Published online Aug 28, 2015. doi: 10.4254/wjh.v7.i18.2162
Peer-review started: May 19, 2015
First decision: June 25, 2015
Revised: July 30, 2015
Accepted: August 10, 2015
Article in press: August 11, 2015
Published online: August 28, 2015
Processing time: 105 Days and 23.7 Hours
The expanded indications of partial grafts in pediatric liver transplantation have reduced waiting list mortality. However, a higher morbidity is observed, including an increased rate of biliary complications (BCs). Factors such as the type of graft, the preservation methods applied, the donor characteristics, the type of biliary reconstruction, and the number of bile ducts in the liver graft influences the occurrence of these complications. Bile leaks and strictures comprise the majority of post-transplant BCs. Biliary strictures require a high grade of suspicion, and because most children have a bileo-enteric anastomosis, its diagnosis and management rely on percutaneous hepatic cholangiography and percutaneous biliary interventions (PBI). The success rates with PBI range from 70% to 90%. Surgery is reserved for patients who have failed PBI. BCs in children after liver transplantation have a prolonged treatment and are associated with a longer length of stay and higher hospital costs. However, with early diagnosis and aggressive treatment, patient and graft survival are not significantly compromised.
Core tip: Biliary complications in children after liver transplantation cause significant morbidity, which affect quality of life, increase hospital costs and jeopardize the liver graft if they are not treated appropriately. Diagnostic and treatment approaches to the different types of complications are highlighted, as are the technical nuances specific to pediatric recipients.
- Citation: Feier FH, da Fonseca EA, Seda-Neto J, Chapchap P. Biliary complications after pediatric liver transplantation: Risk factors, diagnosis and management. World J Hepatol 2015; 7(18): 2162-2170
- URL: https://www.wjgnet.com/1948-5182/full/v7/i18/2162.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i18.2162
Pediatric liver transplantation has expanded over the last several decades due to the utilization of partial grafts, including split and reduced grafts from deceased donors and live-donor grafts. Comparable patient and graft survival to whole liver recipients has been achieved in most centers[1]; however, the reduction in the waiting-list mortality came at a price in regard to post-transplant morbidity, which is mainly represented by the higher incidence of biliary complications (BCs) in recipients of these grafts[1].
BCs are classified into biliary strictures (BS), which can be anastomotic or intrahepatic, bile leaks (BL), bilomas, excluded ducts, stones and cast formation, among others. The overall BC incidence in transplanted children varies from 15% to 40%[2,3]. The type of graft, number of bile ducts and type of biliary reconstruction performed influence the variability in the incidence of BC. The diagnosis, especially in cases of BS, requires a high index of suspicion, and only with early diagnosis and prompt management can high success rates be expected[4]. In children, due to underlying liver disease, the majority of the biliary reconstruction is a bilioenteric (BE) anastomosis performed by a Roux-en-Y. The preferred method to access the biliary anastomosis in cases of BC is using a percutaneous transhepatic cholangiography (PTC) to perform the percutaneous biliary intervention (PBI). PTC and PBI have become the mainstay of diagnosis and treatment in liver transplanted children, with success rates between 70% and 90%[4-6].
The presentation of BC is quite variable. BL usually have a straightforward diagnosis and present early in the post-transplant course. BS has a more indolent evolution and presents later in the post-transplant follow-up. BS demands a high index of suspicion because in the initial phases the clinical picture can be confused with rejection, infection and primary disease recurrence. Early complications (< 30 d post-transplant) are a result of technical issues such as handling and harvesting of the graft, preservation injuries, surgical technique of biliary reconstruction, or even vascular insufficiency[7]. Late complications (> 90 d post-transplant) can appear from anastomotic (AS) and non-anastomotic strictures (NAS). NAS can be related to the use of ABO-incompatible grafts, preservation injury, opportunistic infections, recurrent hepatitis, ductopenic rejection, recurrent primary sclerosing cholangitis (PSC), stones or casts, post-transplant lymphoproliferative disorder or other tumors[7]. Risk factors for AS complications (leaks and strictures) are linked and include a prolonged cold ischemia time, hepatic artery thrombosis (HAT), CMV infection, and chronic rejection. Tissue hypoxia, as occurs in patients with hepatopulmonary syndrome, at level of the anastomosis can increase the rate of BCs following liver transplantation[8]. The presence of multiple bile ducts was found to be an independent risk factor for the development of BC after pediatric liver transplantation and has a higher incidence of BL compared with a single duct (21% vs 9%, respectively)[9].
BL can occur at the anastomosis, T-tube insertion, or cut surface of partial liver grafts. Their incidence after pediatric liver transplantation ranges from 2% to 15%[10,11]. They usually present early in the post-operative course and are diagnosed either by bilious secretion in the abdominal drain or by routine cholangiography in those cases where a t-tube or trans-AS stent was used[7,12]. Bilomas result from small self-limited BL that form collections. They result from the inadvertent division of bile radicals during hilar dissection or parenchymal division in partial grafts. They are diagnosed by ultrasound (US) or computed tomography (CT)[2].
An AS stricture is a segmental narrowing around the anastomosis. The incidence of AS in children varies between 2% and 35%[9,12]. The clinical presentation can be poor, but jaundice, acholic stools, fever and alteration of liver enzymes are common findings[4,12]. A high gamma-glutamyl transferase (GGT) is present in patients with BS[4,13], and an increased GGT value can be related to a greater stricture severity[14]. Usually, the diagnostic workup starts with non-invasive imaging studies, including US, cholangio CT and cholangio magnetic resonance (MR)[7]. US sensitivity is highly variable between studies and ranges from 23% to 92%[2,13,15]. In the pediatric population, US showed only mild biliary dilation in 86.6% of the patients who were diagnosed with severe strictures on PTC[14]. Potthoff et al[16] compared US with cholangiography in adults who underwent liver transplantation and found that US was able to detect a BC in 52.4% of the patients. The sensitivity of US to detect AS was 24%, with a specificity of 100%. The lack of US sensitivity may occur because early strictures do not cause the bile ducts to dilate immediately. Due to denervation of the implanted liver, it takes up to three months for the dilation to be detected by US[16].
Cholangio MR is routinely performed in adult recipients as part of the diagnostic work up of BS. It was shown that cholangio MR sensitivity and specificity can be as high as 90% for the diagnosis of BS[17]. In cases of BE anastomosis, however, the sensitivity of cholangio MR drops to 50%[18]. This is important for the pediatric population because most pediatric patients have a BE anastomosis. However, cholangio MR is helpful when studying patients with two separate anastomoses, without significant dilation on US, and with clinical suspicion of BS. It can also serve as a guide to a PTC for identifying the exact puncture location[4]. Further studies in children are necessary to define the diagnostic yield of cholangio MR for BS.
Liver biopsy can help elucidate the investigation and provide a differential diagnosis[19] (Table 1). Histology consistent with cholestasis due to biliary obstruction has been found in 69%-83% of the patients with BS[6,13].
| Histopathological features | PSC/BS | Chronic rejection | Primary biliary cirrhosis |
| Distribution, severity and composition of portal inflammation | Usually patchy to diffuse; mild neutrophilic, eosinophilic, or occasionally mononuclear predominant | Patchy; usually minimal or mild lymphoplasmacytic | Noticeably patchy and variable intensity; predominantly mononuclear; nodular aggregates and granulomas |
| Presence and type of interface activity | Prominent and defining feature: ductular type with portal and periportal edema | Minimal to absent | Important feature later in disease development: ductular and necroinflammatory-type with copper deposition |
| Bile duct inflammation and damage | Periductal lamellar edema "fibrous cholangitis"; acute cholangitis; multiple intra-portal ductal profiles | Focal ongoing lymphocytic bile duct damage; inflammation wanes with duct loss | Granulomatous or focally severe lymphocytic cholangitis is diagnostic in proper setting |
| Biliary epithelial senescence changes and small bile duct loss | Small bile duct loss associated with ductular reaction | Senescence/atrophy/atypia involve a majority of remaining ducts | Small bile duct loss associated with ductular reaction |
| Perivenular mononuclear inflammation and/or hepatocyte dropout | Absent | Usually present but variable | Variable but generally mild; if present, involves a minority of perivenular regions |
| Lobular findings and necroinflammatory activity | Disarray unusual; neutrophil clusters; ± cholestasis | Variable; if present, concentrated in perivenular regions | Mild disarray; parenchymal granulomas; periportal copper deposition and cholestasis are late features |
| Pattern of fibrosis during progression towards cirrhosis | Biliary pattern | Uncommon; if present usually a venocentric pattern; may evolve to biliary pattern | Biliary pattern |
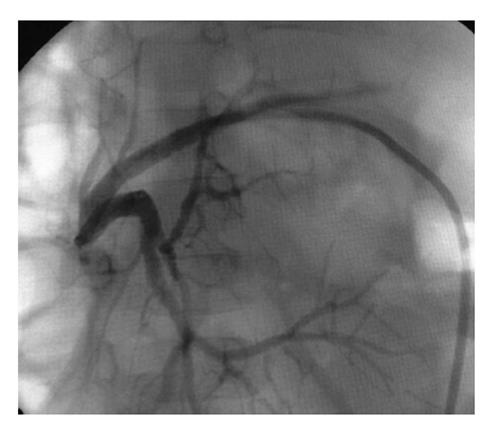
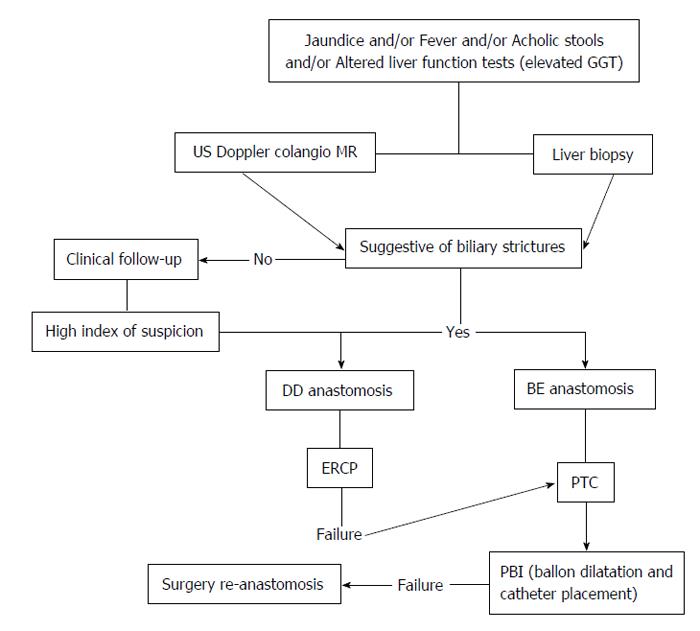
The gold standard for the diagnosis and treatment is endoscopic retrograde cholangiography (ERC) in patients with duct-to-duct (DD) anastomosis and PTC in patients with BE anastomosis (Figure 1). The diagnostic algorithm for BS is shown in Figure 2.
NAS concerns strictures located in both the extrahepatic and intrahepatic biliary systems of the liver graft[20]. The most severe forms of NAS evolve in the case of early HAT and result in partial or complete biliary necrosis. In the case of late HAT or arterial stenosis, the forms are attenuated due to collateral perfusion. Thus, the severity of NAS correlates with the time of manifestation and is most severe in the first year, whereas late HAT may be even clinically unapparent[12]. A patient with NAS has a cholestatic picture with episodes of cholangitis. More than 50% of the cases present in the first year.
NAS can also develop with an open artery, which represents a distinct entity generally referred to as ischemic-type biliary lesions (ITBL)[20]. NAS can be classified as extrahepatic (type I), intrahepatic (type II), or a combination of both. NAS can also be subclassified according to the etiology: NAS secondary to HAT; ITBL secondary to microangiopathic injury (donor factors-preservation injury, prolonged ischemia times, donor after cardiac death); or ITBL secondary to immunogenetic injury (ABO incompatibility, rejection, autoimmune hepatic disease, CMV infection and chemokine polymorphisms)[20].
The incidence of NAS varies between 5% and 25%. Recently, many centers have had an increased incidence due to the more liberal use of extended criteria donors and donors after cardiac death[12]. This is rarely the case in pediatric liver transplantation, where donor selection follows more strict criteria. The rate of NAS after living donor liver transplantation (LDLT) is low, and the cases are usually represented on PSC recurrence.
Risk factors for NAS include the use of UW-solution (with a high-viscosity), Roux-en-Y reconstruction, postoperative CMV infection and PSC as underlying liver disease, cold ischemia time > 10 h, donor age and organ quality (severe macrovascular steatosis)[12]. HAT is a direct cause of NAS because the blood supply to the biliary tree is almost solely arterial and receives no significant contribution from the portal vein in physiological conditions. However, some researchers support the hypothesis that the peribiliary vascular plexus is sustained not only by blood from the hepatic artery but also by blood from the portal vein. Because ITBLs can occur in the absence of HAT, it has been suggested that the portal blood impacts the pathogenesis of ITBL. Patients with partial portal vein thrombosis and intact arterial blood supply have developed ITBL in the segments affected by portal vein thrombosis[20].
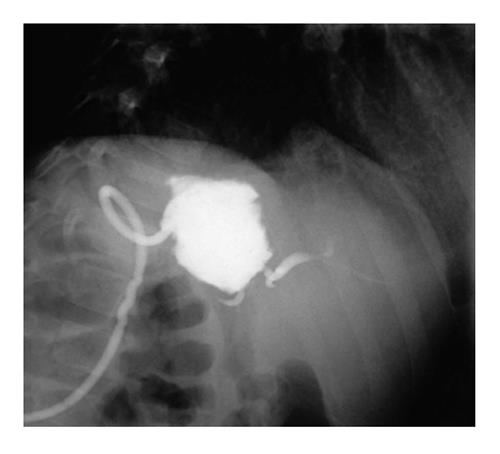
A missing duct or excluded segmental bile duct is a segmental bile duct that is discontinuous with the primary biliary drainage tree of a partial allograft (Figure 3). Its incidence after LDLT can reach 40%[21]. Signs suggestive of a missing duct are persistent bile leak, high direct hyperbilirubinemia and imaging evidence of an isolated dilated bile duct system by US, CT or MR[22]. Additionally, a missing duct should always be suspected when a patient presents signs of cholestasis but has normal stool coloration[4].
Partial grafts always have a cut surface area and occasionally present multiple bile ducts. During the liver resection procedure in live-donor, split or reduced grafts, these secondary bile ducts may be missed and ligated unintentionally. Anatomical variations in biliary drainage exist in which a biliary branch does not communicate with its segmental bile duct and thus drains into another segment[22]. Conzen et al[22] reported four cases of excluded bile ducts after pediatric liver transplantation. All of these patients had their diagnosis confirmed by PTC and initially treated by PBI with external catheter placement. Subsequently, a surgical intervention was performed to include this excluded duct in the BE anastomosis. The drainage catheter was used for guidance and remained postoperatively. All but one patient recovered well. The remaining patient developed ductopenic rejection and underwent re-transplantation[22].
Some important measures can help prevent this complication, such as an adequate preoperative evaluation of the live donor’s biliary tree with a cholangio MR or by means of an intraoperative cholangiography. Additionally, for split and reduced grafts, backtable cholangiography could help identify these secondary ducts[22].
Treatment strategies for BCs are based on the type and severity of the complication and the biliary reconstruction technique applied (DD or BE anastomosis). Non-operative management is the first-line approach, and success can be achieved in 70%-90% of all BS cases[2,4-6].
BL can be treated conservatively by maintaining the abdominal drain if the patient’s condition is stable[4]. Cut surface leaks or small caudate duct leaks usually respond to the conservative approach and close in 5-8 wk[4,7]. Anastomotic leaks, however, require additional intervention. Patients with DD anastomosis are treated with ERC, with reported success rates of 80%-90%[23]. Small leaks can be managed by sphincterotomy alone. ERC management of significant anastomotic BLs is successful approximately 50% of the time, but the remainder of cases requires surgical revision. PTC is used as a second-line therapy, i.e., as a rescue procedure, in patients with DD anastomosis. Early leaks in a clinically unstable patient demand urgent surgical revision. They are usually due to a large defect or even biliary necrosis[11]. Ultimately, most BL will require surgery[4]. Bilomas are usually self-limiting and may be treated by insertion of a percutaneous pigtail catheter. Using all the available resources, the success rates of BL treatment are approximately 85%-100%[12].
Overall 60%-90% of AS can be treated with intervention. In the long term, approximately 10%-20% of such cases require surgical revision. In most centers, surgical intervention is left as last resource for treating AS. Patients with DD anastomosis are approached with ERC with balloon dilatation with or without stent placement[12]. A successful endoscopic treatment can be achieved in 58%-76% of LDLT cases and 80%-90% of deceased donor cases[24]. The complication rate is of 6.6% per procedure, with a cumulative complication rate per patient of 21%[15]. There is less cumulative experience with ERC in children. Dechêne et al[25] reported on 17 children with BC submitted to ERC. All but one patient completed the exam, and those patients were treated with sphincterotomy, balloon dilatation or stent placement. Although the most common complication was bleeding (23.5%), only one required surgical revision. In those patients with AS, the success rate was 100%[25].
Unlike most centers, Darius et al[26] indicated surgery as the first treatment for AS. The primary patency of surgically treated patients was 80%. The other 20% had recurrences and needed a second surgical intervention. The 30-d mortality was 3.4%; 8.5% had major complications, and most required another surgical intervention[26].
The gold-standard treatment for patients with BE anastomosis is PTC with PBI. This is the mainstay of treatment for liver-transplanted children because the vast majority of these children have a BE anastomosis. With an aggressive interventional radiology team, PTC can be successful 76%-89% of the time[2]. To achieve good results, an early indication of the procedure, based on a high index of clinical suspicion, is paramount. PTC can be performed safely, even in the absence of dilated bile ducts. Most of the procedures performed in our institution are in children who are recipients of partial grafts and lack bile duct dilatation. All such procedures are performed under either US guidance or fluoroscopic control[4]. Percutaneous treatment of AS in pediatric patients is considered safe and effective, and in most cases, surgical revision of the anastomosis is not needed[6]. Contraindications to PTC are relative and include uncorrectable coagulopathy, allergy to iodinated contrast, and large volume ascites. To perform the procedure safely, the patient should have an INR ≤ 1.5, platelet count ≥ 50000/dL and a normal partial thromboplastin time[27]. Miraglia et al[6], studying pediatric liver transplant recipients with BS, achieved a trans-stricture biliary catheter placement success rate of 92%. During follow-up, 11% of their patients needed surgical revision of the anastomosis. In 75% of the patients, only one course of treatment was required, 20% required two courses and 5% required three courses of percutaneous stenting and bilioplasty. No life-threatening complications were reported[6].
Moreira et al[13] studied pediatric liver transplant recipients who underwent PTC due to clinical suspicion of BS. They found that 29.7% had normal findings, 15.6% had simultaneous intrahepatic stenosis and 54.7% had isolated AS. One course of percutaneous treatment section was needed in 65.7% of the patients, two courses were required in 20%, three courses were needed in 11.4% and more than three courses were needed in 2.8%. Thirty-four precent had a recurrence at a median 2.2 years of follow-up. At the end of follow-up, 82.6% were symptom-free. Only two patients presented with hemobilia associated with hemodynamic repercussions and were treated successfully by arterial embolization[13]. Some series reported complication rates of approximately 40%[28]. The most common potential complications from PBI included bleeding, fever, bacteremia and sepsis. Minor complications occurred in approximately 11% of cases, and major complications occurred in less than 2%[27].
For those patients who fail ERC and PTC, a novel magnetic compression anastomosis can be created. Transmural compression with two magnets causes gradual ischemic necrosis, thus creating a new anastomosis between the dilated duct and small intestine or bile duct. There are only few cases in which this technique was performed, and further experience is required before it has broader indications[29,30].
There is still no randomized controlled trial for the treatment of AS in pediatric liver transplant recipients. PBI is less invasive and has a lower complication rate. The treatment, however, is long, and the patient needs multiple re-interventions for catheter exchange. A direct surgical approach is obviously more invasive and has higher complication rates, though it does have a shorter treatment period[31]. Quality of life cannot currently be evaluated for each treatment approach based on the personal impressions of the attendants. Most centers are more likely to adopt a less invasive strategy than a more invasive strategy[32].
Treatment of NAS is multidisciplinary, and the success rates with interventional treatment are lower than those observed for anastomotic complications. The involvement of the biliary system is diffuse, and severe forms with cast formation do not respond to endoscopic treatment. Usually, ursodeoxycholic acid is used to increase bile flow and lower lithogenicity. Antibiotic therapy and prophylaxis are often necessary. The difficulty in treating this condition is expressed by the 10-year graft failure rates that can occur in 20%-50% of cases[12].
Technical variant grafts are becoming the most used types of liver grafts in pediatric recipients. However, recipients of technical variant grafts are more likely to develop any type of complication within 30 d than are whole organ recipients. In the study by Diamond et al[1], the incidence of BC within 30 d was 18.8% for split livers, 17.5% for LDLT, 16% for reduced liver grafts and 7.5% for whole liver grafts, and the BC were mostly represented by BL. As for the complications observed at 2 years of follow-up, all of the variant grafts were associated with an increased incidence of BC, and all cases presented with an increased incidence of BL. Recipients of reduced and live-donor grafts also had an increased incidence of intrahepatic BS. Recipients of live-donor grafts had a 2-fold increased incidence of AS[1]. However, no other groups have linked an increased incidence of BC with technical variant grafts and claim that the initial high rate of BC decreases after the initial learning experience[9].
The incidence of BC after pediatric LDLT ranges from 4% to 45%[10,33]. BC occur more frequently after LDLT than deceased donor transplantation and remain the most common and prolonged treatment problem after LDLT[24]. Reding et al[34] reported an incidence of BC of 34% in LDLT vs 14% in deceased donors. The incidence of strictures in LDLT is twice that of deceased donors (24% vs 12%, respectively)[34].
Some aspects of the donor surgery are particularly important, and actions toward refining this technique to lower complication rates in the recipients were taken by groups with large experience in live donation[35]. It is not unusual to have more than one bile duct in the liver graft from either right lobes or left lateral segment grafts (LLS): 40% of LLS grafts require at least 2 biliary anastomosis[9]. It is a common practice to study the donor’s biliary anatomy before electing for surgery and/or during the hepatectomy by means of a transcystic cholangiography. Intraoperative cholangiography can help identify the exact point of bile duct transection. Additionally, preoperative cholangio MR should be performed in the donors, especially in the case of left and right grafts. Cholangio MR accurately described the anatomy in 88.3% of the donors[36]. Another method, by CT cholangiography could accurately define the anatomy in 96% of the donors[37].
An aberrant biliary anatomy and the presence of two or more ducts are significant risk factors for the development of BCs[24]. Generally, donation is precluded if three or more small bile ducts are present. Care should also be taken with the caudate lobe anatomy because BL originating from the caudate lobe are not infrequent and are difficult to manage. A maximum of 5 bile ducts are encountered in the caudate lobe and careful attention should be paid to the performance of continuous suturing and ligation of these radicles[24].
Because ischemia of the bile ducts plays an important role in the development of BC, preserving the blood supply to the bile duct is an important factor to prevent these complications. In LDLT, interruption of the blood supply is thought to be the most important contributing factor for the higher incidence of BC in this population. In a partial liver graft, the blood vessels to the graft bile duct from the common bile duct side are transected. The dissection of the right or left hepatic artery in the donor operation should be restricted to what is absolutely necessary to protect the branches, and the dissection of the hilar duct should be minimal or even avoided. Following the principle of preserving the bile duct blood supply in the donor, Soin et al[35] applied a complete Glissonian approach in the donor’s hepatectomy and reduced the incidence and severity of BC in the recipients from 15.8% to 5.3%.
In the beginning of the LDLT experience, the standard biliary reconstruction procedure was the Roux-en-Y hepaticojejunostomy. It is also the preferred method for children because their bile ducts are too small or the BE anastomosis is mandated because of underlying liver disease. In adult LDLT, the routine use of a DD reconstruction is now applied because of its theoretical advantages: it preserves the sphincter mechanism, decreases the operative time and allows access through ERC[24]. However, it cannot always be performed, as in cases of PSC, biliary atresia, duct size mismatch, tension and twisting of the bile duct as in partial liver grafts.
In recent years, more pediatric groups are adopting the DD reconstruction after pediatric liver transplantation whenever it is technically possible, and some advocate that it provides better outcomes than the BE anastomosis. Tanaka et al[38] compared the results of the two techniques in 60 pediatric LDLT recipients. The overall BC incidence was 20%. Patients in the BE group had more BL (6.5% vs 0%), but patients in the DD group had more strictures (21.4% vs 8.7%). The researchers could not confirm the advantage of the DD anastomosis and noted that when a stricture developed, it was more difficult to treat if the patient had a DD anastomosis; however, ERCP was not applied to treat these patients[38]. Liu et al[11] also recognized problems when performing a DD anastomosis: three patients had to be converted to a BE anastomosis because of kinking and tension. In a smaller cohort, Shirouzu et al[3] showed no difference in the incidence of complications between the techniques. Sakamoto et al[33] reported on 19 pediatric recipients with a DD anastomosis and observed an incidence of BC of 47.4%. They argued that when the biliary stent was routinely placed, the incidence of complications dropped; thus, they recommended the use of stents when performing a DD anastomosis[33].
To date, there is no high-impact study that has proved the superiority of a DD anastomosis in children. Because endoscopic biliary intervention is not widely performed in younger children because of its technical difficulty, the choice between a BE or DD anastomosis should not be driven by the performance of ERC in children. A common problem encountered with the DD in partial liver grafts is the kinking of the anastomosis due to tension during the liver transplant or during follow-up because of the regeneration process. The BE anastomosis may be less susceptible to kinking because the intestinal loop has more mobility[38]. Generally, in patients with a preserved bile duct, such as those with metabolic diseases, liver tumors, cryptogenic cirrhosis an attempt could be made to perform a DD anastomosis. Bile duct size mismatch, tension and kinking of the anastomosis may preclude the performance of a DD, especially in partial liver grafts, because it increases the risk of a bile duct stricture.
Another regularly raised issue is the use of anastomotic stents. The rationale for a stent is the maintenance of the biliary flow despite swelling of the anastomosis as well as easy access for control cholangiography in case of a suspected leak or stricture. However, the stent itself is a foreign body and can induce inflammation and subsequent stricture[24]. Several groups with experience in pediatric liver transplantation have a great variety of techniques for reconstructing the biliary duct[3-5,10,11,26,38-41] (Table 2). It would take a large prospective controlled study to define the best technique for biliary reconstruction.
| Ref. | N | Type of graft | BE/DD | Suture technique | Stent | BC | BS | BL |
| Okajima et al[38] | 6 | LDLT | 0/6 | Interrupted | Yes | 16.6% | 16.6% | 0 |
| Sakamoto et al[12] | 19 | LDLT | 0/19 | Continuous and interrupted | Yes, but not routine | 47.4% | 36.8% | 10.5% |
| Shirouzu et al[3] | 30 | LDLT | 20/10 | Interrupted | Yes | 6.6% | 3.3% | 3.3% |
| Liu et al[10] | 7 | LDLT | 3/4 | Interrupted | No | 14.2% | 0 | 14.2% |
| Anderson et al[5] | 66 | Whole, split and reduced | 51/15 | Continuous and interrupted | No | 26% | 23% | 3% |
| Tanaka et al[37] | 60 | LDLT | 46/14 | Continuous and interrupted/only interrupted | Yes/No | 20% | 11.7% | 5% |
| Haberal et al[39] | 31 | LDLT | 0/31 | - | No | 15.6% | 9.3% | 6.2% |
| Ando et al[9] | 49 | LDLT | 47/2 | Interrupted, wide interval | Yes | 4% | 2% | 2% |
| Chok et al[40] | 78 | LDLT | 74/4 | Continuous posterior/interrupted anterior | No | 16.7% | ||
| Feier et al[4] | 489 | LDLT | - | Continuous and interrupted | No | 14.5% | 9.2% | 6.7% |
| Darius et al[30] | 429 | Whole, split, reduced and LDLT | 395/24 | Interrupted | No | 23% | 13.2% | 3.0% |
Microsurgical techniques are emerging as an alternative to lower the incidence of BC in LDLT. Lin et al[42] started applying microsurgical techniques to biliary reconstruction in LDLT. Their first report showed comparable results between the conventional and microsurgical groups, with overall complication rates of 18.8% and 15.3%, respectively. However, after stratifying the cases and excluding the learning curve, the results with the microsurgical technique improved with an overall incidence of BC of 5.4%[42]. Their long-term results were published later, and the incidence of BC was 9.6%. Their sample was constituted mainly of adult patients, right lobe grafts and single duct openings[43]. Further experience with this type of reconstruction in children with a BE anastomosis could help define the role of microsurgery in this subgroup of patients.
Despite difficult diagnosis and prolonged treatment, BS have a high rate of resolution with non-operative management. BL will ultimately require surgical treatment unless it is caused by a cut surface leak. No significant difference was observed regarding patient or graft survival in the different series[2,4,26]. However, the presence of BC, particularly BL increases the length of stay and hospital costs[2,44]. Technical refinement, especially in technical variant grafts, might be the key to lowering the incidence of BC in pediatric liver transplant recipients.
P- Reviewer: Hill A, Junge N S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, Song C; SPLIT Research Group. Impact of graft type on outcome in pediatric liver transplantation: a report From Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg. 2007;246:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Kling K, Lau H, Colombani P. Biliary complications of living related pediatric liver transplant patients. Pediatr Transplant. 2004;8:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Shirouzu Y, Okajima H, Ogata S, Ohya Y, Tsukamoto Y, Yamamoto H, Takeichi T, Kwang-Jong L, Asonuma K, Inomata Y. Biliary reconstruction for infantile living donor liver transplantation: Roux-en-Y hepaticojejunostomy or duct-to-duct choledochocholedochostomy? Liver Transpl. 2008;14:1761-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Feier FH, Chapchap P, Pugliese R, da Fonseca EA, Carnevale FC, Moreira AM, Zurstrassen C, Santos AC, Miura IK, Baggio V. Diagnosis and management of biliary complications in pediatric living donor liver transplant recipients. Liver Transpl. 2014;20:882-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Anderson CD, Turmelle YP, Darcy M, Shepherd RW, Weymann A, Nadler M, Guelker S, Chapman WC, Lowell JA. Biliary strictures in pediatric liver transplant recipients - early diagnosis and treatment results in excellent graft outcomes. Pediatr Transplant. 2010;14:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Miraglia R, Maruzzelli L, Caruso S, Riva S, Spada M, Luca A, Gridelli B. Percutaneous management of biliary strictures after pediatric liver transplantation. Cardiovasc Intervent Radiol. 2008;31:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Ostroff JW. Management of biliary complications in the liver transplant patient. Gastroenterol Hepatol (N Y). 2010;6:264-272. [PubMed] |
| 8. | Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, Pomier-Layrargues G. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Salvalaggio PR, Whitington PF, Alonso EM, Superina RA. Presence of multiple bile ducts in the liver graft increases the incidence of biliary complications in pediatric liver transplantation. Liver Transpl. 2005;11:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ando H, Kaneko K, Ono Y, Tainaka T, Kawai Y. Biliary reconstruction with wide-interval interrupted suture to prevent biliary complications in pediatric living-donor liver transplantation. J Hepatobiliary Pancreat Sci. 2011;18:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Liu C, Loong CC, Hsia CY, Peng CK, Tsai HL, Tsou MY, Wei C. Duct-to-duct biliary reconstruction in selected cases in pediatric living-donor left-lobe liver transplantation. Pediatr Transplant. 2009;13:693-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 13. | Moreira AM, Carnevale FC, Tannuri U, Suzuki L, Gibelli N, Maksoud JG, Cerri GG. Long-term results of percutaneous bilioenteric anastomotic stricture treatment in liver-transplanted children. Cardiovasc Intervent Radiol. 2010;33:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Teplisky D, Urueña Tincani E, Halac E, Garriga M, Cervio G, Imventarza O, Sierre S. Ultrasonography, laboratory, and cholangiography correlation of biliary complications in pediatric liver transplantation. Pediatr Transplant. 2015;19:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Holt AP, Thorburn D, Mirza D, Gunson B, Wong T, Haydon G. A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation. 2007;84:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Potthoff A, Hahn A, Kubicka S, Schneider A, Wedemeyer J, Klempnauer J, Manns M, Gebel M, Boozari B. Diagnostic value of ultrasound in detection of biliary tract complications after liver transplantation. Hepat Mon. 2013;13:e6003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Katz LH, Benjaminov O, Belinki A, Geler A, Braun M, Knizhnik M, Aizner S, Shaharabani E, Sulkes J, Shabtai E. Magnetic resonance cholangiopancreatography for the accurate diagnosis of biliary complications after liver transplantation: comparison with endoscopic retrograde cholangiography and percutaneous transhepatic cholangiography - long-term follow-up. Clin Transplant. 2010;24:E163-E169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kinner S, Dechêne A, Paul A, Umutlu L, Ladd SC, de Dechêne EM, Zöpf T, Gerken G, Lauenstein TC. Detection of biliary stenoses in patients after liver transplantation: is there a different diagnostic accuracy of MRCP depending on the type of biliary anastomosis? Eur J Radiol. 2011;80:e20-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, Daskal I, El-Monayeri MS, Fontes P, Fung J. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant. 2012;2012:164329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Huang TL, Cheng YF, Chen CL, Chen TY, Lee TY. Variants of the bile ducts: clinical application in the potential donor of living-related hepatic transplantation. Transplant Proc. 1996;28:1669-1670. [PubMed] |
| 22. | Conzen KD, Lowell JA, Chapman WC, Darcy M, Duncan JR, Nadler M, Turmelle YP, Shepherd RW, Anderson CD. Management of excluded bile ducts in paediatric orthotopic liver transplant recipients of technical variant allografts. HPB (Oxford). 2011;13:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Dechêne A, Kodde C, Kathemann S, Treckmann J, Lainka E, Paul A, Gerken G, Feldstein AE, Hoyer PF, Canbay A. Endoscopic treatment of pediatric post-transplant biliary complications is safe and effective. Dig Endosc. 2015;27:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Darius T, Rivera J, Fusaro F, Lai Q, de Magnée C, Bourdeaux C, Janssen M, Clapuyt P, Reding R. Risk factors and surgical management of anastomotic biliary complications after pediatric liver transplantation. Liver Transpl. 2014;20:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Racadio JM, Kukreja K. Pediatric biliary interventions. Tech Vasc Interv Radiol. 2010;13:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Migliazza L, López Santamaría M, Murcia J, Gamez M, Clavijo J, Camarena C, Hierro L, Frauca E, de la Vega A, Diaz M. Long-term survival expectancy after liver transplantation in children. J Pediatr Surg. 2000;35:5-7; discussion 7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Marubashi S, Nagano H, Yamanouchi E, Kobayashi S, Eguchi H, Takeda Y, Tanemura M, Maeda N, Tomoda K, Hikita H. Salvage cystic duct anastomosis using a magnetic compression technique for incomplete bile duct reconstruction in living donor liver transplantation. Liver Transpl. 2010;16:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Muraoka N, Uematsu H, Yamanouchi E, Kinoshita K, Takeda T, Ihara N, Matsunami H, Itoh H. Yamanouchi magnetic compression anastomosis for bilioenteric anastomotic stricture after living-donor liver transplantation. J Vasc Interv Radiol. 2005;16:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Darius T, Reding R. Therapeutic strategy for anastomotic biliary strictures after pediatric liver transplantation: two radically different approaches. Liver Transpl. 2014;20:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Seda Neto J, Chapchap P. When is surgery required for the treatment of biliary complications after pediatric liver transplantation? Liver Transpl. 2014;20:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Sakamoto S, Egawa H, Ogawa K, Ogura Y, Oike F, Ueda M, Yazumi S, Shibata T, Takada Y, Uemoto S. The technical pitfalls of duct-to-duct biliary reconstruction in pediatric living-donor left-lobe liver transplantation: the impact of stent placement. Pediatr Transplant. 2008;12:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Reding R, de Goyet Jde V, Delbeke I, Sokal E, Jamart J, Janssen M, Otte JB. Pediatric liver transplantation with cadaveric or living related donors: comparative results in 90 elective recipients of primary grafts. J Pediatr. 1999;134:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Soin AS, Kumaran V, Rastogi AN, Mohanka R, Mehta N, Saigal S, Saraf N, Mohan N, Nundy S. Evolution of a reliable biliary reconstructive technique in 400 consecutive living donor liver transplants. J Am Coll Surg. 2010;211:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Song GW, Lee SG, Hwang S, Sung GB, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY, Kim BS. Preoperative evaluation of biliary anatomy of donor in living donor liver transplantation by conventional nonenhanced magnetic resonance cholangiography. Transpl Int. 2007;20:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Wang ZJ, Yeh BM, Roberts JP, Breiman RS, Qayyum A, Coakley FV. Living donor candidates for right hepatic lobe transplantation: evaluation at CT cholangiography--initial experience. Radiology. 2005;235:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Tanaka H, Fukuda A, Shigeta T, Kuroda T, Kimura T, Sakamoto S, Kasahara M. Biliary reconstruction in pediatric live donor liver transplantation: duct-to-duct or Roux-en-Y hepaticojejunostomy. J Pediatr Surg. 2010;45:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Okajima H, Inomata Y, Asonuma K, Ueno M, Ishiko T, Takeichi T, Kodera A, Yoshimoto K, Ohya Y. Duct-to-duct biliary reconstruction in pediatric living donor liver transplantation. Pediatr Transplant. 2005;9:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Haberal M, Karakayali H, Atiq A, Sevmis S, Moray G, Ozcay F, Boyvat F. Duct-to-duct biliary reconstruction without a stent in pediatric living-donor liver transplantation. Transplant Proc. 2011;43:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Chok KS, Chan SC, Chan KL, Sharr WW, Tam PK, Fan ST, Lo CM. Bile duct anastomotic stricture after pediatric living donor liver transplantation. J Pediatr Surg. 2012;47:1399-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Lin TS, Concejero AM, Chen CL, Chiang YC, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Jawan B. Routine microsurgical biliary reconstruction decreases early anastomotic complications in living donor liver transplantation. Liver Transpl. 2009;15:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Lin TS, Chen CL, Concejero AM, Yap AQ, Lin YH, Liu CY, Chiang YC, Wang CC, Wang SH, Lin CC. Early and long-term results of routine microsurgical biliary reconstruction in living donor liver transplantation. Liver Transpl. 2013;19:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Englesbe MJ, Dimick J, Mathur A, Ads Y, Welling TH, Pelletier SJ, Heidt DG, Magee JC, Sung RS, Punch JD. Who pays for biliary complications following liver transplant? A business case for quality improvement. Am J Transplant. 2006;6:2978-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |