Published online Oct 8, 2015. doi: 10.4254/wjh.v7.i22.2363
Peer-review started: May 28, 2015
First decision: July 27, 2015
Revised: August 14, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: October 8, 2015
Processing time: 128 Days and 4.1 Hours
The year 2014 marked the beginning of the end of the interferon era and the triumph of the all-oral interferon-free regimens for treatment of hepatitis C virus (HCV) infection. These innovative therapies are safe and yield a cure rate of over 90%. The scientific hepatology community is euphoric about the possibility of elimination and even eradication of HCV infection. However, the current high cost of the new all-oral regimens allows access to treatment only for a restricted number of HCV-infected patients. In addition, many other conditions such as modality of access and delivery of care, inadequate knowledge of HCV epidemiology and political commitments to be undertaken, hamper the fulfillment of the dream to eliminate the virus. Since, such conditions are not impossible to overcome, a global urgent effort must be made to allow a widespread access to the new treatments which will permit in the next years to avoid million of HCV-related deaths.
Core tip: It is begun the era of all-oral direct-acting antiviral drugs for hepatitis C virus (HCV) treatment allowing interferon-free therapeutic regimens. These regimens are safe and yield cure rate greater than 90%. The therapeutic success has posed the basis for HCV elimination, although, confirmation are waiting from real world. However, many conditions hinder the fulfillment of the dream. These conditions are the excessive cost of drugs, the access and delivery of care, the epidemiology of HCV and the political commitments. Thus, an urgent effort must be done to make accessible on large-scale all-oral anti-HCV therapies allowing saving millions of lives.
- Citation: Adinolfi LE, Guerrera B. All-oral interferon-free treatments: The end of hepatitis C virus story, the dream and the reality. World J Hepatol 2015; 7(22): 2363-2368
- URL: https://www.wjgnet.com/1948-5182/full/v7/i22/2363.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i22.2363
In the years 2014-2015 the scientific hepatology community virtually laid the milestone that could mark the beginning of the end of the hepatitis C virus (HCV) story. The milestone marked the advent of the first new oral drugs approved for HCV treatment, namely sofosbuvir and simeprevir, which were quickly followed by several others allowing interferon (IFN)-free therapeutic regimens. These innovative therapies, which yield a cure rate of over 90%, have been approved in many regions of the world, including Europe and the United States. However, success did not come overnight but was the result of a step-by-step escalation in both understanding the molecular mechanisms of HCV replication and in the therapeutic approaches which started at the end of the 1980s, before the discovery of HCV, with IFN alpha affording a response rate of less than 15%[1]. The addition of ribavirin to IFN increased the success rate to about 30%, and then treatment moved into the era of the long-acting pegylated IFNs (pegIFNs). PegIFN plus ribavirin was the standard therapy for about 10 years during which the overall therapeutic success increased to 50%[2]. However, IFN-based treatment was associated with a significant number of severe side effects that limited its use considerably. Many other factors conspired to limit the therapeutic response, further hampering the use of IFN. These included host factors, such as age, body mass index, level of liver fibrosis and steatosis, insulin resistance, ethnicity, the genetic background such as the polymorphism of interleukin-28B, and viral factors, namely HCV genotype and HCV RNA levels. Furthermore, IFN-based regimens required a long therapeutic course, which reduced the adherence to treatment.
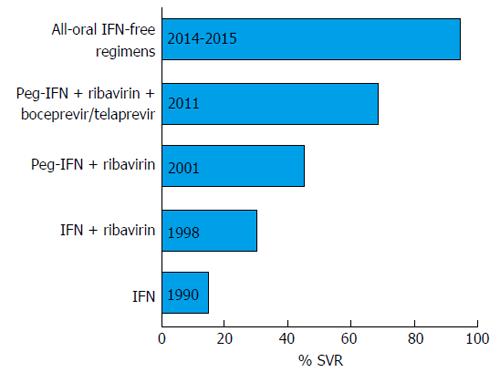
The year 2011 marked the beginning of the era of oral direct-acting antiviral drugs (DAAs). The scenery was complex with the first-generation DAAs, the NS3-A4 protease inhibitors boceprevir and telaprevir, which were approved in combination with pegIFNs and ribavirin for HCV genotype 1 treatment[3]. Although this triple therapy afforded a higher sustained virological response (SVR) rate than pegIFN plus ribavirin, the treatment was short-lived because of the dependence on IFN due to the low barrier to resistance of these first-generation DAAs. Moreover, the use of these DAAs was aggravated by a high rate of serious adverse events and by the scanty possibility to treat patients with advanced liver disease. Fortunately, the research moved fast and new oral HCV DAAs were devised, several of which had proved highly effective in a combined regimen, and finally approved for use in clinical practice (Table 1). Several other DAAs are currently in the drug development pipeline. Figure 1 shows the crucial milestones in the chronic hepatitis C treatment evolution, from IFN-based regimens to all-oral IFN-free combinations.
| DAAs | Class | Generation | Activity |
| Simeprevir | NS3-4A | Second-wave, first generation | Genotypes 1 and 4 |
| Paritaprevir | NS3-4A | Second-wave, first generation | Genotypes 1 and 4 |
| Sofosbuvir | Nucleoside/nucleotide | Nucleotide analogue | Pangenotype |
| analogues | |||
| Dasabuvir | Non-nucleoside inhibitors of the HCV RNA-dependent | Palm domain I inhibitor | Genotype 1 |
| RNA polymerase | |||
| Daclatasvir | NS5A inhibitors | First-generation | Pangenotype |
| Ledipasvir | NS5A inhibitors | First-generation | Genotypes 1, 3, 4, 5, and 6 |
| Ombitasvir | NS5A inhibitors | First-generation | Genotypes 1 and 4 |
The first therapeutic strategy with the new DAAs approved in 2014 was pegIFN plus ribavirin plus sofosbuvir, a nucleoside analogue acting as a false substrate for HCV to stop viral RNA synthesis. Sofosbuvir is active against all HCV genotypes and has a high barrier to resistance. A phase III trial[4] using the triple regimen showed an SVR of 89% and 96% for HCV genotype 1 and 4, respectively, and a phase II trial[5] in treatment-experienced patients showed an SVR of 96% and 83% in genotype 2 and 3, respectively.
In the same year, based on the data of phase III studies[6,7], another triple regimen with pegIFN plus ribavirin and simeprevir, a first-generation NS3-4A protease inhibitor, was approved for the treatment of HCV genotype 1 and 4. The two trials reported an SVR of 80% and showed that in HCV genotype 1a carrying the mutation Q80K in the NS3 protease sequence the response rate was significantly impaired.
The year 2014 also marked the beginning of the end of the IFN era and the triumph of the all-oral IFN-free regimens, which were highly effective and safe and afforded treatment also for patients with advanced cirrhosis. Phase III studies[8,9] showed that the combination sofosbuvir plus ribavirin for 12 wk for HCV genotype 2 achieved an SVR from 88% to 100% depending on whether the patients were treatment-naïve or -experienced and on the absence or presence of cirrhosis, and for 24 wk for genotype 3, achieving an SVR from 60% to 94%, with the poorest results in therapy-experienced cirrhotic patients. The treatment of genotype 3 can be shortened to 12 wk by adding pegIFN to sofosbuvir and ribavirin. A phase III trial[10] showed that the combination sofosbuvir plus daclatasvir, a “pan-genotypic” first-generation NS5A inhibitor, taken for 12 wk produced an SVR in genotype 3-infected patients of 90% for previously untreated patients and 86% for prior non-responders. Thus, at present, this combination seems to be the best option for the treatment of genotype 3 infection. The combination sofosbivir plus daclatasvir also showed good results in HCV genotype 1, 2, 4, 5 and 6.
The regimen sofosbuvir plus simeprevir has been approved for treatment of HCV genotypes 1 and 4. A phase II study[11] showed an SVR of 93%-96% in treatment-naïve and -experienced patients treated for 12 wk. For HCV genotype 1a the Q80K substitution did not seem to influence the response rate of the sofosbuvir and simeprevir combination.
In early 2015 the combination of sofosbuvir and ledipasvir, a first-generation NS5A inhibitor, in a single pill to be administered once a day was available for the treatment of HCV genotypes 1, 3 and 4. The SVR after 12 wk of treatment was as high as 99%[12,13]. Importantly, it was shown that the treatment could be shortened to 8 wk with an SVR of 94%[14]. Patients with genotypes 5 and 6 have also been treated successfully.
The first IFN-free triple combination approved for HCV genotype 1 was ritonavir-boosted paritaprevir and ombitasvir in one pill, and dasabuvir. Several phase III studies[15-18] showed an SVR from 95% to 100%. High SVR rates were also reported for patients with HCV genotype 4 without adding dasabuvir.
All the above-mentioned data are awaiting solid confirmation from the real world. The early real-life data presented seem to confirm the trial results, albeit with a slightly lower SVR[19]. Further studies are necessary to evaluate the effect of treatment in patients with advanced liver disease as well as on the development of hepatocellular carcinoma (HCC). To this regard, preliminary studies seem to indicate that cirrhotic patients should undergo a close follow-up after HCV clearance by DAAs due to the residual possibility of developing HCC. In addition, considering the widespread use, the possibility to shorten the treatment duration should be evaluated.
Overall, the data on safety seem to indicate good tolerability for all of the above combinations. Moreover, it seems that there are no absolute contra-indications to the use of these approved DAAs. However, it is necessary to be prudent with the use of sofosbuvir for patients with severe renal impairment. In addition, the triple combination of ritonavir-boosted paritaprevir, ombitasvir, and dasabuvir has not yet been adequately evaluated in patients with Child-Pugh B decompensated cirrhosis and is contraindicated in patients with Child-Pugh C decompensated cirrhosis. Furthermore, the pharmacokinetics and safety of simeprevir for decompensated cirrhosis have not yet been fully evaluated, and it induces increased bilirubin levels in about 10% of cases. At present, considering the scanty data on the safety of DAAs in advanced liver cirrhosis, they must be used with extreme caution in this subgroup of patients. Moreover, if DAAs are administered to patients taking other drugs, it is mandatory that the drug-to-drug interaction be taken into account[20].
On the basis of the solid data from the trials on the efficacy and safety of all-oral INF-free combinations, the scientific community is euphoric about the elimination and even eradication of chronic hepatitis C. Therefore, now that we have a cure, the question is: In the real world, can we meet the conditions to make the dream come true? At the present, considering that HCV infection and its treatment are tremendously complex situations, the answer cannot be directly affirmative because of the many conditions, not impossible to overcome, that hamper the fulfillment of the dream. These conditions are linked to the elevated cost of drugs, to the access and delivery of care, to the epidemiology of HCV and to the political commitments to be undertaken.
The current high cost of the new all-oral regimen allows access to treatment only for a restricted number of HCV-infected patients; at present, many countries have chosen to treat only patients with advanced liver diseases. The high treatment costs can only be justified by the need to recuperate the research and development costs of the drugs; it has been estimated that the predicted manufacturing costs of HCV DAAs range from United States $100 to $270 per person for a 12-wk treatment course[21]. Thus, action strategies should be evaluated with the aim to reducing the price of the new drugs and allowing extensive access to the new treatments not only in high- but also in middle- and low-income countries, where the highest prevalence of HCV infection is concentrated. By extending access to treatment, a double advantage can be achieved: First, it will enable the drug companies to recover their costs sooner, and second, it will allow substantial individual and public health benefits by reducing both the hepatic and extrahepatic HCV-associated pathologies and the spread of HCV infection worldwide. Global negotiations should be undertaken to include state authorities, drug companies, healthcare authorities and the scientific communities to reduce the cost of treatment, thereby extending access to more patients and preventing the spread of HCV infection. In this respect, the policies adopted by Egypt and India have obtained a price reduction that will allow widespread treatment of HCV-infected patients. Alternatively, it will be necessary to wait 15 years for generic drugs, the time required for drug patents to expire. If this should occur, the question that everyone should ask themselves is: Is it ethical to let someone die of hepatitis C when effective treatment is available but is inaccessible due to the high cost, especially since we know that treatments can be produced at a very low cost?
At present, of the estimated 150-200 million HCV-infected subjects worldwide, only the tip of the iceberg is really known. Consequently, if we presume today that the drug costs could be reduced, we would be able to treat only the prevalent cases of HCV. To achieve the elimination of HCV infection another two conditions have to be considered, the cases that are unaware of their HCV status and the incident cases. As a substantial number of countries do not have a screening program, it is critical to start adequate screening programs to identify subjects to be treated. Furthermore, the spread of HCV infection must be taken in hand to identify incident cases. Therefore, marginalized populations, drug users and intra-family spread of the infection must be monitored. To this regard, the current costs for both the epidemiological and pre-treatment screening is high. To ensure widespread treatment with the new HCV DAAs it is necessary to reduce the costs of diagnostic tests and, perhaps, determine only the HCV genotype to detect viremic subjects and decide the best therapeutic approach.
A further critical point is the delivery of treatment. At present, due to the high cost and the complexity of therapy, treatment is confined to specialist care centers to administer the drugs. However, for several reasons, these centers are not accessible to all infected subjects and in order to implement a successful HCV elimination program, non-specialists in primary care settings could deliver treatment. Due to the complexity of treatment, to be sure that an effective regimen is delivered, specialist supervision in the primary care should be given. Accordingly, Project ECHO could be a model for the management of HCV[22,23] where the decisions on treatment could be made using communication technologies.
The fantastic race for effective treatment for HCV has reached the final straight and the finishing line that marks the fulfillment of the dream can be clearly seen. Although the reality has exceeded all expectations, the Healthcare Authorities do not appear to be ready to take on the challenge for victory. One analysis suggested that it is possible to achieve the elimination of HCV by 2030 if screening programs to identify the HCV-infected populations are implemented and if active management with new oral anti-HCV therapies is made accessible on a large scale[24,25]. However, time is running out and there is an urgent need to reduce liver transplantation, considering that HCV infection is the leading cause of liver transplants, and to save millions of lives. To this regard, it is important to underscore that the current HCV-related death rate ranges from 350000 to 500000 people per year[26]. Furthermore, a recent analysis showed that HCV-related morbidity and mortality are expected to increase in the next 15 years[25], and it is estimated that among the current chronically HCV-infected population with no liver cirrhosis, the HCV-related deaths will peak in 2030-2035[27]. Now that we are in the final straight of the extraordinary race for effective therapy, a concerted effort must be made by the state authorities to press for a reduction in the cost of the drugs and by the scientific communities to improve the knowledge on HCV epidemiology and to plan strategies for the access and delivery of therapies. Widespread access to the new treatments will allow the dream to become reality.
P- Reviewer: Luo GH, Wong GLH S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Hoofnagle JH, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, Peters M, Waggoner JG, Park Y, Jones EA. Treatment of chronic non-A,non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 646] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 3. | Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 803] [Cited by in RCA: 844] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 4. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 5. | Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Dvory-Sobol H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2015;61:769-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 7. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 9. | Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 10. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 11. | Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 12. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1365] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 13. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 14. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 928] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 15. | Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 656] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 16. | Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 548] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 17. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 18. | Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, Müllhaupt B, Horsmans Y, Weiland O, Reesink HW. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359-365.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 19. | Jensen DM, O’Leary JG, Pockros PJ, Sherman KE, Kwo PY, Mailliard ME. Safety and efficacy of sofosbuvir- containing regimens for hepatitis C: real-world experience in a diverse, longitudinal observational cohort. Hepatology. 2014;60:219A. |
| 20. | Available from: http://www.hep-druginteractions.org/interactions.aspx. |
| 21. | Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, Parish B, Burke T, Pak W, Dunkelberg J. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 812] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 23. | Mitruka K, Thornton K, Cusick S, Orme C, Moore A, Manch RA, Box T, Carroll C, Holtzman D, Ward JW. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model--Arizona and Utah, 2012-2014. MMWR Morb Mortal Wkly Rep. 2014;63:393-398. [PubMed] |
| 24. | Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 25. | Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, Örmeci N, Van Vlierberghe H, Gschwantler M, Akarca U. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21 Suppl 1:60-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5243] [Article Influence: 524.3] [Reference Citation Analysis (0)] |
| 27. | Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |