1155 | 462 |
| Casein | 200 | 800 | 200 | 800 |
| Sucrose | 100 | 400 | 100 | 400 |
| Dextrinated starch | 132 | 528 | 132 | 528 |
| Soybean oil | 70 | 630 | 40 | 360 |
| Lard | - | - | 312 | 2808 |
| Cellulose | 50 | - | 50 | - |
| Mineral mix | 35 | - | 35 | - |
| Vitamin mix | 10 | - | 10 | - |
| L-cystine | 3 | - | 3 | - |
| Choline | 2.5 | - | 2.5 | - |
| Total | 1000 | 3948 | 1000 | 5358 |
Twenty-four hours before the end of the protocol, mice were fasted for 6 h, and blood samples were collected from the tails. Glucose was measured using the glucose oxidase method. Insulin (1.5 U/kg) was administered by intraperitoneal injection, and blood samples were collected for serum glucose determination at 0, 10, 15, 20 and 30 min. The rate constant for glucose disappearance during an insulin tolerance test (kITT) was calculated using the formula 0.693/t1/2. The glucose t1/2 was calculated from the slope of the least-square analysis of the plasma glucose concentrations during the linear decay phase.
At the end of protocol, mice were fasted for 12 h and euthanized by xylasine/ketamine overdose (0.1 mL/30 g body weight of 1:1 v/v of 2% xylasine and 10% ketamine), and blood samples were collected in tubes by portal vein or cardiac puncture. Liver was perfused with 15 mL phosphate buffered saline (PBS), collected and weighed. Samples were immediately processed or stored at -80 °C for further analysis.
Aspartate aminotransferase (AST) and ALT serum levels were determined using a commercial kit (LABORLAB, Sao Paulo, Brazil).
Liver and adipose tissue biopsies were homogenized in solubilization buffer containing 100 mmol/L Tris (pH = 7.6), 1% Triton X-100, 150 mmol/L NaCl, 0.1 mg aprotinin, 35 mg/mL PMSF, 10 mmol/L Na3VO4, 100 mmol/L NaF, 10 mmol/L Na4P2O7 and 4 mmol/L EDTA to extract total protein. Liver and adipose tissue extract supernatants were collected and used in ELISA kits (R and D Systems, Inc, Minneapolis, MN, United States) or Multiplex Assay kits (Millipore, Billerica, MA, United States), respectively, according to the manufacturer’s protocol.
Hydrated 4.0 mm sections of paraformaldehyde-fixed, paraffin-embedded liver specimens were stained with hematoxylin-eosin to evaluate liver histology. Additional sections were stained with Masson’s trichrome for fibrosis analysis. For each group, six to nine mouse livers were prepared and stained. An expert pathologist evaluated the stained samples in a blinded fashion. Steatosis, ballooning degeneration and inflammation were histopathologically determined. The percentage of steatotic cells (macrovesicular and microvesicular) was determined and graded as follows: (1) 0: absent; (2) 1: < 25%; (3) 2: 26%-50%; (4) 3: 51%-75%; or (5) 4: > 75% of the parenchyma. Hyperemia, inflammation and fibrosis were evaluated as either present or absent.
Liver tissues were homogenized with in chloroform and methanol (2:1 v/v) and an aqueous solution of NaCl was added[13]. The chloroform layer was dried under N2, the total extract ressupended in PBS and triglycerides were determined using commercial enzymatic kit (LaborClin, Pinhais, PR, Brazil).
Data are expressed as the mean ± SEM. Comparisons among groups of data were made using a one-way ANOVA test followed by the Dunnett multiple comparisons test. Non-parametric data (scores) are expressed as the median (range) and were analyzed using the Mann-Whitney test. An associated probability (P value) of 5% was considered statistically significant.
Animals on a HFD for 12 wk presented significant alterations in body weight. However, pentoxifylline-treated mice showed no change in body weight compared with the matched controls. Pentoxifylline treatment decreased liver weight in obese mice, but the depot of visceral adipose tissue significantly increased. We evaluated blood glucose levels and insulin tolerance at the end of the treatment and did not find any differences between treated animals and untreated animals. AST levels decreased after pentoxifylline treatment, but ALT levels did not change (Table 2).
| Control | HFD | |||
| NT | PTX | NT | PTX | |
| Final BW (g) | 34.2 ± 1.0 | 37.0 ± 2.0 | 51.6 ± 1.5a | 52.0 ± 2.7 |
| Liver (g) | 1.7 ± 0.2 | 1.7 ± 0.0 | 2.6 ± 0.2a | 1.7 ± 0.0c |
| Liver (% of BW) | 4.1 ± 0.0 | 4.6 ± 0.1c | 4.8 ± 0.2a | 3.3 ± 0.0c |
| Visceral adipose tissue (g) | 0.8 ± 0.1 | 0.9 ± 0.2 | 1.8 ± 0.2a | 2.7 ± 0.1c |
| Visceral adipose tissue (% of BW) | 2.2 ± 0.2 | 2.4 ± 0.4 | 3.4 ± 0.3a | 5.4 ± 0.4c |
| Blood glucose (mg/dL) | 120.6 ± 6.8 | 131.0 ± 3.0 | 178.6 ± 10a | 141.0 ± 16 |
| kITT | 4.0 ± 0.6 | 3.6 ± 0.3 | 2.1 ± 0.1a | 1.6 ± 0.3 |
| Insulin (ng/mL) | 0.4 ± 0.1 | 0.4 ± 0.2 | 4.4 ± 2.0a | 4.5 ± 1.2 |
| AST (U/L) | 19.4 ± 4.6 | 9.4 ± 2.3a | 23.2 ± 6.9 | 12.1 ± 1.6c |
| ALT (U/L) | 3.12 ± 0.9 | 9.0 ± 2.0a | 2.25 ± 0.9 | 4.2 ± 1.5 |
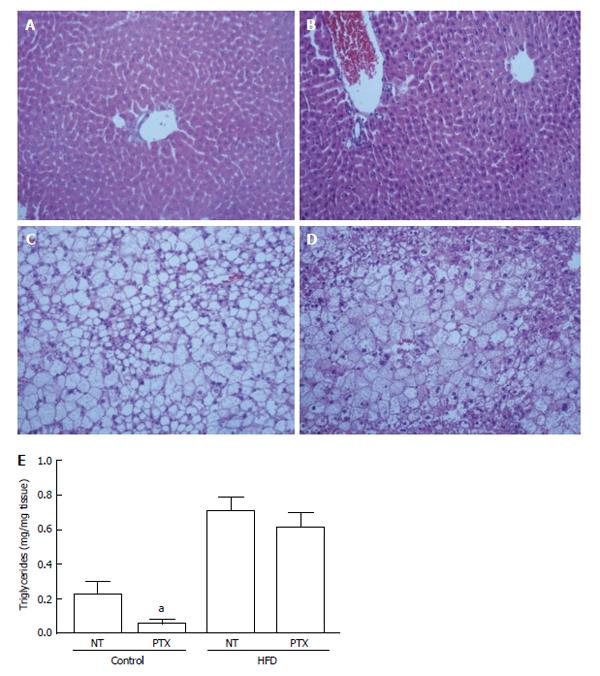
The livers from HFD mice presented pronounced macrosteatosis, microsteatosis, hyperemia and inflammation, features that were not observed in lean mice. We did not observe fibrosis in any of the groups. Pentoxifylline treatment did not alter the livers of lean mice, but it reduced inflammation, and we observed a trend to reduce microsteatosis and hyperemia in HFD mice (Figure 1 and Table 3). However, triglycerides measurement revealed a tendency to reduction in livers from lean mice but not from obese mice (Figure 1).
We evaluated TNF-α, interleukin (IL)-10 and monocyte chemoattractant protein (MCP)-1 protein levels in the livers of untreated obese mice or obese mice treated with pentoxifylline. A high-fat diet increased TNF-α and MCP-1 levels but did not affect IL-10 expression. Pentoxifylline treatment reduced TNF-α level but did not modify hepatic MCP-1 or IL-10 levels (Table 4). Adipose tissue analysis revealed that total plasminogen activator inhibitor (PAI)-1, MCP-1 and leptin levels increased in obese mice. Pentoxifylline treatment significantly decreased TNF-α and IL-6 levels in obese adipose tissue, but increased leptin and PAI-1 in lean adipose tissue (Table 5).
| Control | HFD | |||
| NT | PTX | NT | PTX | |
| TNF-α (pg/mL) | 87.2 ± 2.8 | 75.5 ± 3.9 | 106.1 ± 17.6 | 51.1 ± 9.6c |
| IL-6 (pg/mL) | 227.9 ± 23.3 | 178.5 ± 30.9 | 340.8 ± 51.3 | 166.6 ± 22.5c |
| PAI-1 (pg/mL) | 600.2 ± 32.3 | 1508.6 ± 210.4c | 2646.3 ± 755.1a | 4434.5 ± 1400.1 |
| MCP-1 (pg/mL) | 287.8 ± 17.1 | 253.8 ± 13.3 | 721.5 ± 112.8a | 563.8 ± 109.7 |
| Leptin (ng/mL) | 8.1 ± 0.7 | 23.1 ± 2.9c | 26.6 ± 3.0a | 20.5 ± 3.7 |
| Adiponectin (ng/mL) | 109.1 ± 0.5 | 102.8 ± 0.6 | 106.8 ± 1.7 | 102.8 ± 5.9 |
NAFLD is currently considered a consequence of obesity, and its prevalence in obese subjects is very high. Sedentary life style and consumption of foods with high-fat and high-caloric content are the main contributing factors to obesity[14]. The reduction of body weight and lifestyle changes are the primary recommendations to control NAFDL, and pharmacological interventions aim to induce weight loss (e.g., orlistat and sibutramine), to improve the antioxidant response (e.g., vitamin E and C, ursodeoxycholic acid) or to ameliorate insulin resistance and glucose and lipid metabolism (e.g., metformin, thiazolidinediones)[15]. Pentoxifylline has been considered an alternative treatment to control NAFDL, as it has been recommended in alcoholic fatty liver disease by acting as an anti-inflammatory drug[16]. Pentoxifylline is a methylxanthine derivative that acts as a nonspecific phosphodiesterase inhibitor to promote an increase in cyclic AMP levels and inhibit TNF-α gene transcription[17,18].
A high-fat diet obesity model is suitable to study metabolic and liver disease associated with adipose tissue expansion and to study potential therapeutics to control obesity. Our results show that Swiss mice fed a HFD for 12 wk present increased body weight, increased adiposity, adipose tissue inflammation, insulin resistance, hyperglycemia, steatosis, inflammation and increased TNF-α and MCP-1 levels in the liver. Pentoxifylline treatment did not change the final body weight but did decrease the liver weight. Visceral (epididymal) adipose tissue increased after pentoxifylline treatment, which may explain why we did not observe a decrease in body weight. Pentoxifylline treatment did not improve glucose homeostasis, but some NAFLD features improved, such as hepatic steatosis, inflammation, TNF-α levels, and serum AST levels. Our results are consistent with a previous study, where Sprague-Dawley rats fed a HFD for 16 wk were treated with pentoxifylline for 4 wk (16 mg/kg per day). The results showed decreased AST levels but not ALT, and improvements in basal glucose but not HOMAIR index[19]. Additionally, in a previous study of Sprague-Dawley fed HFD for 10 wk and treated with pentoxifylline for 6 wk (50 mg/kg per day), hepatic steatosis and plasma levels of TNF-α were reduced[20]. In our work, we evaluated both hepatic and adipose tissue TNF-α levels and found that pentoxifylline treatment reduced both. However, glucose homeostasis did not improve, but TNF-α and IL-6 levels decreased. A balance between leptin and adiponectin have been suggested to have a role in metabolic syndrome and type 2 diabetes[21]. Pentoxifylline treatment could not reverse the alterations in the obesity-induced leptin/adiponectin ratio.
Interestingly, we observed increased TNF-α in the liver and increased PAI-1 and leptin in adipose tissue of lean mice after pentoxifylline treatment. A systematic review of pentoxifylline data in patients with NAFLD revealed that AST and ALT plasma levels and liver histological scores were improved in several studies using pentoxifylline. However, pentoxifylline treatment did not inhibit plasma cytokines levels such as IL-6 in all studies[22]. Although pentoxifylline has been shown to inhibit TNF-α, Zein et al[9] reported that pentoxifylline treatment did not inhibit TNF-α plasma levels in NASH patients. The authors suggested that TNF-α plasma levels may not be related to hepatic levels of this cytokine because they observed histological improvement. Both the adipose tissue and liver of lean mice increased pro-inflammatory cytokine production in response to pentoxifylline treatment, an unexpected result that should be further studied. Interestingly, triglycerides levels presented a tendency to reduction in liver of lean mice. Several findings suggested that triglycerides per se are not toxic, on contrary; they protect liver from lipotoxicity by buffering the accumulation of fatty acids. Triglycerides synthesis inhibition improves steatosis but stimulates oxidizing systems that increase hepatic oxidative stress and liver damage[23]. Although, pentoxifylline is able to decrease oxidative stress and to inhibit lipid peroxidation in patients with NASH[24], we did not rule out the possibility that lean mice had an increase in oxidative response due pentoxifylline treatment. We hypothesize that metabolic status, liver metabolism, adiposity or inflammation degree can interfere with the pentoxifylline response, which could explain the controversial data obtained in different clinical studies of NAFLD patients. In this line of reasoning, pentoxifylline is effective in states of hyperinflammation because relevant anti-inflammatory effects can be achieved only in the presence of sufficient adenosine concentrations[25]. Metabolic stress, hypoxia and inflammation are conditions related to increase adenosine extracellular concentrations[26], and thus, could interfere with the pentoxifylline response.
In conclusion, our results showed that pentoxifylline was beneficial in an obesity-associated NAFLD model by improving liver inflammation and adipose tissue inflammation, but it was not able to improve obesity-induced metabolic disturbances. Unexpectedly, pentoxifylline treatment increased pro-inflammatory markers in the liver and adipose tissue of lean mice.
Non-alcoholic fatty liver disease (NAFLD) defines a spectrum of hepatic disorders including steatosis and non-alcoholic steatohepatitis that can progress to end-stage liver disease, such as fibrosis, cirrhosis and hepatic cancer. NAFLD is frequently associated with metabolic syndrome establishment and obesity. Several decades of pharmacological research have resulted in very few options for NAFLD management; therefore, new therapeutic approaches need to be researched.
Pentoxifylline is a non-selective phosphodiesterase inhibitor that has been reported to have antioxidant activity and decrease tumor necrosis factor (TNF)-α gene transcription. Pentoxifylline is prescribed to patients with severe alcoholic hepatitis, which suggest that this drug could also be beneficial to NAFLD. In this study, authors described pentoxifylline effects upon NAFLD using an experimental model of high-fat diet induced obesity in mice. Pentoxifylline was beneficial in obesity-associated NAFLD reducing liver microsteatosis and TNF-α, as well as, serum aspartate aminotransferase levels. However, pentoxifylline treatment in lean mice resulted in pro-inflammatory cytokine production in the liver and adipose tissue, suggesting that pentoxifylline effects could be dependent of additional conditions, as metabolic status, liver metabolism, adiposity or inflammation degree.
NAFLD has reached epidemic proportions nowadays. The current therapy is limited to suggestions of lifestyle changes and metabolic alterations control. Here, authors described beneficial pentoxifylline effects upon NAFLD using an experimental model of high-fat diet induced obesity in mice. Interestingly, the same treatment protocol in lean mice resulted in non-desirable effects upon inflammatory parameters in liver and adipose tissue.
By demonstrating that pentoxifylline has a protective role in liver from obese mice but not from lean mice, this study contributes to a better understanding of conflicting results provides by clinical studies using this therapeutic for NAFLD.
NAFLD defines a spectrum of hepatic disorders including steatosis, non-alcoholic steatohepatitis, liver fibrosis, cirrhosis and hepatic cancer. Pentoxifylline is a non-selective phosphodiesterase inhibitor prescribed to patients with severe alcoholic hepatitis, which suggest that this drug could also be beneficial to NAFLD.
In the current manuscript, Acedo et al reported that administration of pentoxifylline was able to reduce the fat accumulation in liver of obese mice fed by high-fat diet. This study is helpful to better understand the mechanism of pentoxifylline on NAFLD.
P- Reviewer: Jin B, Liaskou E S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Bertolotti M, Lonardo A, Mussi C, Baldelli E, Pellegrini E, Ballestri S, Romagnoli D, Loria P. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20:14185-14204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 232] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 2. | Baran B, Akyüz F. Non-alcoholic fatty liver disease: what has changed in the treatment since the beginning? World J Gastroenterol. 2014;20:14219-14229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 3. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2287] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 4. | Petta S, Amato MC, Di Marco V, Cammà C, Pizzolanti G, Barcellona MR, Cabibi D, Galluzzo A, Sinagra D, Giordano C. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2012;35:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 6. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 7. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16:39-46. [PubMed] |
| 9. | Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277-286. [PubMed] |
| 11. | Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Massart J, Robin MA, Noury F, Fautrel A, Lettéron P, Bado A, Eliat PA, Fromenty B. Pentoxifylline aggravates fatty liver in obese and diabetic ob/ob mice by increasing intestinal glucose absorption and activating hepatic lipogenesis. Br J Pharmacol. 2012;165:1361-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Folch J, Lees M, Sloane stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. [PubMed] |
| 14. | Basaranoglu M, Kayacetin S, Yilmaz N, Kayacetin E, Tarcin O, Sonsuz A. Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:2223-2226. [PubMed] |
| 15. | Schreuder TC, Verwer BJ, van Nieuwkerk CM, Mulder CJ. Nonalcoholic fatty liver disease: an overview of current insights in pathogenesis, diagnosis and treatment. World J Gastroenterol. 2008;14:2474-2486. [PubMed] |
| 16. | Park BJ, Lee YJ, Lee HR. Chronic liver inflammation: clinical implications beyond alcoholic liver disease. World J Gastroenterol. 2014;20:2168-2175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | LeMay LG, Vander AJ, Kluger MJ. The effects of pentoxifylline on lipopolysaccharide (LPS) fever, plasma interleukin 6 (IL 6), and tumor necrosis factor (TNF) in the rat. Cytokine. 1990;2:300-306. [PubMed] |
| 18. | Duman DG, Ozdemir F, Birben E, Keskin O, Ekşioğlu-Demiralp E, Celikel C, Kalayci O, Kalayci C. Effects of pentoxifylline on TNF-alpha production by peripheral blood mononuclear cells in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52:2520-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Wu J, Zhao MY, Zheng H, Zhang H, Jiang Y. Pentoxifylline alleviates high-fat diet-induced non-alcoholic steatohepatitis and early atherosclerosis in rats by inhibiting AGE and RAGE expression. Acta Pharmacol Sin. 2010;31:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Yalniz M, Bahçecioğlu IH, Kuzu N, Celebi S, Ataseven H, Ustündağ B, Ozercan IH, Sahin K. Amelioration of steatohepatitis with pentoxifylline in a novel nonalcoholic steatohepatitis model induced by high-fat diet. Dig Dis Sci. 2007;52:2380-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014;18:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 788] [Article Influence: 43.8] [Reference Citation Analysis (2)] |
| 24. | Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, Hazen SL, Feldstein AE. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Kreth S, Ledderose C, Luchting B, Weis F, Thiel M. Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock. 2010;34:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Gause WC, Leibovich SJ. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |