Published online Oct 18, 2015. doi: 10.4254/wjh.v7.i23.2474
Peer-review started: December 2, 2014
First decision: January 12, 2015
Revised: August 24, 2015
Accepted: September 10, 2015
Article in press: September 16, 2015
Published online: October 18, 2015
Processing time: 321 Days and 22.1 Hours
The prevalence of hepatocellular carcinoma (HCC) has progressively increased in recent years and is now the fifth and the second most common cancer in the World and in Egypt, respectively. Much work has focused in the development of assays for detecting hepatic carcinogensis before the observance of hepatic focal lesions. Particular attention has been directed towards HCC-specific biomarkers for use in the early diagnosis of HCC and in the confirmation of radiological studies. Although a number of biomarkers have been identified, none have been considered reliable indicators of early HCC lesions. This review presents a few of the most relevant HCC biomarkers and suggests improvements to the accuracy of diagnostic assays through their combined use. Furthermore, we present an algorithm for the biomarker-based diagnosis of HCC and highlight its important role in the early prediction of HCC.
Core tip: Alpha-fetoprotein (AFP) has been widely used as a reference biomarker to validate the diagnosis of hepatocellular carcinoma (HCC). However, normal physiological-levels of AFP are observed in approximately one third of HCC cases. Furthermore, a number of HCC positive patients have AFP levels less than the threshold value of 400 ng/mL. These factors make an AFP-based diagnosis of HCC far from reliable. However, high diagnostic accuracy indices have been reported when AFP is combined with other biomarkers such as midkine, golgi protein 73, des-γ-carboxyprothrombin, glypican-3, and gamma-glutamyl transferase.
- Citation: Khattab M, Fouad M, Ahmed E. Role of biomarkers in the prediction and diagnosis of hepatocellular carcinoma. World J Hepatol 2015; 7(23): 2474-2481
- URL: https://www.wjgnet.com/1948-5182/full/v7/i23/2474.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i23.2474
Hepatocellular carcinoma (HCC) has an annual incidence of 7.9% in men and 6.5% in women (fifth and seventh worldwide respectively)[1]. In regions with a high prevalence of hepatitis C virus (HCV) and hepatitis B virus (HBV) infections, the incidence and prevalence of HCC has progressively increased[2]. In Egypt, due to the high prevalence of HCV and HBV infections, the incidence rate of HCC has doubled in the past ten years[3].
Important environmental risk factors for HCC include chronic hepatitis virus infections, alcohol abuse, and non-alcoholic steatohepatitis (NASH). These risk factors are also relevant aetiologic factors for cirrhosis[4]. In Eastern Asia and sub-Saharan Africa the main risk factors for HCC are chronic hepatitis B infection and exposure to aflatoxin B1. In North America, Europe, and Japan, the main risk factors are chronic hepatitis C (CHC) infection and alcohol consumption[5]. HCV increases HCC risk by promoting cirrhosis and causing specific genetic lesions to the infected liver cells[6]. Clinically relevant hepatitis viral infections has been shown to predispose individuals to HCC, similarly, occult hepatitis B infection has been associated with the development of HCC[7].
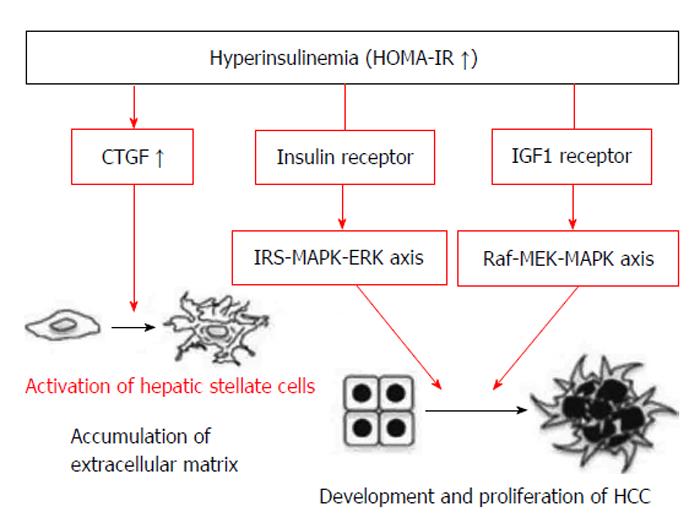
Higher viral loads with prolonged infections have been correlated with the occurrence of HCC and may be due to accumulated risks from chronic oncogenic damage[8]. Daily alcohol consumption and Aflatoxin food contamination have been associated with increased risks of HCC[9,10]. Patients with CHC, increased insulin resistance and high serum adiponectin levels are more likely to develop liver cancer[11]. Metabolic syndrome, a combination of phenomena, including obesity, dyslipidaemia, insulin resistance and type 2 diabetes mellitus (DM), is a known potential risk factor for HCC. Due to obesity or other causes, NASH has been associated with increased risks of HCC[12,13]. DM has been associated with a two to three fold increased risk of HCC. Additionally, DM has been shown to affect the prognosis of HCC after curative therapies[14]. Furthermore, DM type 2 can lead to HCC caused by carcinogenic effects on the liver and other tissues from insulin-like growth factor-1 (IGF-1) due to hyperinsulinaemia and insulin-resistance (Figure 1)[15,16]. The risk for HCC was particularly higher in diabetic patients treated with insulin[17]. However, Yamamoto et al[18], 2012, reported a case in which a dramatic regression of HCC was observed after four weeks of treatment with a dipeptidyl peptidase-4 enzyme (DPP-4) inhibitor in a patient with HCV-related chronic hepatitis. CD8+ T-cells were shown to accumulate around the HCC tissue, indicating that a DPP-4 inhibitor may safely exert beneficial effects on HCV-related HCC through immunity modulation[19]. A 1.7-fold increase in the incidence rates of HCC was reported for individuals with hereditary haemochromatosis confirming preliminary observations in smaller studies in related populations[20]. A correlation was also observed for alcoholics presenting with liver iron overload for increased risks of HCC and C282Y mutation in haemochromatosis[21].
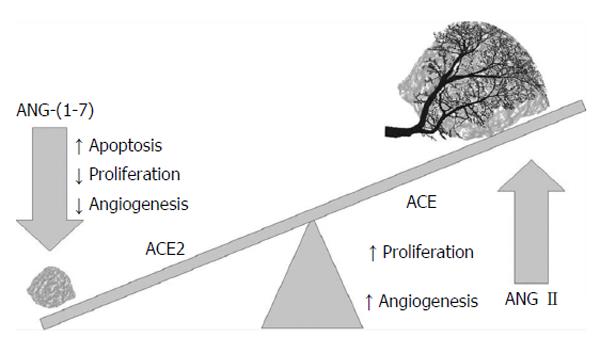
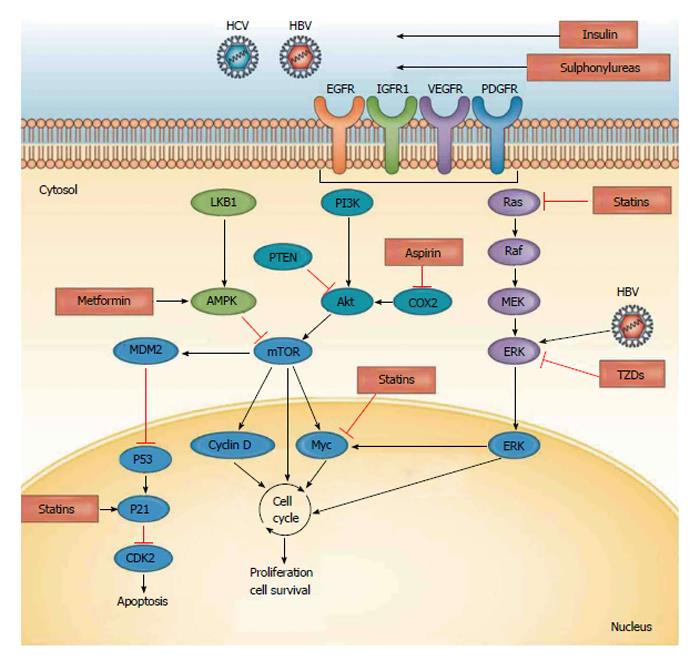
The pathogenesis of HCC remains undetermined. Evidence exists supporting the notion that DNA damage occurs, resulting in the dysregulation of DNA methylation, chromosomal instability, proto-oncogene activation, and tumour suppressor gene inactivation. The renin angiotensin system signalling pathways have been observed to activate, which leads to cell proliferation (Figure 2)[22,23]. Major risk factors for HCC typically lead to liver cirrhosis and the accumulation of genetic and epigenetic changes, such as the activation of oncogenes and the inactivation of tumour suppressor genes. The signalling pathways (e.g., Raf/MEK/ERK, PI3K/AKT, and Wn/β-catenin pathways) are activated through various growth factors. Growth factor receptor signalling typically results in abnormal hepatocyte proliferation and subsequently, tumour angiogenesis (Figure 3)[24,25]. Furthermore, multiple mutations at the chromosomales, genetics and epigenetics levels have been implicated as pathogenetic mechanisms in the development of HCC[26,27]. MicroRNA has been shown to aid in the transcription of HCC oncogenes and (Wnt) signaling, and it plays important roles in the development of HCC through the activation of β catenin, the overexpression of Wnt receptors and the inactivation of E-cadherin[28].
There are three forms of alpha-fetoprotein (AFP) according to electrophoresis lectin-reactivity (AFP-L1, AFP-L2, and AFP-L3). A high percentage of AFP-L3 seems to differentiate HCC from chronic liver diseases and may be an indicator of HCC when the total serum AFP levels are ≥ 200 ng/mL[29].
AFP has been traditionally used as a reference biomarker to screen and support the diagnoses of HCC. However, approximately one-third of HCC patients have normal, physiological-levels of AFP. AFP levels > 200 ng/mL are specific for HCC, and levels > 500 ng/mL correlate with tumour size. AFP-L3 levels above 10% to 15% threshold level (percentage of AFP-L3 over AFP) have been detected in approximately one-third of HCC patients[30]. The use of AFP levels to diagnose HCC patients relies on a threshold value of 200 ng/mL, a sensitivity of 0.310, a specificity of 0.960 and an area under the curve (AUC) of 0.835[31,32].
Des-γ-carboxyprothrombin (DCP) is produced in HCC cell lines, and it is found at significantly higher concentrations than normal in 50% to 60% of all HCC patients and in 15% to 30% of early HCC cases. DCP can be used together with AFP-L3 to diagnose HCC[33]. At a 125 mAU/mL threshold, DCP has high sensitivity (89%), specificity (95%) and AUC (0.797)[34,35] in the prediction of HCC.
AT a 654 ng/mL threshold level, midkine (MDK) serum has higher sensitivity (86.9% vs 51.9%), specificity (86.3% vs 83.9%) and AUC (0.915 vs 0.754) compared with AFP. Therefore, MDK can be used in the diagnosis of AFP-negative HCCs and very early-stages of HCCs. Furthermore, MDK can be used in HCC patients after curative resections to diagnose tumour recurrence[36].
Dickkopf-1 (DKK-1) can be used together with AFP for the diagnosis of HCC and especially for HCC cases with low levels of AFP. DKK-1 can distinguish HCC from non-malignant chronic liver diseases and has sensitivity of 69.1% and a specificity of 90.6%[37].
Golgi protein 73 (GP73) serum levels increase in patients with liver disease and HCC[38]. At a threshold value of RU 10 units, GP73 sensitivity, specificity and AUC are 69%, 75% and 0.914 respectively[39].
The combination of GP73 and AFP (with a 35 ng/mL threshold for AFP and an 8.5 RU threshold for GP73) increased the HCC diagnostic sensitivity to 89.2%, specificity to 85.2%, with an AUC of 0.914[40].
Glypican-3 (GLP-3) is a 60 kDa cell surface-linked heparin sulfate proteoglycan and is not expressed in adult livers[41]. GLP-3 serum levels are higher in HCC patients than in patients with HCV-induced cirrhosis. Furthermore, GLP-3 is more sensitive than AFP for the detection of smaller HCC. The combined use of GLP-3 and AFP has been shown to provide higher sensitivity and specificity than the individual use of each marker[42].
In HCC and liver diseases, significant changes occur in gamma-glutamyl transferase (GGT) serum activity. GGT-II is a hepatoma-specific GGT and an early enzyme marker of precancerous and cancerous processes[43]. At threshold levels of 100 U/L and 100 IU/mL for GGT and AFP, respectively, GGT had a higher sensitivity and a lower specificity than AFP (69.5% vs 43.5%) and (41.9% vs 96.4%), respectively, for the diagnosis of HCC. When AFP and GGT were combined, the sensitivity was 56.5% and specificity was 69.5%[44].
Alpha-l-fucosidase (AFU) is a lysosomal enzyme. Its serum levels have been shown to increase in patients with cirrhosis and HCC[45]. At a threshold of 2.3005 μmol/L per minute, AFU yielded a sensitivity and specificity of 90% and 97.5%, respectively[46].
Transforming growth factor beta-1 (TGF-beta-1) is a cytokine with multiple biological functions. It has a role in cell growth and extracellular matrix formation[47]. With a threshold of 64.33 ng/mL, TGF-beta-1 has a sensitivity of 78.3% and a specificity of 29.5% for the diagnosis of HCC. The combined use of AFP and TGF-beta-1 altered the specificity and the sensitivity to 86.6% and 30.4%, respectively[48].
IGFsI and II are polypeptides that play important roles in hepatic carcinogenesis. The serum levels of IGFI are significantly lower in patients with HCC compared with patients without HCC[49]. At a threshold of 4.1 mg/g IGFI has a 63% sensitivity and a 90% specificity for diagnosings of HCC. The combination of IGFI and AFP increased the sensitivity to 80% and the specificity to 90%[50].
Squamous cell carcinoma antigen (SCCA) levels are persistently elevated in patients with HCC displaying normal, physiological-AFP levels. This property is useful in the early detection and follow-up diagnoses for patients treated for HCC[51]. At a threshold of 0.368 ng/mL, SCCA has an AUC of 0.705, a sensitivity of 84.2% and a specificity of 48.9%[52].
Osteopontin (OPN) is an integrin-binding glycophosphoprotein involved in many cellular functions, such as regulating the survival, migration, invasion, and metastasis of tumour cells[53]. When compared with cirrhosis, CHC, chronic hepatitis B or healthy controls, OPN plasma levels were significantly elevated in HCC patients[54]. At a serum of 557 ng/mL, OPN has sensitivity, specificity and AUC of 26%, 92.5% and 51%, respectively, for the diagnosis of HCC[55,56]. Furthermore, OPN was more valuable when combined AFP-L3[57].
Heat shock proteins (HSP) are stress-induced proteins and belong to the glucose-regulated proteins (GRPs) family. In neoplasms, the expression of HSP has been associated with apoptosis regulation and tumour immune response. The expressions of HSP27, HSP70, HSP90, GRP78, and GRP94 increased in a stepwise pattern as HCC developed from a dysplastic nodules to early HCC and, finally to advanced HCC[58]. In HBV-infected patients, the expressions of GRP78, GRP94, or HSP90 have been significantly correlated with vascular invasion and intrahepatic metastasis. HSP27 has been detected in 90% HCC patients sera and two HBV patients sera, but in none of normal sera[59]. The optimal diagnostic threshold for HSP27 was 456.5 pg/mL. This yielded a sensitivity of 70% and a specificity of 73%, with an AUC of 0.749[60].
Syndican: The serum level of syndecan-1 significantly increased in HCC patients when compared with cirrhotic and control groups. Syndecan-1 levels significantly increased with progressive stages of Barcelona-Clinic Liver Cancer Group[61]. The diagnostic value of the test was significantly increased when combined with AFP.
Interleukin-6: At a threshold of 7.9 pg/mL, interleukin-6 (IL-6) has a sensitivity of 0.83, a specificity of 0.83, and an AUC of 0.810[62].
Higher C-reactive protein and IL-6 levels correlated well with larger tumour sizes, poorer Child-Pugh functions, shorter survival times and more predictable outcomes in patients with HCC receiving loco-regional therapy[63].
In a study by Cabrera et al[64], 2012, sCD25 was found be elevated in HCC patients when compared with those with cirrhosis. At a threshold of 2180 pg/mL, sCD25 based diagnoses of HCC had a sensitivity of 92.3% and a specificity of 37.7%; for the early detection of HCC. sCD25 based diagnoses had a sensitivity of 89.6% and specificity of 39.3%[64].
Vascular endothelial growth factor (VEGF), especially VEGF-A, was found to be elevated in HCC, particularly in advanced tumour stages and metastasis[65]. High serum levels of VEGF indicate poor HCC prognosis[66].
In experimental studies, the sera and tissue levels of the alpha feto protein and glucoregulatory enzymes, HK, GAPDH and G6PD of hexose monophosphate shunt were significantly higher in HCC-bearing animals when compared with normal controls[67]. Glucoregulatory enzymes are promising targets as biomarkers for the prediction of HCC in humans (Table 1).
| Marker | Threshold | Sensitivity | Specificity | AUC | Ref. |
| AFP | 200 ng/mL | 0.310 | 0.960 | 0.835 | [31,32] |
| DCP | 7.5 ng/mL | 0.600 | 0.940 | 0.797 | [34,35] |
| GP73 | 10 RU | 0.69 | 0.75 | 0.914 | [39] |
| AFP-L3 | 10% | 0.410 | 0.990 | 0.710 | [30] |
| MDK | 654 ng/mL | 86.9% | 86.3% | 0.915 | [36] |
| AFU | 2.3005 μmol/L | 90% | 0.975 | [46] | |
| AFP + GP73 | 7.4 RU | 0.770 | 0.840 | 0.932 | [40] |
| GGT + AFP | 100 U/L + 100 IU/mL | 0.57 | 0.70 | - | [44] |
| SCCA | 0.368 ng/mL | 0.84 | 0.49 | 0.705 | [52] |
| HSP27 | 456.5 pg/mL | 0.70 | 0.73 | 0.649 | [70] |
| IL-6 | 7.9 pg/mL | 0.83 | 0.83 | 0.810 | [62] |
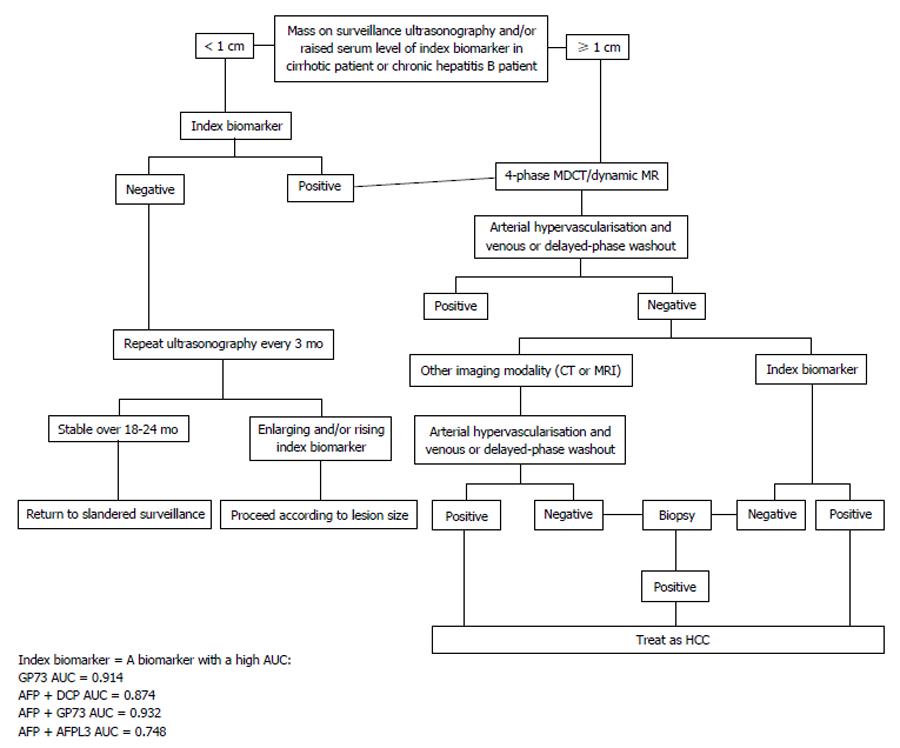
HCC nodules 1 cm or smaller are difficult to diagnose by imaging and require further tested. Nodules exceeding 1 cm can be diagnosed by imaging computed tomography (CT) or magnetic resonance imaging (MRI) with contrast. The uptake during the arterial phase and contrast washout during the venous or delayed phases provides diagnostic clues for HCC[68]. However, the conventional practices and methods for diagnosing HCC are cumbersome and invasive. Nodular lesions showing an atypical imaging patterns indicative of HCC on one of the dynamic scans (CT or MRI) are validated with the other dynamic scan (CT or MRI). Any liver nodules ≥ 2 cm showing atypical imaging pattern on both dynamic scans (CT and MRI) subsequently require histological confirmation[69]. However, the use of biomarkers with high HCC diagnoses accuracy indices can preclude the need for these invasive and hazardous liver biopsies (Figure 4).
Multiple biological markers are available to aid in the diagnosis of HCC. However, their individual use does not provide sufficient sensitivity and specificity. As presented in this review, the combined use of more than one biomarker may increase the predictive accuracy of HCC diagnoses in cirrhotic patients.
P- Reviewer: Chen JL, Passadakis PS, Romanelli RG, Wang B S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2506] [Article Influence: 192.8] [Reference Citation Analysis (2)] |
| 3. | El-Garem H, Hafez HA, Foaud A, Akel A, Eldien MA, EldienAtia M, Salah M, Khatteb H, Osman H, Ragab K. Tissue Biomarkers in the Early Detection of Hepatocellular Carcinoma among Egyptian Patients with Chronic Hepatitis C: A Possible Genetic Profile. Br J Med Med Res. 2013;3:1858-1870. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] |
| 5. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 6. | Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, Lee LP, Lin ZY, Hsieh MY, Wang LY. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat. 2014;21:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2363] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 9. | Schwartz LM, Persson EC, Weinstein SJ, Graubard BI, Freedman ND, Männistö S, Albanes D, McGlynn KA. Alcohol consumption, one-carbon metabolites, liver cancer and liver disease mortality. PLoS One. 2013;8:e78156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Magnussen A, Parsi MA. Aflatoxins, hepatocellular carcinoma and public health. World J Gastroenterol. 2013;19:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (4)] |
| 11. | Khattab MA, Eslam M, Mousa YI, Ela-adawy N, Fathy S, Shatat M, Abd-Aalhalim H, Kamal A, Sharawe MA. Association between metabolic abnormalities and hepatitis C-related hepatocellular carcinoma. Ann Hepatol. 2012;11:487-494. [PubMed] |
| 12. | Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, Chen CJ. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Thompson KJ, Lau KN, Johnson S, Martinie JB, Iannitti DA, McKillop IH, Sindram D. Leptin inhibits hepatocellular carcinoma proliferation via p38-MAPK-dependent signalling. HPB (Oxford). 2011;13:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Zhang H, Gao C, Fang L, Yao SK. Increased international normalized ratio level in hepatocellular carcinoma patients with diabetes mellitus. World J Gastroenterol. 2013;19:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Donadon V, Balbi M, Perciaccante A, Casarin P, Zanette . Insulin Resistance and Hyperinsulinemia in Patients with Chronic Liver Disease and Hepatocellular Carcinoma Clinical Medicine. Endocrinol Diabetes. 2009;2:25-33. |
| 16. | Eslam M, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, Romero-Gomez M. Use of HOMA-IR in hepatitis C. J Viral Hepat. 2011;18:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Schlesinger S, Aleksandrova K, Pischon T, Jenab M, Fedirko V, Trepo E, Overvad K, Roswall N, Tjønneland A, Boutron-Ruault MC. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. 2013;24:2449-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Yamamoto S, Tokuhara T, Nishikawa M, Nishizawa S, Nishioka T, Nozawa A, Takahashi A, Watanabe Y, Wada R, Wakasa K. Spontaneous regression of hepatocellular carcinoma after improving diabetes mellitus: possibly responsible for immune system. Kanzo. 2012;53:164-167. |
| 19. | Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. 2013;19:2298-2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 129] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 20. | Fracanzani AL, Fargion S, Stazi MA, Valenti L, Amoroso P, Cariani E, Sangiovanni A, Tommasini M, Rossini A, Bertelli C. Association between heterozygosity for HFE gene mutations and hepatitis viruses in hepatocellular carcinoma. Blood Cells Mol Dis. 2005;35:27-32. [PubMed] |
| 21. | Nahon P, Sutton A, Rufat P, Ziol M, Thabut G, Schischmanoff PO, Vidaud D, Charnaux N, Couvert P, Ganne-Carrie N. Liver iron, HFE gene mutations, and hepatocellular carcinoma occurrence in patients with cirrhosis. Gastroenterology. 2008;134:102-110. [PubMed] |
| 22. | Aucejo F, Hanouneh I, Carey WD. Hepatocellular Carcinoma Text-based CME cases. Disease Management Project Clinical Decisions. 2013; Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hematology-oncology/hepatocellular-carcinoma/Default.htm. |
| 23. | Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Levrero M, Pediconi N, vossio S, Schinzari V, Guerrieri F, Palescandolo E. Molecular pathogensis. Hepatocellular Carcinoma A Practical Approach. 2009;9-25. [DOI] [Full Text] |
| 25. | Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 26. | Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 27. | Toffanin S, Hoshida Y, Lachenmayer A, Villanueva A, Cabellos L, Minguez B, Savic R, Ward SC, Thung S, Chiang DY. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastroenterology. 2011;140:1618-1628.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Wei W, Cheng N, Wang K, Li B, Jiang X, Sun S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology. 2012;56:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 29. | Mayo Clinic. Test ID: L3AFP. Alpha-Fetoprotein (AFP) L3% and Total, Hepatocellular Carcinoma Tumor Marker, Serum. Available from: http://www.mayomedicallaboratories.com/test-catalog/Clinical and Interpretive/88878. |
| 30. | Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010;55:2744-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Hu B, Tian X, Sun J, Meng X. Evaluation of individual and combined applications of serum biomarkers for diagnosis of hepatocellular carcinoma: a meta-analysis. Int J Mol Sci. 2013;14:23559-23580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 515] [Article Influence: 21.5] [Reference Citation Analysis (4)] |
| 33. | Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 34. | Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 35. | Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Zhu WW, Guo JJ, Guo L, Jia HL, Zhu M, Zhang JB, Loffredo CA, Forgues M, Huang H, Xing XJ. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res. 2013;19:3944-3954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 37. | Prieto PA, Cha CH. DKK1 as a serum biomarker for hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2013;2:127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol. 2012;5:874-881. [PubMed] |
| 39. | Zhou Y, Yin X, Ying J, Zhang B. Golgi protein 73 versus alpha-fetoprotein as a biomarker for hepatocellular carcinoma: A diagnostic meta-analysis. BMC Cancer. 2012;12:12-17. [DOI] [Full Text] |
| 40. | Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 41. | Liu H, Li P, Zhai Y, Qu CF, Zhang LJ, Tan YF, Li N, Ding HG. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol. 2010;16:4410-4415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 42. | Badr EA, Korah TE, Ghani AA, El-Sayed S, Badr S. Role of serum glypican-3 in the diagnosis and differentiation of small hepatocellular carcinoma from hepatitis-C virus cirrhosis. Alexandria J Med. 2014;50:221-226. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Zhu J, Jiang F, Ni HB, Xiao MB, Chen BY, Ni WK, Lu CH, Ni RZ. Combined analysis of serum γ-glutamyl transferase isoenzyme II, α-L-fucosidase and α-fetoprotein detected using a commercial kit in the diagnosis of hepatocellular carcinoma. Exp Ther Med. 2013;5:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Stefaniuk P, Mikuła T, Krygier R, Dusza M, Cianciara J, Wiercińska-Drapało A. Combination of alpha-fetoprotein with gamma-glutamyl transferase as the complementary biomarkers useful in early diagnosis of hepatocellular carcinoma. Hepatology. 2010;6:40-44. |
| 45. | Fawzy Montaser M, Amin Sakr M, Omar Khalifa M. Alpha-L-fucosidase as a tumour marker of hepatocellular carcinoma. Arab J Gastroenterol. 2012;13:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Gan Y, Liang Q, Song X. Diagnostic value of alpha-L-fucosidase for hepatocellular carcinoma: a meta-analysis. Tumour Biol. 2014;35:3953-3960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Hussein MM, Ibrahim AA, Khattab NF, El Fouly RF, El-Fouly NF. Serum Transforming Growth Factor Beta1 in Hepatitis C Virus Related Chronic Liver Disease and Hepatocellular Carcinoma Patients. Med J Cairo Univ. 2010;78:279-286. |
| 48. | Yasmin Anum MY, Looi ML, Nor Aini AH, Merican I, Wahidah A, Mohd Radzi AH, Nor Azizah A, Othman NH. Combined assessment of TGF-beta-1 and alpha-fetoprotein values improves specificity in the diagnosis of hepatocellular carcinoma and other chronic liver diseases in Malaysia. Med J Malaysia. 2009;64:223-227. [PubMed] |
| 49. | Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, Precone DF, Cioffi M, Gaeta GB, Caporaso N. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer. 2002;95:2539-2545. [PubMed] |
| 50. | Lopez JB. Recent developments in the first detection of hepatocellular carcinoma. Clin Biochem Rev. 2005;26:65-79. [PubMed] |
| 51. | Hussein MM, Ibrahim AA, Abdella HM, Montasser IF, Hassan MI. Evaluation of serum squamous cell carcinoma antigen as a novel biomarker for diagnosis of hepatocellular carcinoma in Egyptian patients. Indian J Cancer. 2008;45:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Giannelli G, Marinosci F, Trerotoli P, Volpe A, Quaranta M, Dentico P, Antonaci S. SCCA antigen combined with alpha-fetoprotein as serologic markers of HCC. Int J Cancer. 2005;117:506-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: an effector and an effect of tumor metastasis. Curr Mol Med. 2010;10:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 55. | Wan HG, Xu H, Gu YM, Wang H, Xu W, Zu MH. Comparison osteopontin vs AFP for the diagnosis of HCC: a meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:706-714. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Lee HJ, Yeon JE, Suh SJ, Lee SJ, Yoon EL, Kang K, Yoo YJ, Kim JH, Seo YS, Yim HJ. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver. 2014;8:177-185. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Hafez SS, El Sebai1 AA, Abd AL Aziz MM, Mahmoud MAB, El Maraghy MO, Khalifa MO and Musa NI. Alfa-Fetoprotein L3 Subfraction and Osteopontin: Novel Markers for the Diagnosis of Hepatocellular Carcinoma. J Am Sci. 2013;9:322-328. |
| 58. | Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005;11:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005;5:4581-4588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Gruden G, Carucci P, Lolli V, Cosso L, Dellavalle E, Rolle E, Cantamessa A, Pinach S, Abate ML, Campra D. Serum heat shock protein 27 levels in patients with hepatocellular carcinoma. Cell Stress Chaperones. 2013;18:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Metwaly HA, Al-Gayyar MM, Eletreby S, Ebrahim MA, El-Shishtawy MM. Relevance of serum levels of interleukin-6 and syndecan-1 in patients with hepatocellular carcinoma. Sci Pharm. 2012;80:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, Jung HS. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Cabrera R, Fitian AI, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Liu C, Nelson DR. Serum levels of soluble CD25 as a marker for hepatocellular carcinoma. Oncol Lett. 2012;4:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Kemik O, Sumer A, Kemik SA, Purisa S, Tuzun S. Circulating levels of VEGF family and their receptors in hepatocellular carcinoma. Bratisl Lek Listy. 2010;111:485-488. [PubMed] |
| 66. | Zhan P, Qian Q, Yu LK. Serum VEGF level is associated with the outcome of patients with hepatocellular carcinoma: a meta-analysis. Hepatobiliary Surg Nutr. 2013;2:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 67. | Abdel-Hamid NM, Ramadan MF, Amgad SW. Glycoregulatory Enzymes as Early Diagnostic Markers during Premalignant Stage in Hepatocellular Carcinoma. Am J Canc Prev. 2013;1:14-19. |
| 68. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3594] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 69. | Kumar A, Acharyay SK, Singhz SP, Saraswatx VA, Arora A, Dusejak A, Jain , Premashish Kar MK, Kumaryy M, Kumaranzz V. The Indian National Association for Study of the Liver (INASL) Consensus on Prevention, Diagnosis and Management of Hepatocellular Carcinoma in India: The Puri Recommendations. J Clin Exp Hepatol. 2014;4 S3:S3-S26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |