Published online Oct 28, 2015. doi: 10.4254/wjh.v7.i24.2543
Peer-review started: May 5, 2015
First decision: June 3, 2015
Revised: September 7, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: October 28, 2015
Processing time: 179 Days and 0.6 Hours
In the era of highly effective direct acting antiviral (DAA) drugs for the treatment of chronic hepatitis C (CHC) infection, where eradication is almost ensured with minimal side effects, all hepatitis C carriers should benefit theoretically. In the real world setting however, only a small proportion will benefit at this time point due to the multiple barriers to accessing therapy. Given that universal treatment is unlikely, treatment with DAAs will likely be restricted to those with the highest health benefits, and for those who can afford the high expense of a treatment course. Those with the highest unmet needs include those who have failed previous interferon-based therapy or who are interferon-ineligible with evidence of active disease, those with advance liver disease, and those with recurrence of hepatitis C after liver transplantation. In the future, the focus should be on increasing access to treatment for those infected with CHC.
Core tip: Chronic hepatitis C has become an easily curable disease with new direct acting antivirals (DAAs). However, due to multiple barriers to therapy, only those with highest unmet clinical needs including those with prior treatment failure, cirrhosis, and post-liver transplant, will likely receive therapy. DAAs have been shown to be highly efficacious in these groups.
- Citation: Fung J. Era of direct acting antivirals in chronic hepatitis C: Who will benefit? World J Hepatol 2015; 7(24): 2543-2550
- URL: https://www.wjgnet.com/1948-5182/full/v7/i24/2543.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i24.2543
An estimated 170 million people worldwide are chronically infected with the hepatitis C virus (HCV), affecting 2-3 percent of the world population, and constituting a major health burden globally[1,2]. For those with chronic hepatitis C (CHC), approximately 20% will progress to the development of liver cirrhosis[3]. This process usually takes several decades to occur although disease progression can be accelerated by the presence of co-existing liver disease[4], co-infection with other viruses such as human immunodeficiency virus[5-8], and also with alcohol intake[9-11]. Once cirrhosis is established, further complications may occur with liver decompensation and the development of hepatocellular carcinoma (HCC)[12]. In fact, the history of HCV is relatively short, with the discovery of the blood-borne virus in 1989 just over 25 years ago. Major routes of transmission include transfusion of unscreened contaminated blood products[13], and persons who inject drugs using contaminated drug paraphernalia[14].
In the early 1990s, standard interferon (IFN) alpha-2b was used to treat CHC infection. However, the curative rate with standard IFN monotherapy was dismal, with only approximately 15% achieving a sustained virological response (SVR)[15,16]. The addition of ribavirin enhanced the SVR to 33% and 41% for 24 and 48 wk of therapy respectively[17-19]. The subsequent introduction of pegylated IFN (Peg-IFN) in combination with ribavirin (RBV) improved the SVR rate to an estimated 50%[20,21]. The length of treatment ranged from 24-48 wk, and together with the SVR, was dependent on the genotype of the HCV[22]. Despite significant side effects, the modest SVR rate, the need for parenteral injections, and prolonged duration of therapy, Peg-IFN and RBV (PR) remained the standard of care for over a decade from the turn of the new millennium.
Over the last several years, there has been an exponential increase in therapeutic agents approved for CHC infection. These agents, collectively known as direct acting antivirals (DAAs), work by inhibiting specific stages of the HCV replication cycle. Classes of drugs include NS3/4A protease inhibitors, non-nucleoside polymerase inhibitors, NS5B nucleos(t)ide polymerase inhibitors, NS5A inhibitors, and cyclophilin inhibitors. In 2011, boceprevir and telaprevir were approved for the treatment of GT1 patients, improving the SVR to 68%-75% for treatment-naive patients[23,24], and 51%-59% in treatment-experienced patients[25,26]. Despite the modest improvement, these first generation DAAs still required the use of PR, resulting in a complicated treatment regimen and potential for significant side effects. In 2013, sofosbuvir and simeprevir were approved. The use of simeprevir or sofosbuvir with PR therapy for 12 wk achieved an SVR of approximately 80% and 90% respectively[27-29]. An all-oral combination of simeprevir and sofosbuvir achieved an overall SVR12 rate of 92%, proving evidence of the efficacy of an all-oral regimen[30]. In 2014, 3 all-oral combination regimens were approved, including sofosbuvir + simeprevir, sofosbuvir + ledipasvir, and ombitasvir + paritaprevir + ritonavir + dasabuvir. All 3 regimens were demonstrated to be highly effective, with SVR rates approaching 100%[31-35].
Within a space of a few years, the treatment of CHC has seen a dramatic shift in paradigm. CHC infection has emerged from a disease that has been difficult to cure with prolonged parenteral therapy to one that is easily curable with a short duration of oral antiviral therapy with minimal side effects.
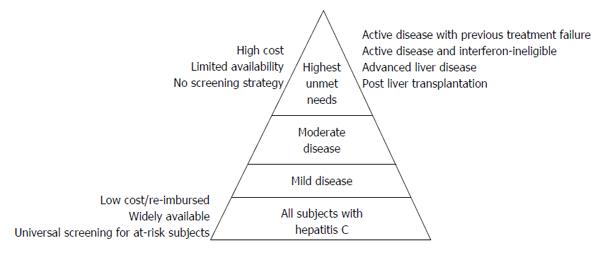
Given the high SVR rates achieved, and the favorable side effects profile of DAAs, it stands to reason that all CHC carriers should benefit from these newly approved all-oral combination DAA therapies. Moreover, with the high cure rates observed, for the first time, there is a glimpse of opportunity to eradicate HCV completely. In reality however, only a small fraction of CHC patients will likely receive DAAs at this time point, as shown in Figure 1. This is due to the fact that there are multiple barriers to HCV treatments that currently exist, precluding patients from receiving the best treatment available[36,37]. The barriers most inherent to DAAs include the availability of these new agents and the high cost associated with a treatment course[38]. Until these become widely available, and at an affordable cost, the benefits of DAAs will likely be restricted to those with the highest unmet needs. These include patients who have failed previous IFN-based therapies with evidence of disease progression, those who are ineligible for IFN therapy with progressive disease, those with established cirrhosis, those on the liver transplant waiting list, and those who have had a liver transplant (Figure 1).
For those who have failed previous IFN-based therapies, combination DAAs offers the best chance of achieving SVR. In the COSMOS trial of 80 HCV GT1 patients who were null responders treated with simeprevir and sofosbuvir +/- ribavirin for 24 wk, the SVR achieved was 90%[30]. A trial (the SAPPHIRE 2 study) of 297 HCV GT1 patients without cirrhosis who had failed PR therapy and treated with 12 wk of ritonavir-boosted paritaprevir + ombitasvir + dasabuvir + RBV resulted in an overall SVR rate of 96.3%[39]. In the ION-2 study, 440 HCV GT1 previously treated patients given sofosbuvir + ledipasvir +/- RBV I for 12-24 wk resulted in a SVR rate of ≥ 94%[31]. Despite previously failing to respond to IFN-based regimens, combinations DAAs have been demonstrated to be highly effective for this group of patients.
There may be many different reasons as to why patients may be ineligible for IFN-based treatment. These include those who are intolerant to IFN or have hypersensitivity to polyethylene glycol, have underlying autoimmune hepatitis or other severe autoimmune disorders[40,41], history of significant psychiatric disorder such as depression[42], and pre-existing cardiac disease[43]. Those patients with significant anemia, neutropenia, or thrombocytopenia may also be contra-indicated, as therapy with IFN can potentially compound the pre-existing cytopenic state[44-46]. Patients with established severe liver disease are also contra-indicated for IFN-based therapy, as there is a risk of decompensation and liver failure with the use of IFN[47].
Therefore, for those with evidence of active hepatitis and disease progression, and whom IFN is contra-indicated or tolerated poorly, the use of combination DAAs is the only therapeutic option in this setting.
As mentioned previously, those patients with established cirrhosis, especially with evidence of advance liver disease (Child Pugh B or C), are not eligible for IFN-based therapy as there is an increase risk of precipitating liver decompensation and failure[48]. Prior to the availability of all-oral combination of DAAs regimen, there was no specific antiviral therapy available for this group. Management of these patients with evidence of active hepatitis and disease progression was often frustrating for clinicians where the only outcome was either decompensation leading to death or liver transplantation. Ironically, this group of patient perhaps is the group that would need effective antiviral therapy the most.
Several studies have shown that DAAs are highly effective in patients with established cirrhosis. In the TURQUOISE II trial of 380 compensated cirrhotic patients infected with HCV genotype 1 using ritonavir-boosted paritaprevir + ombitasvir + dasabuvir + RBV, the SVR rate was noted to be 92% and 96% with 12 and 24 wk of treatment respectively[49]. A multicenter double blind study using ledipasvir + sofosbuvir for 24 wk and ledipasvir + sofosbuvir + RBV for 12 wk in compensated HCV genotype 1 patients, the SVR12 rates were 97% and 96% respectively[50]. In the SOLAR-2 study of 108 decompensated patients with HCV GT 1/4, treatment with ledipasvir + sofosbuvir + RBV for 12 and 24 wk were associated with a SVR rate of 87% and 89% respectively[51].
As hepatitis C recurrence is universal for those patients who are viremic at the time of liver transplantation, there is enormous impetus to eradicate or treat HCV prior to transplantation[52-54]. The indications for liver transplantation for CHC patients include those with decompensated cirrhosis and those with HCC[55]. For those with decompensated cirrhosis, as discussed in the previous section, treatments using DAAs has been shown to be effective. There are several rationales for treating wait-listed patients. Firstly, there is a theoretical opportunity that for those that achieve SVR, liver synthetic function may be restored sufficiently to the point whereby liver transplantation is no longer required (similar to that observed for patients with chronic hepatitis B)[56,57]. Secondly, achieving SVR or complete viral suppression will prevent or reduce the rate of disease progression and further decompensation[58,59]. Thirdly, by achieving viral suppression or SVR prior to liver transplantation, the recurrence rate after liver transplantation will be significantly improved, and even prevented for those who achieve SVR prior to their liver transplant[60]. In a phase II open-label study of 61 patients with HCV of any genotype and compensated cirrhosis with HCC were treated up to 48 wk of sofosbuvir + RBV before liver transplantation. Of these, 43 had undetectable HCV RNA at the time of transplantation, of which 30 (70%) had virological response at 12 wk post transplant. The recurrence was related inversely to the number of consecutive days of undetectable HCV RNA before transplantation[61]. There has also been reported case of decompensated patient improving to the point of being delisted after treatment with sofosbuvir and ribavirin for 24 wk[62].
For patients with recurrent hepatitis C after liver transplantation, the disease progression is accelerated, with approximately 20% developing graft cirrhosis by 5 years[63-66]. The use of long-term immunosuppression or large doses of pulse steroid during episodes of acute cellular rejection may increase the severity of graft hepatitis[63]. Treatment with combination Peg-IFN and RBV is often poorly tolerated after transplantation, and has been associated with poor SVR rates of approximately 30%[67]. For those who do not tolerate therapy with evidence of active hepatitis and disease progression, those who do not respond to therapy with evidence of disease progression, and those with severe fibrosing cholestatic hepatitis, the rate of graft failure is high, leading to graft decompensation and death or retransplantation[68,69].
Treatment with DAAs has been shown to improve significantly the SVR rates in post transplant patients. The use of early DAAs including telaprevir and boceprevir in combination with Peg-IFN and RBV improved the SVR rate to 41%-51%[70]. Not surprisingly, there were significant side effects observed with these regimens. Furthermore, significant drug interactions occur between telaprevir/boceprevir with the immunosuppressive therapy[71,72]. All-oral combination DAAs have recently been shown to be highly effective in post transplant patients. In the CORAL-I trial of ritonavir-boosted paritaprevir + ombitasvir + dasabuvir with RBV, the SVR12 rate was 97% after 24 wk of therapy[73]. Significant dose reduction of immunosuppression must also be undertaken when using this combination because of significant drug interactions. In another study using sofosbuvir and RBV after liver transplantation for 24 wk, the SVR12 rate was 70%[74]. In the SOLAR-1 trial using ledipasvir + sofosbuvir + RBV for 12 or 24 wk, the SVR rates was 96% and 98% respectively for non-cirrhotic patients, and 96% for those with compensated cirrhosis[75].
One key aspect of using DAAs after transplantation is that treatment should be given early at the time of hepatitis recurrence[76]. Despite the excellent virological response, the clinical benefit of DAAs when administered late at the time of advanced disease with graft decompensation may be negated. The SVR rates have been shown to be lower for these patients, and despite complete viral suppression, patients may still succumb due to the poor general condition with high susceptibility to infections[51,74].
With SVR rates approaching 100% with all-oral combination DAAs, there is no doubt that therapy is highly effective. Due to the high replicative rate of the HCV, and coupled with a lack of proofreading mechanism of the RNA-polymerase enzyme, a large number of variants are produced[77,78]. Treatment with DAAs may select out those pre-existing resistance-associated variants with lowered susceptibility to the drugs, resulting in potential treatment failure[79-81]. Although treatment failure rates are low, as evident by the consistently high SVR rates achieved with combination DAAs, the development of resistance may potentially be a significant problem in the future as increasing number of patients are being treated.
Due to the high cost of DAAs, questions are raised regarding the cost-effectiveness of these new regimens. There are now several studies demonstrating that the new DAAs are cost-effective across a wide range of patients, including those with early disease[82-84]. However, in the real world, with millions of eligible patients for antiviral therapy, the cost of therapy would be prohibitive. The previous sections have identified subsets of chronic HCV carriers with perhaps the highest unmet needs currently, and with the highest health benefit to cost ratio. For this group of patients, the cost of managing decompensated liver cirrhosis or liver transplantation far exceeds the cost of a course of DAAs.
The introduction of DAAs over the last several years has revolutionized the treatment landscape for CHC infection. Currently, SVR can be achieved in almost all patients treated with combination all-oral regimens with minimal side effects. Therefore, these newly approved DAAs will certainly improve almost every aspect of patient outcome, but only for those who have access to treatment. Although the high costs remain a major hurdle for many, other barriers exist. These barriers include the lack of an effective screening strategy to identify all chronic HCV carriers, medical eligibility for therapy, stigmatization of HCV carriers, and also the attribution of the patient and health care provider. The possibility of treatment being available to all CHC subjects with complete eradication of HCV remains a distant prospect, and can only be achieved with universal screening of at-risk subjects, and making treatment widely available and affordable to all (Figure 1). In the current review, those infected the CHC with the highest unmet needs are discussed. These are patients for whom treatment is likely to incur the highest health benefit. As treatment becomes increasingly affordable and more widely available, more patient groups will stand to benefit. In the era of highly effective DAAs where treatment efficacy is no longer an issue, the focus should now be on increasing access and removing barriers to therapy.
P- Reviewer: Mihaila RG, Waisberg J, Wang Y, Zhu F S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1145] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 2. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 3. | Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383-398, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 267] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 710] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 6. | Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis. 2001;183:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Soto B, Sánchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, Rey C, Abad MA, Rodríguez M, Sales Gilabert M. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 466] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 9. | Corrao G, Aricò S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998;27:1730-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Degott C, Valla D, Erlinger S, Rueff B. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 231] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 957] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 13. | Aach RD, Stevens CE, Hollinger FB, Mosley JW, Peterson DA, Taylor PE, Johnson RG, Barbosa LH, Nemo GJ. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991;325:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 352] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Thorpe LE, Ouellet LJ, Hershow R, Bailey SL, Williams IT, Williamson J, Monterroso ER, Garfein RS. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, Goodman Z, Banks SM, Hoofnagle JH. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 863] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 16. | Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Perrillo RP, Carey W, Jacobson IM, Payne J, Dienstag JL. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1213] [Cited by in RCA: 1130] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 17. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1640] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 18. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2435] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 19. | Reichard O, Norkrans G, Frydén A, Braconier JH, Sönnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998;351:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 443] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 21. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 22. | Fung J, Lai CL, Yuen MF. Treatment of Chronic Hepatitis C with Different Genotypes. New York: Springer 2008; 130-147. |
| 23. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 24. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 25. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 26. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [PubMed] |
| 27. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 28. | Manns M, Marcellin P, Poordad F, Stanislau Affonso de Araujo E, Buti M, Horsmans Y, Janczewska EJ, Villamil F, Peeters M, Lenz O. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype-1 infection in treatment-naive patients: results from QUEST-2, a phase III trial. J Hepatol. 2013;58:S568. [DOI] [Full Text] |
| 29. | Jacobson I, Dore GJ, Foster GR, Fried MW, Radu M, Rafalskiy VV, Moroz M, Craxi A, Peeters M, Lenz O. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype-1 infection in treatment-naive patients: results from QUEST-1, a phase III trial. J Hepatol. 2013;58:S574. |
| 30. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 31. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 32. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1365] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 33. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 928] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 34. | Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359-365.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 35. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 548] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 36. | Searson G, Engelson ES, Carriero D, Kotler DP. Treatment of chronic hepatitis C virus infection in the United States: some remaining obstacles. Liver Int. 2014;34:668-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | McGowan CE, Monis A, Bacon BR, Mallolas J, Goncales FL, Goulis I, Poordad F, Afdhal N, Zeuzem S, Piratvisuth T. A global view of hepatitis C: physician knowledge, opinions, and perceived barriers to care. Hepatology. 2013;57:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Reau NS, Jensen DM. Sticker shock and the price of new therapies for hepatitis C: is it worth it? Hepatology. 2014;59:1246-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourlière M, Sulkowski MS, Wedemeyer H, Tam E, Desmond P. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 445] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 40. | García-Buey L, García-Monzón C, Rodriguez S, Borque MJ, García-Sánchez A, Iglesias R, DeCastro M, Mateos FG, Vicario JL, Balas A. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology. 1995;108:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Lörke J, Erhardt A, Häussinger D. Induction of autoimmune hepatitis by pegylated interferon alfa-2b in chronic hepatitis C. Clin Gastroenterol Hepatol. 2004;2:xx. [PubMed] |
| 42. | Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Sonnenblick M, Rosin A. Cardiotoxicity of interferon. A review of 44 cases. Chest. 1991;99:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Soza A, Everhart JE, Ghany MG, Doo E, Heller T, Promrat K, Park Y, Liang TJ, Hoofnagle JH. Neutropenia during combination therapy of interferon alfa and ribavirin for chronic hepatitis C. Hepatology. 2002;36:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Peck-Radosavljevic M, Wichlas M, Pidlich J, Sims P, Meng G, Zacherl J, Garg S, Datz C, Gangl A, Ferenci P. Blunted thrombopoietin response to interferon alfa-induced thrombocytopenia during treatment for hepatitis C. Hepatology. 1998;28:1424-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Wang Q, Miyakawa Y, Fox N, Kaushansky K. Interferon-alpha directly represses megakaryopoiesis by inhibiting thrombopoietin-induced signaling through induction of SOCS-1. Blood. 2000;96:2093-2099. [PubMed] |
| 47. | Everson GT. Treatment of hepatitis C in patients who have decompensated cirrhosis. Clin Liver Dis. 2005;9:473-486, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 49. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 50. | Bourlière M, Bronowicki JP, de Ledinghen V, Hézode C, Zoulim F, Mathurin P, Tran A, Larrey DG, Ratziu V, Alric L. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis. 2015;15:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 51. | Flamm SL, Everson GT, Charlton M, Denning JM, Arterburn S, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal NH. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: preliminary results of a propsective, multicenter study. Hepatology. 2014;60:320A. |
| 52. | Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, Combs C, Fennessy S, Roberts JP, Ascher NL. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317-322. [PubMed] |
| 53. | Rosen HR. Hepatitis C virus in the human liver transplantation model. Clin Liver Dis. 2003;7:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 381] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 55. | Adam R, McMaster P, O’Grady JG, Castaing D, Klempnauer JL, Jamieson N, Neuhaus P, Lerut J, Salizzoni M, Pollard S. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 56. | Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Kim WR, Terrault NA, Pedersen RA, Therneau TM, Edwards E, Hindman AA, Brosgart CL. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137:1680-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, de Franchis R, Almasio PL, Maisonneuve P. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | D’Ambrosio R, Aghemo A, Rumi MG, Primignani M, Dell’Era A, Lampertico P, Donato MF, De Nicola S, Prati GM, de Franchis R. The course of esophageal varices in patients with hepatitis C cirrhosis responding to interferon/ribavirin therapy. Antivir Ther. 2011;16:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Forns X, García-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, García-Valdecasas JC, Navasa M, Rimola A, Rodés J. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 61. | Curry MP, Forns X, Chung RT, Terrault NA, Brown R, Fenkel JM, Gordon F, O’Leary J, Kuo A, Schiano T. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100-107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 62. | Ruiz I, Feray C, Pawlotsky JM, Hézode C. Patient with decompensated hepatitis C virus-related cirrhosis delisted for liver transplantation after successful sofosbuvir-based treatment. Liver Transpl. 2015;21:408-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, Lau JY, Williams R. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996;110:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 352] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 64. | Féray C, Caccamo L, Alexander GJ, Ducot B, Gugenheim J, Casanovas T, Loinaz C, Gigou M, Burra P, Barkholt L. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. European Concerted Action on Viral Hepatitis (EUROHEP) Group. Gastroenterology. 1999;117:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 268] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14 Suppl 2:S36-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 66. | Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, Córdoba J, Herola A, Ascher N, Mir J. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000;32:673-684. [PubMed] |
| 67. | Gane EJ, Agarwal K. Directly acting antivirals (DAAs) for the treatment of chronic hepatitis C virus infection in liver transplant patients: “a flood of opportunity”. Am J Transplant. 2014;14:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Satapathy SK, Sclair S, Fiel MI, Del Rio Martin J, Schiano T. Clinical characterization of patients developing histologically-proven fibrosing cholestatic hepatitis C post-liver transplantation. Hepatol Res. 2011;41:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Narang TK, Ahrens W, Russo MW. Post-liver transplant cholestatic hepatitis C: a systematic review of clinical and pathological findings and application of consensus criteria. Liver Transpl. 2010;16:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Coilly A, Dumortier J, Botta-Fridlund D, Latournerie M, Leroy V, Pageaux G-P, Giostra EG, Moreno C, Roche B, Lebray P. Sustained virologic response after protease inhibitor-based therapy for hepatitis C recurrence after liver transplantation: a multicentric European experience. Hepatology. 2013;58:316A. |
| 71. | Hulskotte E, Gupta S, Xuan F, van Zutven M, O’Mara E, Feng HP, Wagner J, Butterton J. Pharmacokinetic interaction between the hepatitis C virus protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteers. Hepatology. 2012;56:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 72. | Werner CR, Egetemeyr DP, Lauer UM, Nadalin S, Königsrainer A, Malek NP, Berg CP. Telaprevir-based triple therapy in liver transplant patients with hepatitis C virus: a 12-week pilot study providing safety and efficacy data. Liver Transpl. 2012;18:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, Gordon F, Levitsky J, Terrault NA, Burton JR. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 74. | Charlton M, Gane E, Manns MP, Brown RS, Curry MP, Kwo PY, Fontana RJ, Gilroy R, Teperman L, Muir AJ. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 75. | Reddy KR, Everson GT, Flamm SL, Denning JM, Arterburn S, Brandt-Sarif T, Pang PS, McHutchison JG, Curry MP, Charlton M. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with post transplant recurrence: preliminary results of a prospective, multicenter study. Hepatology. 2014;60:200A-201A. |
| 76. | Pellicelli AM, Montalbano M, Lionetti R, Durand C, Ferenci P, D’Offizi G, Knop V, Telese A, Lenci I, Andreoli A. Sofosbuvir plus daclatasvir for post-transplant recurrent hepatitis C: potent antiviral activity but no clinical benefit if treatment is given late. Dig Liver Dis. 2014;46:923-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 77. | Herrmann E, Neumann AU, Schmidt JM, Zeuzem S. Hepatitis C virus kinetics. Antivir Ther. 2000;5:85-90. [PubMed] |
| 78. | Martell M, Esteban JI, Quer J, Genescà J, Weiner A, Esteban R, Guardia J, Gómez J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225-3229. [PubMed] |
| 79. | Paolucci S, Fiorina L, Mariani B, Gulminetti R, Novati S, Barbarini G, Bruno R, Baldanti F. Naturally occurring resistance mutations to inhibitors of HCV NS5A region and NS5B polymerase in DAA treatment-naïve patients. Virol J. 2013;10:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 80. | Krishnan P, Tripathi R, Schnell G, Reisch T, Beyer J, Irvin M, Xie W, Larsen L, Cohen D, Podsadecki T. Resistance Analysis of Baseline and Treatment-Emergent Variants in Hepatitis C Virus Genotype 1 in the AVIATOR Study with Paritaprevir-Ritonavir, Ombitasvir, and Dasabuvir. Antimicrob Agents Chemother. 2015;59:5445-5454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 81. | McCormick AL, Wang L, Garcia-Diaz A, Macartney MJ, Webster DP, Haque T. Prevalence of baseline polymorphisms for potential resistance to NS5A inhibitors in drug-naive individuals infected with hepatitis C genotypes 1-4. Antivir Ther. 2015;20:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis. 2015;61:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 83. | Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, Avorn J, Choudhry NK. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 84. | Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61:1860-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |