Published online Aug 28, 2015. doi: 10.4254/wjh.v7.i18.2147
Peer-review started: April 30, 2015
First decision: July 25, 2015
Revised: August 10, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: August 28, 2015
Processing time: 124 Days and 7.4 Hours
Hepatocellular carcinoma (HCC) is best treated by liver transplantation, but the applicability of transplantation is greatly limited. Tumor resection in partial hepatectomy is hence resorted to. However, in most parts of the world, only 20%-30% of HCCs are resectable. The main reason for such a low resectability is a future liver remnant too small to be sufficient for the patient. To allow more HCC patients to undergo curative hepatectomy, a variety of ways have been developed to increase the resectability of HCC, mainly ways to increase the future liver remnants in patients through hypertrophy. They include portal vein embolization, sequential transarterial chemoembolization and portal vein embolization, staged hepatectomy, two-staged hepatectomy with portal vein ligation, and Associating Liver Partition and Portal Vein Ligation in Staged Hepatectomy. Herein we review, describe and evaluate these different ways, ways that can be life-saving.
Core tip: There are different ways to increase the resectability of hepatocellular carcinoma by increasing the volume of the future liver remnant (FLR) through hypertrophy. Portal vein embolization features the the embolization of the ipsilateral side of the portal vein which supplies the liver lobe harboring the tumor, either in an open or percutaneous manner. Sequential transarterial chemoembolization and portal vein embolization is a way to augment the effect of portal vein embolization and prevent tumor progression. Staged hepatectomy is mainly for liver tumors with bilobar involvement and colorectal liver metastasis and is often aided by effective adjuvant chemotherapy. Its aim is to strike a balance between complete tumor removal and preservation of the FLR. Two-staged hepatectomy with portal vein ligation is also mainly for liver tumors with bilobar involvement and colorectal liver metastasis. In the first-stage operation, tumor in the liver portion which is designated as the FLR is cleared, and portal vein ligation is performed. The liver parenchyma is transected only in the second-stage operation. Associating Liver Partition and Portal Vein Ligation in Staged Hepatectomy is used to speed up hypertrophy in the hope that the FLR will grow large enough for a safe hepatectomy before tumor progression occurs. It features right portal vein ligation and in-situ splitting of the intended transection surface down to the inferior vena cava. In the first-stage operation, the anterior approach is encouraged and the Pringle maneuver is discouraged, and the hilar plate is left untouched.
- Citation: She WH, Chok KS. Strategies to increase the resectability of hepatocellular carcinoma. World J Hepatol 2015; 7(18): 2147-2154
- URL: https://www.wjgnet.com/1948-5182/full/v7/i18/2147.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i18.2147
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the most common primary liver malignancy[1,2]. Most cases of HCC in Asia are related to hepatitis B, which is prevalent in the region[3]. The best treatment for HCC is liver transplant because it removes both the tumor and the diseased liver, and a 5-year post-transplant survival rate of > 70% is expected[4-7]. Unfortunately, its applicability is limited by the shortage of liver grafts[8]. Moreover, only patients who have HCC within selection criteria (e.g., the Milan criteria[9], the University of California, San Francisco criteria[10] are eligible for liver transplant. A study reported that for patients with HCC within the Milan criteria, the 5-year survival rate was 81% with living donor liver transplant and 72.8% with partial hepatectomy[11]. In the face of perpetual liver graft shortage, hepatectomy remains an important curative measure as it can achieve a satisfactory survival outcome.
Hepatectomy has been evolving and is getting more technically challenging as surgeons are pushing limits. They are trying to operate on HCCs larger and larger and with more and more nodules, but a R0 resection is always the ultimate goal. The applicability of hepatectomy is often limited by an inadequate future liver remnant (FLR) or a marginal liver, especially in patients with underlying hepatitis or cirrhosis. The success of hepatectomy depends on many factors, which include status of the tumor, the patient’s clinical status and underlying liver function, and the size of the FLR of the patient[12-14]. Aggressive hepatectomy may still be beneficial for patients who have advanced HCC with large or multiple tumors or intrahepatic venous invasion if they are properly selected[15]. Curative hepatectomy is the first-line treatment for HCC at many centers. The resectability of HCC often rises with the volume of the FLR, and therefore different measures are employed for increasing such volume. Moreover, a larger FLR would probably mean better overall and disease-free survival.
In the management of HCC, liver resection for tumor clearance is the first-line curative treatment for patients with preserved liver function[11,14,16]. Major hepatectomy can be performed safely nowadays with careful patient selection[12,14], better understanding of the liver anatomy[17], improvement of surgical techniques, and advances of surgical instruments. Widely adopted techniques include the hanging maneuver[18], the anterior approach for avoidance of mobilization and rupture of large tumors[19,20], the Pringle maneuver[21], and meticulous control of central venous pressure for reduction of reduce blood loss[22]. Widely employed instruments include Cavitron Ultrasound Surgical Aspirator, hydrojet[23,24], the Harmonic scalpel, LigaSure, Harmonic Ace, and Thunderbeat[25]. Although complications and perioperative mortalities still occur, the rates are acceptable[13,14,26,27]. However, major hepatectomy may not be suitable for patients with marginal liver function or a relatively small FLR. The University of Hong Kong uses indocynaine green clearance test as an important tool to assess their patients’ preoperative liver function[28]. Unfortunately, there is no perfect test for the prediction of postoperative mortality[29,30]. For risk stratification for major hepatectomy, usually a combination of assessment modalities is adopted, which usually includes measurement of the disease’s Child-Pugh grading, indocynaine green clearance test, renal function test by creatinine level check, and platelet count.
Location of tumors is a decisive factor in surgical planning. The amount of liver removed in hepatectomy decides the volume of the liver remnant. Major hepatectomy can only be offered to patients with an adequate FLR and adequate post-resection liver function. To avoid massive bleeding and vascular insult to the liver, preservation or reconstruction of major hepatic veins in addition to meticulous surgical skills is needed[29]. A patient’s liver volume can be measured by tracing the liver contour in the cross-sectional image on computed tomography volumetry[31], and a patient’s standard liver volume can be derived from his weight and height with different formulae[32,33]. The volume of his FLR can then be calculated. Patients with cirrhosis have relatively poor liver function, and thus need a larger FLR[34-37] to lower the risk of liver failure. At The University of Hong Kong, for patients who have Child-Pugh A cirrhosis and an indocynaine green retention rate ≤ 20% at 15 min, an FLR > 30% of the estimated standard liver volume is preferred for right hepatectomy, and an FLR > 35% of the estimated standard liver volume is preferred for extended right hepatectomy or right trisectionectomy. Patients who have cirrhosis and an inadequate FLR have a high risk of post-hepatectomy liver failure[37,38].
Different types of injury (e.g., ischemia/reperfusion, resection) will induce a hypertrophic response called the atrophy-hypertrophy complex in a liver remnant. Hypertrophy is simultaneously caused by increased endothelial shear stress, hepatocellular swelling, and activated growth factors/cytokines due to increased portal flow[39]. The idea of portal vein embolization (PVE) is to occlude a liver segment or lobule so as to bring about its ischemia[40,41] and consequent atrophy, thereby inducing hypertrophy of the part of liver not atrophied.
PVE is indicated for patients who are considered for right or extended right hepatectomy but with a relatively small FLR. By PVE, the size of an FLR can be increased. PVE features the embolization of the ipsilateral side of the portal vein which supplies the liver lobe harboring the tumor, either in an open or percutaneous manner, thereby inducing hypertrophy of the FLR[42,43]. To date, there is still no straight value on the minimum volume of an FLR which allows major hepatectomy to be performed safely. An FLR > 35% of the estimated standard liver volume has been recommended for patients with cirrhosis, steatosis, or chronic hepatitis[28,36,37,44-48]. PVE is rarely required before extended left hepatectomy or left trisectionectomy, since the right posterior section usually constitutes about 30% of the total liver volume[49,50]. The technique for embolizing the segment-4 portal vein is crucial; if the vein is not properly blocked, suboptimal hypertrophy may results.
The FLR volume will be reassessed 4-8 wk after PVE[51,52]. Rapid growth of the FLR is anticipated in the first 3-4 wk. Generally, an 8%-30% enlargement over 2-6 wk is expected[43,52-55]. Hypertrophy is usually slower in the presence of cirrhosis[56]. Studies comparing major hepatectomy with and without preceding PVE reported that comparable and even superior long-term outcomes were achieved with PVE[45,57-63]. With PVE, patients who would have been considered inoperable in the past because of their small FLR have the option of hepatectomy with reasonable long-term surgical outcomes.
PVE can be performed in an open or percutaneous manner. Open right portal vein ligation often renders the subsequent surgery difficult due to vascular or fibrotic adhesions around the hilar structure. Open transileocolic PVE is performed with cannulation of the ileocolic vein in addition to embolization of the right portal vein in an antegrade manner or percutaneous portal vein cannulation and retrograde embolization. Ipsilateral percutaneous PVE is generally preferred because of the low invasiveness and an easier access to segment-4 portal vein branches[64-66]. Different ways of PVE all carry a risk of complication, such as main portal vein thrombosis. Prompt surgical intervention or anticoagulation is needed if the embolic agent crosses the contralateral side of the portal vein, which would cause liver failure in the case of bilateral PVE, resulting in death[67]. Hemorrhage or catastrophic bleeding at the puncture site may also occur, which also requires prompt surgical intervention. In addition, PVE induces inflammatory response near the hilar structure, which may increase the difficulty in dissection in the subsequent hepatectomy and raise the surgical risk.
PVE can be given to HCC patients with underlying cirrhosis, but hepatic regeneration and thus hypertrophy of the FLR would be impaired in the presence of cirrhosis[68-70]. On the other hand, it is likely that the arterial flow increases compensatorily in segments with PVE, thereby stimulating tumor progression as HCC is a hypervascular tumor supplied by the hepatic artery blood flow[71-73]. To augment the effect of PVE and prevent tumor progression, the treatment sequential transarterial chemoembolization and PVE is used[57]. Studies comparing patients who received this treatment and patients who did not found that patients who did showed a higer rate of hypertrophy of FLR and a bigger increase in their FLR[57,58], and the rate of tumor progression was lower as tumor necrosis was evident[74]. This treatment is not without risk; it could cause ischemic parenchymal damage[75], but overall, it is feasible and safe, and it allows HCC patients who would otherwise be denied hepatectomy to undergo curative resection with reasonable postoperative 5-year overall and disease-free survival[57,58,76].
Staged hepatectomy is mainly for HCC with bilobar involvement and colorectal liver metastasis, and is often aided by effective adjuvant chemotherapy[77-79]. In staged hepatectomy, two or more planned hepatectomies are performed at different time to achieve a R0 resection. It is distinguished from unplanned repeat hepatectomies for recurrent disease[80]. Its aim is to strike a balance between complete tumor removal and preservation of the FLR. The chance of postoperative liver failure can be reduced if bilobar tumors are removed in a staged manner. The preserved portion of the liver should be relatively spared by the disease with sufficient FLR and adequate vascular inflow and outflow[81]. However, there is always the chance that the tumor tissue is cut across during the first-stage procedure, resulting in tumor spillage and peritoneal metastasis, and rendering the planned second-stage procedure unfeasible. Besides, tumors may grow despite temporary chemotherapy during the hepatic regenerative period, which may also preclude further operation. Repeat resection is technically demanding, as not only all the dissection planes have already been disturbed, adhesiolysis can also be very difficult. Adhesiolysis near the liver hilum and the inferior vena cava is particularly challenging, as massive bleeding may occur. Staged hepatectomy for HCC was not common[79]; it was mostly for colorectal liver metastasis[78,82-85].
This treatment requires two laparotomies and is also mainly for HCC with bilobar involvement and colorectal liver metastasis. In the first laparotomy, tumor in the liver portion which is designated as the FLR is cleared, and portal vein ligation is performed. Other required resection such as that of colorectal primary tumor is also done in the first laparotomy. The liver parenchyma is transected only in the second laparotomy but not in the first. The portal vein ligation is to induce hypertrophy of the FLR, allowing hepatectomy in the second-stage procedure and decreasing the risk of postoperative liver failure. Portal vein ligation has been found to be as effective as PVE[86]. However, open portal vein ligation poses the risk of adhesion formation over the hilum, which may increase the difficulty of dissection in the second-stage operation.
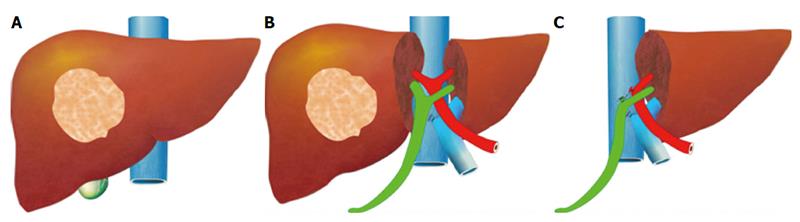
For hepatectomy, one of the limiting factors is inadequate volume of the FLR. Although the aforesaid methods are effective in inducing hypertrophy of the FLR, it takes several weeks for it to reach a satisfactory volume[43]. Tumor progression may occur before the FLR is large enough for the planned hepatectomy to be conducted. If a major vessel such as the ipsilateral portal vein is invaded by tumor, the tumor will progress in terms of days and contralateral deposition and metastasis of the tumor will occur, rendering the tumor inoperable[72,73,87]. Associating Liver Partition and Portal Vein Ligation in Staged Hepatectomy (ALPPS) is one of the main surgical innovations in recent years. The procedure, which was invented by chance, was initially carried out by Dr. Hans Schlitt from Germany in an intended extended right hepatectomy for hilar cholangiocarcinoma[88]. In the surgery, palliative left hepaticojejunostomy was performed because the FLR was small, with division of the liver parenchyma along the falciform ligament and ligation of the right portal vein. On day 8 after the surgery, computed tomography was performed. To Dr. Schlitt’s surprise, the left lateral section had grown enormously in size. The diseased portion of the liver was subsequently removed in another surgery. This novel technique was later termed “ALPPS”[89]. The idea of ALPPS is to speed up hypertrophy of the FLR (the left lobe or the left lateral section) by right portal vein ligation and in-situ splitting of the intended transection surface down to the inferior vena cava (Figure 1). Generally, the FLR regenerates to a volume adequate for a safe hepatectomy in days.
ALPPS was initially applied to relatively normal livers, such as in the case of colorectal liver metastasis. Later it was also applied to livers with steatosis or cirrhosis[88,90-94]. A 70% increase in FLR volume has been reported[95]. ALPPS is better than conventional PVE when the rate and the percentage of hypertrophy are concerned[96,97]. The shorter the interval between the two operations is, the less mature the adhesions would be, and hence the second operation would also be easier.
Most of the reported cases of ALPPS are on non-cirrhotic livers, and there has not been any report on the rate of hypertrophy in cirrhotic livers. However, one would anticipate that some patients would not have adequate hypertrophy of the contralateral side and hence the second stage operation is not possible. ALPPS carries certain risks. The right hepatic artery could be injured, and liver failure could occur after right portal vein ligation. The Pringle maneuver would pose a further risk of liver injury and is thus not recommended. In the first-stage operation, adoption of the anterior approach allows liver transection without mobilization of the right lobe, thereby minimizing adhesion formation[98], and the hilar plate is left untouched so as to minimize the chance of biliary complication. Bile leakage from the transection surface can result in biloma and increases the chance of infection and thus the risk of sepsis, which may forbid the second-stage operation. ALPPS is very technically challenging and demanding, and therefore should not be carried out by inexperienced surgeons.
ALPPS should be carried out with a curative intent. It is indicated for patients who have a large tumor load and a marginal FLR[96], even with tumor invasion of major vessels, such as the portal vein[92]. ALPPS renders some inoperable tumors potentially operable.
Complication and mortality are inevitable with any surgery; ALPPS is no exception. Perioperative mortality rates of 12%-28% have been reported, which are overall higher than those of conventional major hepatectomy[95,96,99,100]. A complication rate high at 50% has been recorded[99,101]. Complications include ascites, bile leakage, persisting cholestasis and sepsis, wound infection, and other inflammatory and infective complications. ALPPS increases operability at the price of a heightened morbidity and mortality. Keeping morbidity and mortality at the minimum requires careful patient selection, meticulous surgical technique, and accurate decision as to proceeding to the second-stage operation or not.
The long-term outcome of ALPPS is still unknown. Long-term overall survival and disease-free survival are still pending. Further studies as well as input from different centers are required but not yet available. However, ALPPS has improved the operative rate, and it is hoped that it will improve the overall and disease-free survival of patients. Nonetheless, larger trials are needed to document its efficacy especially for those patient with HCC and background cirrhosis.
There are revolutional changes of surgical methods to increase the resectability of HCCs, and various ways to increase the volume of the FLR of patients considered for major hepatectomy have been developed. Improvement in surgical techniques also allows patients to benefit from surgical resection with safety. Treatment modalities are always evolving for the better. Hopefully, ALPPS will continue to develop and long-term results will be available in the near future.
P- Reviewer: Peng B, Smith RC S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3280] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 2. | Kim do Y, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11827] [Article Influence: 844.8] [Reference Citation Analysis (4)] |
| 4. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1566] [Article Influence: 92.1] [Reference Citation Analysis (1)] |
| 5. | Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol. 2010;101:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, Ogawa K, Sakamoto S, Ogura Y, Egawa H. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Tamura S, Sugawara Y, Kokudo N. Living donor liver transplantation for hepatocellular carcinoma: the Japanese experience. Oncology. 2011;81 Suppl 1:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Lo CM, Fan ST, Liu CL, Chan SC, Wong J. The role and limitation of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2004;10:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5297] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 10. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1691] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 11. | Fan ST, Poon RT, Yeung C, Lam CM, Lo CM, Yuen WK, Ng KK, Liu CL, Chan SC. Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg. 2011;98:1292-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 15. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 542] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 16. | Cauchy F, Soubrane O, Belghiti J. Liver resection for HCC: patient’s selection and controversial scenarios. Best Pract Res Clin Gastroenterol. 2014;28:881-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg. 1999;16:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Fan ST, Liu CL. Anterior approach for major right hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704-711; discussion 711-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 334] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 367] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 23. | Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814-22, discussion 822-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, Ijichi M, Hasegawa K. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Milsom J, Trencheva K, Monette S, Pavoor R, Shukla P, Ma J, Sonoda T. Evaluation of the safety, efficacy, and versatility of a new surgical energy device (THUNDERBEAT) in comparison with Harmonic ACE, LigaSure V, and EnSeal devices in a porcine model. J Laparoendosc Adv Surg Tech A. 2012;22:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 211] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 558] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Manizate F, Hiotis SP, Labow D, Roayaie S, Schwartz M. Liver functional reserve estimation: state of the art and relevance for local treatments: the Western perspective. J Hepatobiliary Pancreat Sci. 2010;17:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 313] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Chan SC, Lo CM, Chok KS, Sharr WW, Cheung TT, Tsang SH, Chan AC, Fan ST. Validation of graft and standard liver size predictions in right liver living donor liver transplantation. Hepatol Int. 2011;5:913-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 703] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 34. | Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, Tuorto S, Blumgart LH, Fong Y. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 39. | Kim RD, Kim JS, Watanabe G, Mohuczy D, Behrns KE. Liver regeneration and the atrophy-hypertrophy complex. Semin Intervent Radiol. 2008;25:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Lemasters JJ, Ji S, Thurman RG. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981;213:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 42. | Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 310] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 44. | Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 45. | Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, Curley SA, Abdalla EK, Vauthey JN. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: Perioperative outcome and survival. Surgery. 2009;145:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Ribero D, Curley SA, Imamura H, Madoff DC, Nagorney DM, Ng KK, Donadon M, Vilgrain V, Torzilli G, Roh M. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol. 2008;15:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Mullin EJ, Metcalfe MS, Maddern GJ. How much liver resection is too much? Am J Surg. 2005;190:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Yanaga K, Honda H, Ikeda Y, Nishizaki AT, Yamamoto K, Sugimachi K. Significance of liver size in hepatic surgery. HPB Surg. 1997;10:195-199; discussion 199-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Abdalla EK, Denys A, Chevalier P, Nemr RA, Vauthey JN. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Kutsuna Y, Hayakawa N, Yamamoto H. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Corrêa D, Schwartz L, Jarnagin WR, Tuorto S, DeMatteo R, D’Angelica M, Allen P, Brown K, Fong Y. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg. 2010;145:351-354; discussion 354-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 53. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680; discussion 680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 54. | Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Hayakawa N, Yamamoto H. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology. 1995;21:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Imamura H, Shimada R, Kubota M, Matsuyama Y, Nakayama A, Miyagawa S, Makuuchi M, Kawasaki S. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 57. | Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Seo DD, Lee HC, Jang MK, Min HJ, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ, Ko GY. Preoperative portal vein embolization and surgical resection in patients with hepatocellular carcinoma and small future liver remnant volume: comparison with transarterial chemoembolization. Ann Surg Oncol. 2007;14:3501-3509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, Emile JF, Bismuth H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 61. | Tanaka H, Hirohashi K, Kubo S, Shuto T, Higaki I, Kinoshita H. Preoperative portal vein embolization improves prognosis after right hepatectomy for hepatocellular carcinoma in patients with impaired hepatic function. Br J Surg. 2000;87:879-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Wakabayashi H, Ishimura K, Okano K, Izuishi K, Karasawa Y, Goda F, Maeba T, Maeta H. Is preoperative portal vein embolization effective in improving prognosis after major hepatic resection in patients with advanced-stage hepatocellular carcinoma? Cancer. 2001;92:2384-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 63. | Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, Aikou T, Komokata T, Nakamura N, Sakata R. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Nagino M, Nimura Y, Kamiya J, Kondo S, Kanai M. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Gibo M, Unten S, Yogi A, Nakayama T, Ayukawa Y, Gibo S, Murayama S, Takara M, Shiraishi M. Percutaneous ipsilateral portal vein embolization using a modified four-lumen balloon catheter with fibrin glue: initial clinical experience. Radiat Med. 2007;25:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Madoff DC, Abdalla EK, Gupta S, Wu TT, Morris JS, Denys A, Wallace MJ, Morello FA, Ahrar K, Murthy R. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Shaw CM, Madoff DC. Acute Thrombosis of Left Portal Vein during Right Portal Vein Embolization Extended to Segment 4. Semin Intervent Radiol. 2011;28:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30-39. [PubMed] |
| 69. | Chen MF, Hwang TL, Hung CF. Human liver regeneration after major hepatectomy. A study of liver volume by computed tomography. Ann Surg. 1991;213:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, Furukawa K, Tanaka T, Tomoda F, Tanaka W. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology. 1993;18:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Nagino M, Nimura Y, Kamiya J, Kanai M, Hayakawa N, Yamamoto H. Immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization: CT demonstration. AJR Am J Roentgenol. 1998;171:1037-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Elias D, De Baere T, Roche A, Mducreux J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 73. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 246] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | de Graaf W, van Lienden KP, van den Esschert JW, Bennink RJ, van Gulik TM. Increase in future remnant liver function after preoperative portal vein embolization. Br J Surg. 2011;98:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 75. | Okabe H, Beppu T, Ishiko T, Masuda T, Hayashi H, Otao R, Hasita H, Okabe K, Sugiyama S, Baba H. Preoperative portal vein embolization (PVE) for patients with hepatocellular carcinoma can improve resectability and may improve disease-free survival. J Surg Oncol. 2011;104:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Xu C, Lv PH, Huang XE, Wang SX, Sun L, Wang FA, Wang LF. Safety and efficacy of sequential transcatheter arterial chemoembolization and portal vein embolization prior to major hepatectomy for patients with HCC. Asian Pac J Cancer Prev. 2014;15:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-520; discussion 520-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 671] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 78. | Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 536] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 79. | Moussa ME, Bean AG, Habib NA. Repeated resection for malignant liver tumours. Ann R Coll Surg Engl. 1995;77:364-368. [PubMed] |
| 80. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 586] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 81. | Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 82. | Jamal MH, Hassanain M, Chaudhury P, Tran TT, Wong S, Yousef Y, Jozaghi Y, Salman A, Jabbour S, Simoneau E. Staged hepatectomy for bilobar colorectal hepatic metastases. HPB (Oxford). 2012;14:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Tsai S, Marques HP, de Jong MC, Mira P, Ribeiro V, Choti MA, Schulick RD, Barroso E, Pawlik TM. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford). 2010;12:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 84. | Togo S, Nagano Y, Masui H, Tanaka K, Miura Y, Morioka D, Endo I, Sekido H, Ike H, Shimada H. Two-stage hepatectomy for multiple bilobular liver metastases from colorectal cancer. Hepatogastroenterology. 2005;52:913-919. [PubMed] |
| 85. | Shimada H, Tanaka K, Masui H, Nagano Y, Matsuo K, Kijima M, Ichikawa Y, Ike H, Ooki S, Togo S. Results of surgical treatment for multiple (> or =5 nodules) bi-lobar hepatic metastases from colorectal cancer. Langenbecks Arch Surg. 2004;389:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasguen N, Sibert A, Vilgrain V, Belghiti J. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg. 2008;12:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J Hepatol. 2006;45:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Baumgart JLS, Lang H. A new method for induction of liver hypertrophy prior to right trisectionectomy: a report of three cases. HPB (Oxford). 2011;71-72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 280] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 90. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-836; discussion 836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 91. | Cavaness KM, Doyle MB, Lin Y, Maynard E, Chapman WC. Using ALPPS to induce rapid liver hypertrophy in a patient with hepatic fibrosis and portal vein thrombosis. J Gastrointest Surg. 2013;17:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Vennarecci G, Laurenzi A, Santoro R, Colasanti M, Lepiane P, Ettorre GM. The ALPPS procedure: a surgical option for hepatocellular carcinoma with major vascular invasion. World J Surg. 2014;38:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Vennarecci G, Laurenzi A, Levi Sandri GB, Busi Rizzi E, Cristofaro M, Montalbano M, Piselli P, Andreoli A, D’Offizi G, Ettorre GM. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol. 2014;40:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Levi Sandri GB, Lai Q, Rayar M, Sulpice L. ALPPS procedure for hepatocellular carcinoma with macrovascular thrombosis: a new opportunity? J Hepatol. 2015;62:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 928] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 96. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 97. | Ielpo B, Quijano Y, Vicente E. Pearls and pitfalls on ALPPS procedure: new complications in a new technique. Updates Surg. 2014;66:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Chan AC, Pang R, Poon RT. Simplifying the ALPPS procedure by the anterior approach. Ann Surg. 2014;260:e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 100. | Knoefel WT, Gabor I, Rehders A, Alexander A, Krausch M, Schulte am Esch J, Fürst G, Topp SA. In situ liver transection with portal vein ligation for rapid growth of the future liver remnant in two-stage liver resection. Br J Surg. 2013;100:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 101. | Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien PA. Systematic Review and Meta-Analysis of Feasibility, Safety, and Efficacy of a Novel Procedure: Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. Ann Surg Oncol. 2015;22:3109-3120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |