Published online Sep 18, 2015. doi: 10.4254/wjh.v7.i20.2264
Peer-review started: July 7, 2015
First decision: July 27, 2015
Revised: August 13, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 18, 2015
Processing time: 75 Days and 11.9 Hours
Bacterial translocation (BT) refers to the passage of viable bacteria or bacterial products from the intestinal lumen, through the intestinal epithelium, into the systemic circulation and extraintestinal locations. The three principal mechanisms that are thought to be involved in BT include bacterial overgrowth, disruption of the gut mucosal barrier and an impaired host defence. BT is commonly observed in liver cirrhosis and has been shown to play an important role in the pathogenesis of the complications of end stage liver disease, including infections as well as hepatic encephalopathy and hepatorenal syndrome. Due to the importance of BT in the natural history of cirrhosis, there is intense interest for the discovery of biomarkers of BT. To date, several such candidates have been proposed, which include bacterial DNA, soluble CD14, lipopolysaccharides endotoxin, lipopolysaccharide-binding protein, calprotectin and procalcitonin. Studies on the association of these markers with BT have demonstrated not only promising data but, oftentimes, contradictory results. As a consequence, currently, there is no optimal marker that may be used in clinical practice as a surrogate for the presence of BT.
Core tip: The exact mechanism behind bacterial translocation in patients with cirrhosis has not been fully elucidated. The discovery of reliable biomarkers for this phenomenon would be of significant clinical importance, as bacterial translocation is closely associated with the development of severe complications. Various molecules have been identified as candidates for serving as markers of bacterial translocation in this patient population. This mini-review attempts to summarize the most recent available data regarding the potential use of such markers as clinical and prognostic tools in the management of end-stage liver disease.
- Citation: Koutsounas I, Kaltsa G, Siakavellas SI, Bamias G. Markers of bacterial translocation in end-stage liver disease. World J Hepatol 2015; 7(20): 2264-2273
- URL: https://www.wjgnet.com/1948-5182/full/v7/i20/2264.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i20.2264
Bacterial infections are frequent complications of cirrhosis and have been associated with significantly increased mortality rate[1]. Spontaneous bacterial peritonitis (SBP), urinary tract infections, pneumonia and sepsis are the most common infections, with gram-negative and gram-positive bacteria being equally detected as the causative organisms. In particular, cirrhotic patients with gastrointestinal bleeding have higher risk for developing bacterial infections during hospitalization and, thus, antibiotic prophylaxis is recommended in this scenario[2,3].
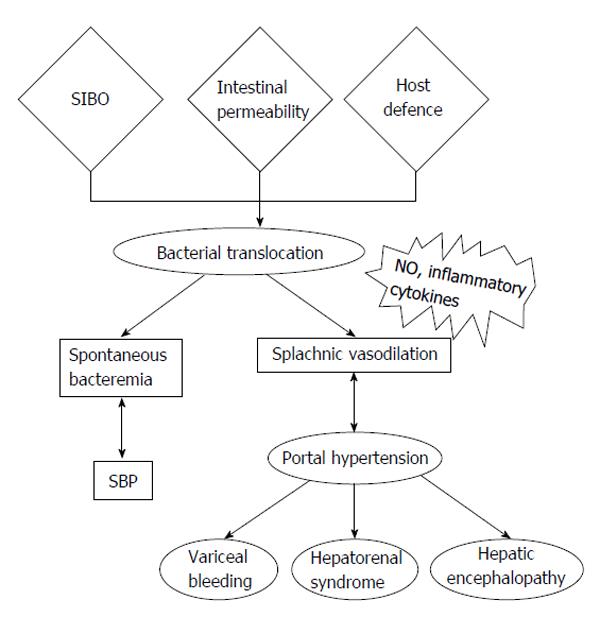
Bacterial translocation (BT) refers to the entry of viable bacteria or their products into the regional lymph nodes, the systemic circulation, and possibly extraintestinal organs. The origin of such microorganisms is the enteric flora and translocation occurs via a defective mucosal barrier[4]. BT is considered the key step in the pathogenesis of SBP and bacteremia in cirrhotic patients, as well as a critical factor that triggers host immune responses and secretion of inflammatory mediators, which, ultimately, mediate the hemodynamic changes that are present in portal hypertension and cirrhosis[5]. The main three mechanisms involved in BT include bacterial overgrowth, physical disruption of the gut mucosal barrier and an impaired host defence (Figure 1)[6]. In the present article, we will briefly review the pathogenesis of BT and analyze the literature regarding surrogate markers for this condition.
Small intestinal bacterial overgrowth (SIBO) is multifactorial and may be the result of defective gastric acid secretion and compromised small intestinal motility, as well as dysregulated mucosal and systemic immunity. The currently accepted criterion for the diagnosis of SIBO is the presence of > 105 colony-forming units/mL of coliform bacteria in aspirates from the proximal jejunum. Alternatively, breath tests have been used as sensitive and simpler tools for diagnosis of bacterial overgrowth, by measuring an increase in breath hydrogen or methane concentration, produced from intestinal bacterial fermentation after glucose or lactulose ingestion[7]. Experimental data has shown that cirrhotic rats with SIBO had a significantly higher rate of BT and slower intestinal transit than those without SIBO[8]. In clinical studies, the prevalence of SIBO in cirrhotic patients was found to be significantly higher than that in non-cirrhotic controls, and that it is related to the severity of liver disease[9]. Furthermore, the incidence of SIBO was higher in patients with a previous history of SBP than in SBP-naïve patients[10].
Structural and functional alterations of the gut mucosa that lead to increased intestinal permeability to bacteria and their products have been described in cirrhosis[11]. Bile secretions may also play a role in the prevention of BT by inhibiting bacterial overgrowth, exerting a trophic effect on intestinal mucosa and neutralizing endotoxin[12]. Increased intestinal permeability has been linked to the progression of liver disease and the complications of cirrhosis[13]. However, increased intestinal permeability cannot fully account for the pathophysiology of BT; moreover, it is not clear whether these structural changes are the cause or the result of BT[5].
The intestinal immune system is comprised of Peyer’s patches, the mesenteric lymph nodes (MLNs) and a large number of cells distributed throughout the lamina propria and epithelium of the intestine[14,15]. Patients with cirrhosis exhibit systemic immune alterations that may promote the development of infections and BT. Advanced cirrhosis is associated with decrease in the cellular and humoral components of immune response, decreased activity of the reticuloendothelial system, decreased phagocytic capacity of Kupffer cells, as well as restricted recruitment of leucocytes in response to inflammatory stimuli due to portal hypertension-associated splanchnic hyperaemia[16-18]. The inflammatory response induced by BT, with the synthesis of cytokines, particularly tumor necrosis factor-alpha (TNF-α), interleukins and nitric oxide (NO) also increases intestinal barrier permeability, which, in turn, favours BT, thus creating a feedback in which BT promotes its own causative mechanisms[5].
Bacterial translocation is also associated with systemic complications and deterioration of the hyperdynamic circulation in cirrhosis (Figure 1). Human studies have shown that cirrhotic patients with increased levels of lipopolysaccharide binding protein (LBP), a marker of BT, are found to have a significant immune and haemodynamic derangement, which is ameliorated by norfloxacin administration, by causing selective intestinal decontamination and inhibiting BT[19]. Additionally, the presence of bacterial DNA (bDNA), another marker of BT, in patients with cirrhosis and ascites, has been correlated with aggravation of peripheral vasodilation and with worsening of intrahepatic endothelial dysfunction[20]. Kidney tissue in cirrhosis shows increased expression of the toll-like receptor 4 (TLR4), nuclear factor κB (NF-κB), and TNF-α molecules, which makes the renal system further susceptible to the effects of cirrhosis during BT, highlighting the fact that BT contributes to the development of hepatorenal syndrome (HRS)[21]. Moreover, it has been reported that the non- absorbable antibiotic rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites, by suppressing intestinal bacterial overgrowth and preventing BT[22]. It has been also suggested that cirrhotic patients may have compromised ability to upregulate sufficient dilatory forces [i.e., endothelial nitric oxide synthase (NOS), inducible NOS, and heme oxygenase-1] to counterbalance the constrictive effect of endothelin-1 upon a secondary insult of endotoxemia. This is indicated by the presence of lipopolysaccharides (LPS) endotoxin during BT, and the net effect of this phenomenon is the establishment of increased intrahepatic resistance[23]. Endotoxemia may also be a trigger factor for variceal bleeding, either by worsening liver function or causing an acute increase in portal hypertension[24]. Infection and the resulting systemic inflammatory response (SIRS) are considered important factors contributing to worsening hepatic encephalopathy (HE) in cirrhotic patients[25]. BT-associated inflammatory response may therefore have a role in the pathogenic mechanisms involved in HE. Consistent with this are the results from studies showing either improvement of minimal encephalopathy in cirrhotic patients receiving rifaximin, or the effectiveness of probiotics for secondary prophylaxis of HE, overall suggesting that BT plays an important role in the pathogenesis of HE[26].
Considering the eminent role of BT in the progression of liver disease and the subsequent complications of cirrhosis, it is not surprising that the elucidation of the underlying mechanisms as well as the discovery of possible markers of BT, that may be easily measured and have prognostic value for the severity of liver disease, has been at the center of attention of many research groups. In this review, we aimed to summarize the most prominent of the proposed markers of BT, such as bDNA, LPS, LBP, sCD14, calprotectin and procalcitonin (Table 1).
| Origin | Comments | Ref. | |
| Bacterial DNA | Bacteria | Pros: Long half-life, association with cytokine production, hemodynamic changes and prognosis | Bellot et al[20], Francés et al[31], El-Naggar et al[32], González-Navajas et al[33], Zapater et al[34], Jun et al[35], Fagan et al[36] |
| Cons: Variable rates of detection (maybe depending on methodology used), lower prevalence in outpatients | Rincón et al[39], Sersté et al[40], Feng et al[41], Fujita et al[42], Vlachogiannakos et al[43], Mortensen et al[44], Mortensen et al[45], Appenrodt et al[46] | ||
| LPS | Gram (-) bacteria | Pros: Correlation with TNF-α, stage of cirrhosis, prognostic value for severity of liver damage | Hanck et al[52], Lin et al[54], Chan et al[55] |
| Cons: Short half-life, variation of detection rates | Bellot et al[5], Fukui et al[53], Kaser et al[56], Stadlbauer et al[57] | ||
| LBP | Acute phase protein triggered by LPS | Pros: Long half-life, correlation with TNF-RI, TNF-α, IL-6, and hyperdynamic circulation | Albillos et al[19], Albillos et al[59] |
| Cons: Low detection rates, elevated in systemic infection from Gram (-) bacteria | Albillos et al[19], Albillos et al[59] | ||
| sCD14 | Monocytes, liver | Pros: Prognostic marker of disease progression in HBV/HCV/HIV, NAFLD and alcoholic liver disease, correlation with liver fibrosis, easily measured | Landmann et al[61], Tuomisto et al[62], Sandler et al[63], Balagopal et al[64], French et al[65], Ogawa et al[66], Campos et al[67] |
| Calprotectin | Neutrophils | Pros: Easily measured, fecal levels associated with stage of liver disease, SBP and HE, ratio of ascites calprotectin/total protein may be better | Gundling et al[77], Lutz et al[81] |
| Cons: Plasma levels do no distinguish cirrhotic patients from healthy controls, weak association with alcoholic liver disease | Homann et al[75], Homann et al[76], Montalto et al[78] | ||
| Procalcitonin | Neutrophils, liver, thyroid | Pros: Ascitic levels may differentiate between cirrhotic subgroups | Attar et al[97] |
| Cons: No correlation with HE, conflicting results depending on etiology of liver disease | Spahr et al[92], Elefsiniotis et al[94], Rahimkhani et al[95], Villarreal et al[96] | ||
| ANCAs (IgA) | Neutrophils | Pros: Associated with ascites and advanced cirrhosis, predicts time to the first infectious complication | Papp et al[99] |
| Cons: Single study |
The occurrence of bacterial-derived material in extraintestinal locations has been long recognized in clinical and experimental cirrhosis. At the time of liver surgery, one third of cirrhotic patients with Child-Pugh stage C demonstrate infected MLNs; this percentage is reduced to the level of non-cirrhotic patients after selective intestinal decontamination[27]. In contrast, most of BT episodes in cirrhotics remain undetected as its diagnosis relies on blood or ascitic fluid cultures, which are more often negative than positive. This may be the result of bacterial opsonization which renders bacteria nonviable in routine cultures. Despite the process of opsonization, bacterial components might remain in biological fluids and could be detected with more sensitive analytical methods. Taken together, these findings emphasize the need for the discovery of novel and reliable markers with high sensitivity and specificity for the diagnosis of BT[28].
The application of bDNA as a marker of BT was initially shown in animal models of experimental cirrhosis. Specifically, in cirrhotic rats the presence of bDNA of a certain bacterial species in blood, ascites or pleuritic fluid was always associated with its simultaneous presence in MLNs. Moreover, the presence of bDNA was associated with marked inflammatory responses[29]. Importantly, these findings occurred independently of the blood culture status (positive or negative).
Several studies in humans have now tested the validity of molecular detection of bDNA as a surrogate marker of BT. Using a polymerase chain reaction (PCR) - based method, Such et al[28] reported that bDNA in serum and ascitic fluid was present in 32% of cirrhotic patients with culture negative ascites, and that this likely represented episodes of single clone translocation and systemic seeding. E. coli was the most frequently identified microorganism, while S. aureus was responsible less frequently. The same group showed that bacteria persist in the blood of cirrhotic patients during variable periods of time after the completion of therapeutic paracentesis, therefore suggesting that this phenomenon is related to the existence of repeated episodes of BT from the intestinal lumen. The presence of identical sequences of nucleotides in all bDNA PCR fragments detected in every patient, strongly supports the existence of the repeated episodes of BT being caused by the same bacteria specie[30]. The presence of bDNA in patients with decompensated cirrhosis has also been associated with marked activation of peritoneal macrophages, as evidenced by NO synthesizing ability along with enhanced interferon-γ, TNF-α, interleukin (IL)-2, and IL-12 production[31]. Serum and ascitic fluid TNF-α levels were significantly higher in patients with bDNA compared to those without this marker on admission. Additionally, the relative risk of death, HRS and SBP was higher in patients with bDNA[32]. A sub-group of patients with translocation of Gram-positive microorganisms showed increased proinflammatory cytokines unrelated to endotoxin[33]. Furthermore, the presence of bDNA in patients with cirrhosis during an episode of ascites was shown to be an indicator of poor prognosis. When considering only patients with MELD score < 15, mortality was significantly higher in those positive for bDNA. In this study, SBP developed independently of the bDNA status at admission[34]. The presence of bDNA was also associated with peripheral vasodilation and deterioration of intrahepatic endothelial function[20]. Another study highlighted the strong correlation between SIBO and the presence of bDNA in the peripheral blood of patients with cirrhosis[35]. Moreover, the high bDNA detection frequency was recently confirmed as it was shown that it was detected in ascitic fluid from 23 of 25 patients with culture-negative, non-neutrocytic ascites. Again, bDNA levels were a poor prognostic factor for a 6-mo clinical outcome. High bDNA burden was also associated with reduced major histocompatibility complex class II expression on macrophages isolated from ascites[36-38].
Studies on the importance of bDNA detection in cirrhotics have occasionally created contradictory results. bDNA was identified by gel electrophoresis of a multiplex PCR-based product which amplified selected prokaryotic nucleic acids and was detected in 5/5 culture-positive neutrocytic, 1/6 culture-negative neutrocytic and 8/56 culture-negative non-neutrocytic samples. Three-month mortality was increased in the presence of ascitic bDNA only for patients with a MELD score > 15[39]. Contrary to hospitalized patients, bDNA was rarely detected in ascitic fluid and serum of asymptomatic outpatients with cirrhosis and non-neutrocytic ascites, after repeated paracentesis[40]. This finding may account for the markedly low prevalence of SBP in cirrhotic outpatients, when compared with their hospitalized counterparts. In another study, bacterial-specific 16S ribosomal RNA was not detected in blood samples from systemic or splanchnic circulation of cirrhotic patients on days 0 and 29 after rifaximin administration. In this study, plasma bDNA concentration did not correlate with systemic hemodynamic parameters[43]. Furthermore, bDNA did not correlate with markers of inflammation, such as C-reactive protein, TNF-α, IL-6 and IL-8. Additionally, it did not accurately predict the presence of SBP[44]. bDNA was also found to be largely unrelated to a panel of markers of inflammation and without association with portal pressure in patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt insertion[45]. No correlation between detection of bDNA in ascites and SBP was found[46]. In another study, administration of a probiotic mixture improved the hepatic and systemic haemodynamics in cirrhotic patients, but these changes were not related to the detection of bDNA[39].
In all, these contradictory results may be accounted for by differences in the tested populations and, also, in the specific methodologies used for the detection of bDNA[41,42]. These discrepancies obviate the need for larger studies which will include well-characterized patient subpopulations, including chronic hepatitis, non-alcoholic fatty liver disease (NAFLD), as well as compensated and decompensated cirrhosis. Moreover, standardisation of methodology is required in order to determine the applicability of bDNA usage in clinical practice as a surrogate marker for BT. Finally, testing of larger cohorts of patients may define the significance of detectable bDNA as a prognostic tool for systemic responses and survival in cirrhosis.
LPS or endotoxin is a lipopolysaccharide, which is part of the outer membrane of Gram-negative bacteria. In circulation, it is recognized by LBP. The LPS-LBP complex binds to membrane CD14 (mCD14) on myeloid cells or to circulating CD14 (soluble CD14, sCD14)[47,48] and promotes a cascade of inflammatory responses via myeloid differentiation-2/TLR4 activation of NF-κB[49,50]. Kupffer cells, which are specialized macrophages located in the liver, are activated when exposed to LPS and can produce a spectrum of cytokines and reactive oxygen intermediates, including the proinflammatory cytokine TNF-α. Thus, LPS is a potent stimulator of Kupffer cell TNF-α production, and this pathway has been implicated in the pathogenesis of many types of liver injury. It has also been proposed that through this mechanism hepatic stellate cells are activated towards the production of inflammatory and adhesion molecules, thus inflicting liver damage[51].
Due to its aforementioned properties, LPS was one of the first molecules that was proposed as a marker of BT. The working hypothesis is that its presence in the sera of cirrhotic patients directly indicates endotoxemia from Gram negative bacteria. In alcoholic cirrhosis, LPS has been found to correlate with TNF-α levels and with the stage of liver disease (Child-Pugh score); thus, it has been suggested that LPS may be a key player in the progression of alcoholic liver disease[52]. Levels of LPS in plasma of patients with alcoholic cirrhosis were higher than in non-alcoholics, pointing to a critical role for alcohol consumption in the development of endotoxemia and liver damage[53]. Taking into consideration that LPS plasma levels of cirrhotic patients are elevated compared to chronic hepatitis and the reported correlation with the stage of cirrhosis, it is probable that LPS may serve as a prognostic factor of disease severity[54] and short-term survival of cirrhotic patients[55]. It should be noted, however, that not all studies have demonstrated a positive correlation between serum LPS and stage of cirrhosis[53,56]. Therefore, LPS has not been clearly established as a reliable marker of BT[57]. This may be due to the short half-life of LPS or to the interference of various factors with the detection of LPS[5].
LBP is a 65 kDa, acute phase protein that is predominantly produced in the liver by hepatocytes. Bacterial LPS triggers the production of LBP and the peripheral levels of LBP are significantly elevated in the setting of bacterial presence. LBP is known to specifically bind and transfer bacterial LPS, and the LPS-LBP complex binds mCD14 on myeloid cells or to sCD14[58]. Consequently, several studies have tested the validity of the expression of LBP as an indicator of a systemic response to LPS, and, thus, as an indirect marker of BT. Albillos et al[19] demonstrated that BT, expressed by elevated plasma levels of LBP, leads to derangement of patients with decompensated cirrhosis. Cirrhotic patients with high LBP levels had enhanced expression of sCD14 and sTNF-RI as well as elevated circulating levels of TNF-α and IL-6. Furthermore, an intense hyperdynamic circulatory state was described in this population. Interestingly, LPS was detectable only in one third of the patients with high LBP. The latter observation strengthens the suggestion that LPS cannot easily serve as a marker of BT, due to its short half-life, so transient episodes of bacteremia may remain undiagnosed. On the other hand, the subsequent expression of LBP after endotoxemia is detectable for a much longer period. Moreover, a study performed by the same group suggested that patients with high LBP circulating levels have increased susceptibility to infections[59]. Different studies have proposed that increased levels of LBP in cirrhosis are the result of chronic endotoxemia and that also no difference occurs in the expression of LBP between alcoholic and non-alcoholic cirrhosis. Kaser et al[56] reported a strong correlation between the two binding proteins of LPS, LBP and sCD14. Although its longer half-life makes LBP a more attractive marker of BT than LPS, its use has certain drawbacks. First, systemic LBP elevation exists only in response to Gram-negative bacteria, and, second, it may not only be present in BT, but also in systemic infection resulting in SIRS (systemic inflammatory response syndrome)[60].
Recently, the target molecule of the complex LPS-LBP, CD14 was also proposed as a surrogate marker of BT. mCD14 is expressed mainly by macrophages and at a lesser extent by neutrophils and dendritic cells. sCD14 is secreted by hepatocytes and monocytes. The expression of sCD14 is upregulated as a result of the presence of LPS. CD14 acts as a co-receptor along with TLR-4 for the detection of bacterial LPS[61]. LPS induces the CD14/TLR4 complex endocytosis in human monocytes and macrophages and the consequent NF-κB activation. Tuomisto et al[62] showed a significant correlation between the levels of bDNA in the liver and the local expression of CD14 in alcoholic liver disease. These findings emphasize the role of CD14 as a marker of BT. Sandler et al[63] proposed that sCD14 may not only be a marker of BT, but also a prognostic marker of disease progression in HBV and HCV infection. Specifically, sCD14 in patients with severe fibrosis was highly elevated not only in peripheral blood, but also within the hepatic parenchyma, measured by CD14 (+) hepatic cells. Regardless of the etiology of cirrhosis, microbial translocation as identified by the presence of sCD14, is believed to play a key role in the progression of liver disease[56]. Studies concerning cirrhosis in HIV-infected patients support this hypothesis[64]. French et al[65] provided evidence that in HIV/hepatitis C virus (HCV)-coinfection, levels of sCD14 and IL-6 were mostly elevated in patient with disease progression than in non-progressors. Studies in patients with NAFLD reached similar results, suggesting a positive correlation between serum sCD14, hepatic CD14 expression and liver inflammation[66]. Attention has also been drawn towards polymorphisms of the promoter region of the CD14 gene, shedding light to the role of CD14/-159TT genotype in the progression of liver injury in alcoholic liver disease[67]. Taking into account the aforementioned studies, it appears that sCD14 may represent a promising marker of BT as it can easily be measured in circulation and correlates with progression of liver disease.
Calprotectin is a calcium and zinc binding protein with a molecular weight of 36 kDa[68]. It has been estimated that it may account for more than 60% of the soluble cytosolic proteins in human neutrophil granulocytes[69]. Moreover, it was shown that calprotectin measurements in fecal samples not only correlate with the degree of neutrophil migration in the gastrointestinal mucosa[70], but also serve as reliable surrogate marker of intestinal inflammation[71]. As a result, fecal calprotectin has been studied in depth in gastrointestinal disorders, and has assumed an important role in monitoring the activity and response to treatment in patients with inflammatory bowel disease[72,73].
The pathogenesis of BT in patients with cirrhosis has been associated with alterations in gut mucosal immune responses and intestinal permeability. In addition, neutrophil infiltrates are detected in the gastrointestinal mucosa of cirrhotics. Consequently, calprotectin has been investigated as a possible diagnostic marker for the existence and natural history of SBP and HE. Initial studies conducted by a Danish research group, focused on the possible prognostic significance of calprotectin levels in the plasma and ascitic fluid samples from patients with end-stage liver disease. The authors did not find a significant difference between healthy controls and patients with cirrhosis (irrespectively if liver disease was compensated or decompensated), a finding that was confirmed in additional studies[74,75]. On the other hand, they reported that high plasma calprotectin levels were an indicator of poor survival in alcohol-related cirrhosis[74]. The most important finding, however, regarding the role of calprotectin in relation to BT, was that during follow up of the patients higher calprotectin levels were an independent predictor of recurrent bacterial infections[76].
Arguably the most important study regarding the role of fecal calprotectin in the diagnosis of BT complications, is the one conducted by Gundling et al[77]. They investigated the relationship between fecal calprotectin levels and the onset and course of SBP and HE. They confirmed that patients with cirrhosis had significantly elevated fecal calprotectin levels when compared to healthy controls. Moreover, this increase correlated with the severity of liver disease (assessed by Child-Pugh and MELD scores). Even more significantly, higher calprotectin values were associated with advanced stages of HE, the presence of SBP, as well as extraintestinal infections. Finally, calprotectin strongly correlated with serum ammonia levels[77]. The aforementioned observations reinforced the hypothesis that fecal calprotectin may be a reliable surrogate marker for BT and provide important assistance in the diagnosis and clinical management of patients with decompensated liver disease. It should be noted that the finding of a possible selective calprotectin upregulation in alcoholic liver disease that was reported in initial studies, was not confirmed in a longitudinal study of active alcoholics, where alcoholics and controls had similar fecal calprotectin measurements[78].
It was recently reported that ascitic calprotectin may be utilized (with the help of a point-of-care assay) to reliably predict an elevated polymorphonuclear count (> 250) in ascitic fluid, allowing for faster diagnosis of SBP[79]. Another study by Alempijević et al[80] focusing exclusively on HE confirmed that fecal calprotectin levels were positively correlated with HE grading according to the West-Haven grouping criteria, although it did not show a correlation with serum ammonia levels as Gundling et al[77] did. Finally, the ratio of ascites calprotectin to total protein was proposed as a better marker than ascitic fluid calprotectin alone for use in the diagnosis and prognosis of SBP. The authors report satisfactory sensitivity and specificity for this new marker, as well as a statistically significant correlation of higher values with poor 30-d survival[81].
In all, calprotectin remains a promising surrogate marker for BT in cirrhosis. It demonstrates many advantages, especially in its fecal measurement, as it is a non-invasive, quick and relatively easy to perform assay, with proven clinical value in other disease states[82].
Procalcitonin (PCT) is a 116 amino acid propeptide of calcitonin. It has been established as a valuable biomarker for the diagnosis and monitoring of bacterial infections to the point that is being used as a guide for antibiotic use[83]. Concerning advanced liver disease, PCT has been studied for the past 15 years regarding its potential for the diagnosis of SBP in decompensated patients and subsequently for its utility as an indirect marker of BT. As the liver is believed to be a key source of PCT, there were initially concerns that hepatic impairment may result in downregulated serum PCT levels. Although, this was not proven to be the case[84], and early reports were encouraging about the use of PCT in the diagnosis of SBP, its potential role remains currently unclear as several studies provided conflicting results[85-93]. A solution has been proposed with the use of an ultra-sensitive PCT assay[87], and possible explanations for the discrepancies noted between studies include higher baseline levels in patient with alcoholic[94] or specific viral-related[95] causes of cirrhosis and the presence of other bacterial infections[96]. Furthermore, it was reported that PCT levels in the ascitic fluid, but not in serum may differentiate between different cirrhotic subgroups, reflecting a possible localized role in the interplay between ascites and BT[97]. Interestingly, in contrast to calprotectin, PCT has not been found to correlate with the presence of HE, another possible important component of the BT phenotype in cirrhosis. It should be also noted, that in several of the PCT-related studies the role of CRP was assessed as well and it was usually found to have an inferior potential as a SBP marker when compared to PCT[91,98].
Finally, as part of the potential of established autoimmune and inflammatory markers of BT, a recent Hungarian study is worth mentioning. Therein, it was reported that the prevalence of anti-neutrophil cytoplasmic antibodies (ANCA) of the IgA subtype was higher in patients with cirrhosis in comparison to patients with non-cirrhotic chronic liver disease or healthy controls. Moreover, the presence of IgA ANCA was associated with the presence of ascites and advanced cirrhosis. Even more significant was the finding that, during follow up, patients with IgA ANCA had not only a higher complication risk but also IgA ANCA positivity correlated with a shorter time to the first infectious complication[99]. As IgA antibodies are linked to the gut immune system, these results may reflect a component in the pathogenesis of BT involving alterations in the local immune system and the intestinal barrier. In spite of the promise shown by these observations, further research is required to elucidate if IgA ANCA may serve as a possible marker of BT.
BT plays an important role in the pathogenesis of end stage liver disease complications. Therefore, its early and reliable detection would be significant for accurately identifying a particular subset of patients with potentially adverse prognosis, who may benefit from increased vigilance and aggressive management. Several biological molecules (bacterial DNA, soluble CD14, LPS/endotoxin, lipopolysaccharide-binding protein, calprotectin, procalcitonin) have been tested as potential biomarkers for BT, each with its own merits and flaws (Table 1). The literature available on the subject is intriguing and expanding. In all, while no single marker has emerged as optimal for the identification of BT, there is an obvious need for better designed and more focused research. These studies will not only enable us to understand the underlying mechanisms of BT, but may also allow for implementing a timely intervention in patients with liver cirrhosis. This may, in turn, alter the natural history of this ominous disease and improve its currently unfavorable prognosis.
This research has been co-financed by the European Union (European Social Fund) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework - Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund, of which Dr. Kaltsa was a recipient.
P- Reviewer: Breitkopf-Heinlein K, Dambrauskas Z, Dolle L, Mortensen C S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 2. | Deschênes M, Villeneuve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol. 1999;94:2193-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 627] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 4. | Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403-411. [PubMed] |
| 5. | Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Pérez-Paramo M, Muñoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Yang CY, Chang CS, Chen GH. Small-intestinal bacterial overgrowth in patients with liver cirrhosis, diagnosed with glucose H2 or CH4 breath tests. Scand J Gastroenterol. 1998;33:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Such J, Guardiola JV, de Juan J, Casellas JA, Pascual S, Aparicio JR, Solá-Vera J, Pérez-Mateo M. Ultrastructural characteristics of distal duodenum mucosa in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2002;14:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Bertók L. Bile acids in physico-chemical host defence. Pathophysiology. 2004;11:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutiérrez A, Carnices F, Palazón JM. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482-1486. [PubMed] |
| 14. | MacDonald TT, Pettersson S. Bacterial regulation of intestinal immune responses. Inflamm Bowel Dis. 2000;6:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Heel KA, McCauley RD, Papadimitriou JM, Hall JC. Review: Peyer’s patches. J Gastroenterol Hepatol. 1997;12:122-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1989;6:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Bolognesi M, Merkel C, Bianco S, Angeli P, Sacerdoti D, Amodio P, Gatta A. Clinical significance of the evaluation of hepatic reticuloendothelial removal capacity in patients with cirrhosis. Hepatology. 1994;19:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Panés J, Pérez-del-Pulgar S, Casadevall M, Salas A, Pizcueta P, Bosch J, Anderson DC, Granger DN, Piqué JM. Impaired mesenteric leukocyte recruitment in experimental portal hypertension in the rat. Hepatology. 1999;30:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Shah N, Dhar D, El Zahraa Mohammed F, Habtesion A, Davies NA, Jover-Cobos M, Macnaughtan J, Sharma V, Olde Damink SW, Mookerjee RP. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Baveja R, Keller S, Yokoyama Y, Sonin N, Clemens MG, Zhang JX. LPS-induced imbalanced expression of hepatic vascular stress genes in cirrhosis: possible mechanism of increased susceptibility to endotoxemia. Shock. 2002;17:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, Lee WM, Blei AT. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 252] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Guarner C, González-Navajas JM, Sánchez E, Soriando G, Francés R, Chiva M, Zapater P, Benlloch S, Muñoz C, Pascual S. The detection of bacterial DNA in blood of rats with CCl4-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006;44:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Francés R, Benlloch S, Zapater P, González JM, Lozano B, Muñoz C, Pascual S, Casellas JA, Uceda F, Palazón JM. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Francés R, Muñoz C, Zapater P, Uceda F, Gascón I, Pascual S, Pérez-Mateo M, Such J. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | El-Naggar MM, Khalil el-SA, El-Daker MA, Salama MF. Bacterial DNA and its consequences in patients with cirrhosis and culture-negative, non-neutrocytic ascites. J Med Microbiol. 2008;57:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | González-Navajas JM, Bellot P, Francés R, Zapater P, Muñoz C, García-Pagán JC, Pascual S, Pérez-Mateo M, Bosch J, Such J. Presence of bacterial-DNA in cirrhosis identifies a subgroup of patients with marked inflammatory response not related to endotoxin. J Hepatol. 2008;48:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Zapater P, Francés R, González-Navajas JM, de la Hoz MA, Moreu R, Pascual S, Monfort D, Montoliu S, Vila C, Escudero A. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology. 2008;48:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Fagan KJ, Rogers GB, Melino M, Arthur DM, Costello ME, Morrison M, Powell EE, Irvine KM. Ascites bacterial burden and immune cell profile are associated with poor clinical outcomes in the absence of overt infection. PLoS One. 2015;10:e0120642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Francés R, Zapater P, González-Navajas JM, Muñoz C, Caño R, Moreu R, Pascual S, Bellot P, Pérez-Mateo M, Such J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Mostafa MS, El-Seidi EA, Kassem AM, Shemis MA, Saber M, Michael MN. Detection of ascitic fluid infections in patients with liver cirrhosis and ascites. Arab J Gastroenterol. 2011;12:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Rincón D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, Salcedo M, Francés R, Matilla A, Catalina MV. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Sersté T, Bert F, Leflon-Guibout V, Chauvet C, Marcon E, Asselah T, Francoz C, Durand F, Lebrec D, Valla D. Detection of bacterial DNA in serum and ascitic fluid of asymptomatic outpatients with cirrhosis and non-neutrocytic ascites. Liver Int. 2011;31:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Feng Y, Chen CL, Chen TH, Liang YH, Chen HL, Lin CY, Chiu CH. Application of next-generation sequencing to study ascitic microbiome in cirrhotic patients with or without spontaneous bacterial peritonitis. J Microbiol Immunol Infect. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Fujita T. Is bacterial DNA a surrogate marker of bacterial translocation in cirrhosis? J Hepatol. 2008;49:145-146; author reply 146-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Vlachogiannakos J, Daikos G, Thalheimer U, Burroughs AK, Ladas SD. Is bacterial DNA a better marker than endotoxin of bacterial translocation in decompensated cirrhosis? Hepatology. 2011;53:2140-2141; author reply 41-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Mortensen C, Jensen JS, Hobolth L, Dam-Larsen S, Madsen BS, Andersen O, Møller S, Bendtsen F. Association of markers of bacterial translocation with immune activation in decompensated cirrhosis. Eur J Gastroenterol Hepatol. 2014;26:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Mortensen C, Karlsen S, Grønbæk H, Nielsen DT, Frevert S, Clemmesen JO, Møller S, Jensen JS, Bendtsen F. No difference in portal and hepatic venous bacterial DNA in patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt insertion. Liver Int. 2013;33:1309-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Appenrodt B, Lehmann LE, Thyssen L, Gentemann M, Rabe C, Molitor E, Trebicka J, Stüber F, Sauerbruch T. Is detection of bacterial DNA in ascitic fluid of clinical relevance? Eur J Gastroenterol Hepatol. 2010;22:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Frey EA, Miller DS, Jahr TG, Sundan A, Bazil V, Espevik T, Finlay BB, Wright SD. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665-1671. [PubMed] |
| 48. | Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 609] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 50. | Le Roy D, Di Padova F, Adachi Y, Glauser MP, Calandra T, Heumann D. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J Immunol. 2001;167:2759-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 509] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 52. | Hanck C, Rossol S, Böcker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol. 1998;33:606-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 350] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 268] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Chan CC, Hwang SJ, Lee FY, Wang SS, Chang FY, Li CP, Chu CJ, Lu RH, Lee SD. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997;32:942-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Kaser A, Ludwiczek O, Waldenberger P, Jaschke W, Vogel W, Tilg H. Endotoxin and its binding proteins in chronic liver disease: the effect of transjugular intrahepatic portosystemic shunting. Liver. 2002;22:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Stadlbauer V, Davies NA, Wright G, Jalan R. Endotoxin measures in patients’ sample: how valid are the results? J Hepatol. 2007;47:726-727; author reply 727-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Schumann RR, Kirschning CJ, Unbehaun A, Aberle HP, Knope HP, Lamping N, Ulevitch RJ, Herrmann F. The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell Biol. 1996;16:3490-3503. [PubMed] |
| 59. | Albillos A, de-la-Hera A, Alvarez-Mon M. Serum lipopolysaccharide-binding protein prediction of severe bacterial infection in cirrhotic patients with ascites. Lancet. 2004;363:1608-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Myc A, Buck J, Gonin J, Reynolds B, Hammerling U, Emanuel D. The level of lipopolysaccharide-binding protein is significantly increased in plasma in patients with the systemic inflammatory response syndrome. Clin Diagn Lab Immunol. 1997;4:113-116. [PubMed] |
| 61. | Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762-1769. [PubMed] |
| 62. | Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 63. | Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220-1230, 1230.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 64. | Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, Kirk GD, Mehta SH, Cox AL, Thomas DL. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 65. | French AL, Evans CT, Agniel DM, Cohen MH, Peters M, Landay AL, Desai SN. Microbial translocation and liver disease progression in women coinfected with HIV and hepatitis C virus. J Infect Dis. 2013;208:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Ogawa Y, Imajo K, Yoneda M, Kessoku T, Tomeno W, Shinohara Y, Kato S, Mawatari H, Nozaki Y, Fujita K. Soluble CD14 levels reflect liver inflammation in patients with nonalcoholic steatohepatitis. PLoS One. 2013;8:e65211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Campos J, Gonzalez-Quintela A, Quinteiro C, Gude F, Perez LF, Torre JA, Vidal C. The -159C/T polymorphism in the promoter region of the CD14 gene is associated with advanced liver disease and higher serum levels of acute-phase proteins in heavy drinkers. Alcohol Clin Exp Res. 2005;29:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000;356:1783-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, Dale I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 70. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 633] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 71. | Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:524-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Burri E, Beglinger C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Rev Gastroenterol Hepatol. 2014;8:197-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 73. | Sipponen T. Diagnostics and prognostics of inflammatory bowel disease with fecal neutrophil-derived biomarkers calprotectin and lactoferrin. Dig Dis. 2013;31:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Homann C, Garred P, Graudal N, Hasselqvist P, Christiansen M, Fagerhol MK, Thomsen AC. Plasma calprotectin: a new prognostic marker of survival in alcohol-induced cirrhosis. Hepatology. 1995;21:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Homann C, Christensen E, Schlichting P, Philipsen EK, Graudal NA, Garred P. Ascites fluid and plasma calprotectin concentrations in liver disease. Scand J Gastroenterol. 2003;38:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Homann C, Garred P, Graudal NA, Hasselqvist P, Christiansen M, Fagerhol MK, Thomsen AC. [Plasma calprotectin. A new prognostic marker of survival in alcoholic liver cirrhosis]. Ugeskr Laeger. 1996;158:2980-2984. [PubMed] |
| 77. | Gundling F, Schmidtler F, Hapfelmeier A, Schulte B, Schmidt T, Pehl C, Schepp W, Seidl H. Fecal calprotectin is a useful screening parameter for hepatic encephalopathy and spontaneous bacterial peritonitis in cirrhosis. Liver Int. 2011;31:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Montalto M, Gallo A, Ferrulli A, Visca D, Campobasso E, Cardone S, D’Onofrio F, Santoro L, Covino M, Mirijello A. Fecal calprotectin concentrations in alcoholic patients: a longitudinal study. Eur J Gastroenterol Hepatol. 2011;23:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Burri E, Schulte F, Muser J, Meier R, Beglinger C. Measurement of calprotectin in ascitic fluid to identify elevated polymorphonuclear cell count. World J Gastroenterol. 2013;19:2028-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Alempijević T, Štulić M, Popovic D, Culafic D, Dragasevic S, Milosavljevic T. The role of fecal calprotectin in assessment of hepatic encephalopathy in patients with liver cirrhosis. Acta Gastroenterol Belg. 2014;77:302-305. [PubMed] |
| 81. | Lutz P, Pfarr K, Nischalke HD, Krämer B, Goeser F, Glässner A, Wolter F, Kokordelis P, Nattermann J, Sauerbruch T. The ratio of calprotectin to total protein as a diagnostic and prognostic marker for spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites. Clin Chem Lab Med. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Montalto M, Gallo A, Santoro L, D’Onofrio F, Landolfi R, Gasbarrini A. Role of fecal calprotectin in gastrointestinal disorders. Eur Rev Med Pharmacol Sci. 2013;17:1569-1582. [PubMed] |
| 83. | Assink-de Jong E, de Lange DW, van Oers JA, Nijsten MW, Twisk JW, Beishuizen A. Stop Antibiotics on guidance of Procalcitonin Study (SAPS): a randomised prospective multicenter investigator-initiated trial to analyse whether daily measurements of procalcitonin versus a standard-of-care approach can safely shorten antibiotic duration in intensive care unit patients--calculated sample size: 1816 patients. BMC Infect Dis. 2013;13:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Bota DP, Van Nuffelen M, Zakariah AN, Vincent JL. Serum levels of C-reactive protein and procalcitonin in critically ill patients with cirrhosis of the liver. J Lab Clin Med. 2005;146:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Connert S, Stremmel W, Elsing C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z Gastroenterol. 2003;41:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Cai ZH, Fan CL, Zheng JF, Zhang X, Zhao WM, Li B, Li L, Dong PL, Ding HG. Measurement of serum procalcitonin levels for the early diagnosis of spontaneous bacterial peritonitis in patients with decompensated liver cirrhosis. BMC Infect Dis. 2015;15:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Marciano S, Haddad L, Martínez AP, Posadas ML, Piñero F, Mora GJ, Guerrero LN, Ridruejo E, Mandó OG, Giunta DH. Ultra-sensitive procalcitonin may help rule out bacterial infections in patients with cirrhosis. Ann Hepatol. 2014;13:541-547. [PubMed] |
| 88. | Lesińska M, Hartleb M, Gutkowski K, Nowakowska-Duława E. Procalcitonin and macrophage inflammatory protein-1 beta (MIP-1β) in serum and peritoneal fluid of patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Adv Med Sci. 2014;59:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Cekin Y, Cekin AH, Duman A, Yilmaz U, Yesil B, Yolcular BO. The role of serum procalcitonin levels in predicting ascitic fluid infection in hospitalized cirrhotic and non-cirrhotic patients. Int J Med Sci. 2013;10:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Su DH, Zhuo C, Liao K, Cheng WB, Cheng H, Zhao XF. Value of serum procalcitonin levels in predicting spontaneous bacterial peritonitis. Hepatogastroenterology. 2013;60:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 91. | Li CH, Yang RB, Pang JH, Chang SS, Lin CC, Chen CH, Chen HY, Chiu TF. Procalcitonin as a biomarker for bacterial infections in patients with liver cirrhosis in the emergency department. Acad Emerg Med. 2011;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Spahr L, Morard I, Hadengue A, Vadas L, Pugin J. Procalcitonin is not an accurate marker of spontaneous bacterial peritonitis in patients with cirrhosis. Hepatogastroenterology. 2001;48:502-505. [PubMed] |
| 93. | Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, Guyomarch S, Tardy B, Bertrand JC. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000;26:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Elefsiniotis IS, Skounakis M, Vezali E, Pantazis KD, Petrocheilou A, Pirounaki M, Papatsibas G, Kontou-Kastellanou C, Moulakakis A. Clinical significance of serum procalcitonin levels in patients with acute or chronic liver disease. Eur J Gastroenterol Hepatol. 2006;18:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Rahimkhani M, Einollahi N, Khavari Daneshvar H, Dashti N. Survey of serum procalcitonin in cirrhotic patients. Acta Med Iran. 2013;51:153-156. [PubMed] |
| 96. | Villarreal E, Vacacela K, Gordon M, Calabuig C, Alonso R, Ruiz J, Kot P, Babiloni D, Ramírez P. Usefulness of procalcitonin for diagnosing infection in critically ill patients with liver cirrhosis. Med Intensiva. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Attar BM, Moore CM, George M, Ion-Nedelcu N, Turbay R, Zachariah A, Ramadori G, Fareed J, Van Thiel DH. Procalcitonin, and cytokines document a dynamic inflammatory state in non-infected cirrhotic patients with ascites. World J Gastroenterol. 2014;20:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, de Lucca Schiavon L, Dantas-Corrêa EB. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599-607. [PubMed] |
| 99. | Papp M, Sipeki N, Vitalis Z, Tornai T, Altorjay I, Tornai I, Udvardy M, Fechner K, Jacobsen S, Teegen B. High prevalence of IgA class anti-neutrophil cytoplasmic antibodies (ANCA) is associated with increased risk of bacterial infection in patients with cirrhosis. J Hepatol. 2013;59:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |