Published online Aug 18, 2015. doi: 10.4254/wjh.v7.i17.2053
Peer-review started: February 25, 2015
First decision: June 3, 2015
Revised: June 22, 2015
Accepted: June 30, 2015
Article in press: July 2, 2015
Published online: August 18, 2015
Processing time: 178 Days and 18.9 Hours
Hepatocellular carcinoma (HCC) is the main cause of death in patients with cirrhosis, with an increasing incidence worldwide. Sorafenib is the choice therapy for advanced HCC. Over time several randomized phase III trials have been performed testing sunitinib, brivanib, linifanib and other molecules in head-to-head comparison with Sorafenib as first-line treatment for advanced-stage HCC, but none of these has so far been registered in this setting. Moreover, another feared vacuum arises from the absence of molecules registered as second-line therapy for patients who have failed Sorafenib, representing an urgent unmet medical need. To date all molecules tested as second-line therapies for advanced hepatocellular carcinoma, failed to demonstrate an increased survival compared to placebo. What are the possible reasons for the failure? What we should expect in the near future?
Core tip: Hepatocellular carcinoma (HCC) is the main cause of death in patients with cirrhosis with an increasing incidence worldwide. Sorafenib is the choice therapy for advanced HCC. Since then no other molecule has been registered as first-line therapy in this setting and one more vacuum arises from the absence of molecules registered as second-line therapy for patients who have failed Sorafenib. What are the reasons and what we should expect in the near future?
- Citation: Maida M, Iavarone M, Raineri M, Cammà C, Cabibbo G. Second line systemic therapies for hepatocellular carcinoma: Reasons for the failure. World J Hepatol 2015; 7(17): 2053-2057
- URL: https://www.wjgnet.com/1948-5182/full/v7/i17/2053.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i17.2053
Hepatocellular carcinoma (HCC) is the main cause of death in patients with cirrhosis with an increasing incidence worldwide and a poor prognosis even when treatments have been considered as potentially radical[1,2].
The natural history of this tumour is severe and extremely heterogeneous, due to the complex interplay between its biological characteristics and the frequent presence of an underlying chronic liver disease[3,4]. As part of this biological and clinical heterogeneity, several lines of evidence based on microarray technology point out how heterogeneity can be explained, least in part, from the identification of several molecular signatures (WNT, TGFβ, MAPK, EGFR, IGF-R, MET/HGF) able to predict prognosis and survival of HCC patients[5-8]. In this regard, a recent work remarked on the importance of genetic predisposition testing, in a clinical setting, a five-gene hepatic transcriptomic signature (angiopoietin-2, NETO2, DLL4, ESM1, NR4A1) able to identify patients with extremely rapid tumour growth and ominous prognosis[9].
In the absence of an ideal prognostic model, treatment algorithms for patients with HCC in Europe and North America have been assessed on the basis of the Barcelona Clinic Liver Cancer (BCLC) staging classification for HCC. It is currently the only staging system that includes an integrated assessment of liver disease, tumor extension, and presence of constitutional symptoms, providing in the meantime an indication of the first-line treatment. It classify stages of disease into five subgroups, from 0 to D, each associated with a specific therapy and prognosis[10].
As well known, the worst prognosis is allocated to patients with end stage disease (BCLC D). They cannot benefit from any specific cancer therapy due to the poor life expectance (median survival less than 3 mo), and could only receive the best available supportive care.
Besides this, patients classified as advanced stage (BCLC C) have a better prognosis of the above, but sill represents a critical group of the whole HCC population. In this subset surgical or loco-regional therapies are contraindicated and systemic therapies remains the only treatment option.
Previously, no effective therapy was offered for the treatment of patients at this stage, a scenario that was partially subverted in 2007 by the advent of Sorafenib, an oral multikinase inhibitor that, by blocking cell proliferation and angiogenesis of the tumour, has shown an improvement of survival according to two pivotal randomized controlled trials (RCTs)[11,12].
What happened next, up to now? About eight years after its introduction Sorafenib has then certainly innovated the clinical scenario of HCC, providing a practical treatment option in a subset of patients, which until then could not benefit from any therapy, but ultimately it has not represented the best desirable therapeutic progress for advanced HCC. Some lines of evidences have attenuated the effectiveness of Sorafenib and its safety profile in clinical practice compared to with those reported in the pivotal trials. Data from a field practice prospective study in Italy, Sorafenib Italian Assessment (SOFIA), confirmed the efficacy of Sorafenib with a lower safety profile compared to that of the phase III trial, showing also a significant proportion of patients who required a dose adjustment with an increased survival rate in those patients who received dose-adjusted Sorafenib (400 mg daily) for ≥ 70% of the treatment period, due to adverse events (AEs) or comorbidities, compared to those that received a full-dosed regimen (800 mg daily)[13]. Moreover the cost-effectiveness analysis based from the SOFIA study, showed that dose-adjusted Sorafenib therapy, compared to full-dose, is a cost-effective treatment for advanced HCC[14].
What came out after Sorafenib era? To date, as for the “first-line” scenario, different drugs have been tested, two different trial designs have been adopted for advanced HCC. The first one was the head-to-head comparison with Sorafenib, which is generally applied only if the effectiveness of a new agent shown to be very promising in early-phase trials.
Over time several randomized phase III trials have been performed testing sunitinib, brivanib, linifanib and other molecules in head-to-head comparison with Sorafenib as first-line treatment for advanced-stage HCC, but none of these has so far been registered in this setting[15-18]. Is important to note that, despite their safety and efficacy unfavorable results, many of these phase III trials were designed with the purpose of demonstrating the non-inferiority on Sorafenib. Anyway, non-inferiority studies have no ethical foundation, since they do not guarantee any possible advantage to patients and only favour pharmaceutical companies’ interests. For these reasons, non-inferiority trials in oncology should be avoided, especially when testing first-line therapies[15,19].
A second modality for first line therapy is to test a new drug in combination with Sorafenib vs Sorafenib alone. This modality has been adopted in different RCTs but failed to show a benefit in term of survival, and non of these combinations has been registered for advanced HCC.
In conclusion, Sorafenib remains the only drug for patients with advanced HCC, and dose-tailored to AEs and comorbidities, appears the only therapeutic innovation with Sorafenib.
Moreover, another feared vacuum arises from the absence of molecules registered as second-line therapy for patients who failed Sorafenib. In fact, in the last years, three randomized phase III trials testing brivanib, everolimus and ramucirumab as second-line therapies for advanced hepatocellular carcinoma, failed to demonstrate an increased survival compared to placebo[20-22]. Following these failures and from clinical practice, we have learned that patients who failed Sorafenib therapy represent a fragile and extremely heterogeneous population from which emerges a complex prognosis.
In this line, a study by Reig et al[23] clearly demonstrated a substantial differences in survival rates among progressors during Sorafenib therapy related to the pattern of HCC progression. The study shows a worse prognosis for patients developing new extrahepatic tumour lesions compared to those with expanding pre-existing lesions or new intra-hepatic nodules, only. While post progression survival of patients under Sorafenib is driven by tumour progression pattern, less known are the factors able to affect prognosis when therapy is discontinued due to other reasons.
In this regard, a recent study has been performed with the aim to identify predictors of survival on a sample of two-hundred and sixty HCC patients who discontinued Sorafenib therapy for any reasons[24]. Overall median post Sorafenib survival (PSS) was 4.1 mo, while median PSS was 9.3, 4.6 and 1.6 mo for BCLC B, BCLC C and BCLC D stage, respectively. Performance status (PS) (HR = 2.4), prothrombin time (HR = 2.9), macrovascular invasion (HR = 1.8), extrahepatic spread (HR = 1.6), alpha-fetoprotein ≥ 400 ng/mL (HR = 1.4) and reason for Sorafenib discontinuation were find to be independent predictors of worse survival by multivariate analysis. Between all causes for Sorafenib interruption the best prognosis was assessed for patients who interrupted for AEs, followed by tumour progression and then by liver function worsening group (liver decompensation vs AEs HR = 2.6, tumor progression vs AEs HR = 1.5).
Within the whole court, 200 patients (77%) with Child-Pugh score up to 7, were considered eligible for inclusion in second-line experimental therapy. In this subset, the presence of macrovascular invasion and extrahepatic spread, PS and the reason for Sorafenib discontinuation, were found to be independent predictors of mortality by multivariate analysis.
Therefore discontinuation due to adverse events in the absence of PS impairment and vascular or extrahepatic diffusion of the tumor, estimates the best post Sorafenib survival in compensated patients, emphasizing the role of these predictors in stratifying patients potentially eligible for second-line studies[24].
This adds further weight to the need to change the current design of second-lines trials, focusing on the importance of stratification among the clinical and biological heterogeneity of cancer after exposure to first-line systemic therapy.
This, in the near future, the genetic signature will likely provide a great contribution for prognostic profiling of patients with advanced HCC and then for a proper planning and design of first and second line clinical trials. In this line, a recent multicentric, randomised, placebo-controlled, double-blind, phase 2 study testing Tivantinib, a selective oral inhibitor of MET, vs placebo, as second-line therapy for advanced HCC, showed that, regardless of treatment, patients with MET high-expression tumours had significantly shorter overall survival compared to the MET low-expression subgroup[25].
Waiting for new effective therapies and further advances in genetic prognostic characterization of the tumor, the evidence that PSS depends on the reasons of therapy discontinuation could support clinicians in counselling and management of these patients.
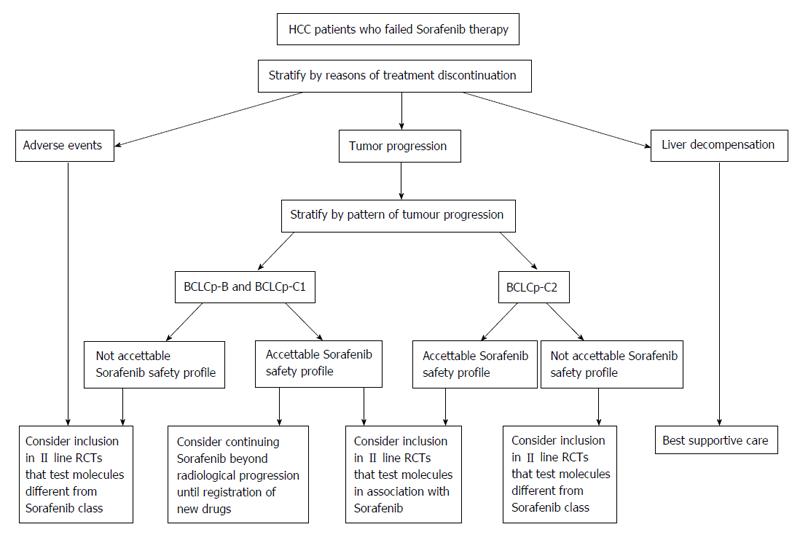
In this line, patients who discontinue therapy for adverse events may be considered for inclusion in II line RCTs that test molecules different from Sorafenib class. The same way as these, can be managed those patients who discontinue therapy for tumor progression with a poor experienced Sorafenib safety profile.
Contrariwise, another strategy that could be offered to patients with radiological progression and good Sorafenib safety profile, is to continue Sorafenib therapy until symptomatic progression, or to consider inclusion in II line RCTs that test molecules in association with Sorafenib. In this group of progressor patients, the decision making process, could be supported by stratification using “BCLC staging system upon progression”. On the contrary the patients who suspend for hepatic failure may only receive the best supportive care, since they have a poor prognosis (Figure 1).
In conclusion, it is clear by now, especially from the clinical point of view, the importance of a correct identification of the reason for Sorafenib discontinuation, in order to obtain an optimal management of HCC patients.
On the other hand, despite this and the proposed strategy, we are still facing with a scenario showing us the failure of the of first and second line systemic therapy trials, leaving Sorafenib as the last outpost for the treatment of advanced stage, a picture almost unchanged over the past seven years. What to expect from the future?
P- Reviewer: Chen CH, Du Z, Snyder N S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 3. | Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Cabibbo G, Maida M, Genco C, Parisi P, Peralta M, Antonucci M, Brancatelli G, Cammà C, Craxì A, Di Marco V. Natural history of untreatable hepatocellular carcinoma: A retrospective cohort study. World J Hepatol. 2012;4:256-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 746] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 6. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1004] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 7. | Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 687] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Kim SM, Leem SH, Chu IS, Park YY, Kim SC, Kim SB, Park ES, Lim JY, Heo J, Kim YJ. Sixty-five gene-based risk score classifier predicts overall survival in hepatocellular carcinoma. Hepatology. 2012;55:1443-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Villa E, Critelli R, Lei B, Marzocchi G, Cammà C, Giannelli G, Pontisso P, Cabibbo G, Enea M, Colopi S. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2866] [Article Influence: 110.2] [Reference Citation Analysis (1)] |
| 11. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10237] [Article Influence: 602.2] [Reference Citation Analysis (2)] |
| 12. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4638] [Article Influence: 272.8] [Reference Citation Analysis (0)] |
| 13. | Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Cammà C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A, Gasbarrini A, Villa E, Zavaglia C, Bruno R. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Genco C, Cabibbo G, Maida M, Brancatelli G, Galia M, Alessi N, Butera G, Genova C, Romano P, Raineri M. Treatment of hepatocellular carcinoma: present and future. Expert Rev Anticancer Ther. 2013;13:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 17. | Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 18. | Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, Hsu CH, Hu TH, Heo J, Xu J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 19. | Garattini S, Bertele’ V. Non-inferiority trials are unethical because they disregard patients’ interests. Lancet. 2007;370:1875-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 484] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 21. | Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 22. | Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 652] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 23. | Reig M, Rimola J, Torres F, Darnell A, Rodriguez-Lope C, Forner A, Llarch N, Ríos J, Ayuso C, Bruix J. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58:2023-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Iavarone M, Cabibbo G, Biolato M, Della Corte C, Maida M, Barbara M, Basso M, Vavassori S, Craxì A, Grieco A. Predictors of survival of patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |